Cell Membrane Adaptations Mediate β-Lactam-Induced Resensitization of Daptomycin-Resistant (DAP-R) Staphylococcus aureus In Vitro
Abstract
:1. Introduction
2. Materials and Methods
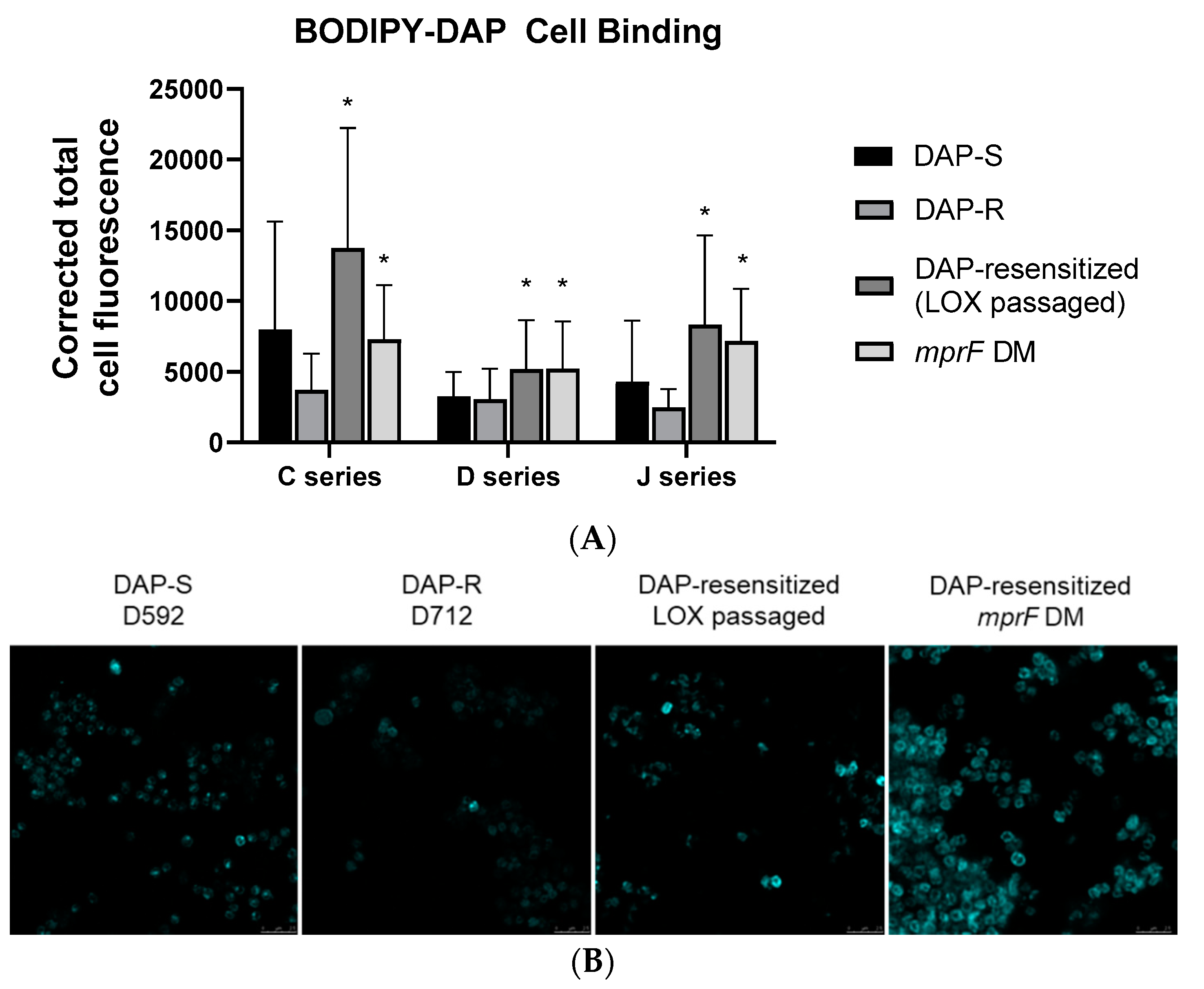
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fowler, V.G., Jr.; Miro, J.M.; Hoen, B.; Cabell, C.H.; Abrutyn, E.; Rubinstein, E.; Corey, G.R.; Spelman, D.; Bradley, S.F.; Barsic, B.; et al. Staphylococcus aureus endocarditis: A consequence of medical progress. JAMA 2005, 293, 3012–3021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Hal, S.J.; Jensen, S.O.; Vaska, V.L.; Espedido, B.A.; Paterson, D.L.; Gosbell, I.B. Predictors of Mortality in Staphylococcus aureus Bacteremia. Clin. Microbiol. Rev. 2012, 25, 362–386. [Google Scholar] [CrossRef] [Green Version]
- Kullar, R.; Sakoulas, G.; Deresinski, S.; Van Hal, S.J. When sepsis persists: A review of MRSA bacteraemia salvage therapy. J. Antimicrob. Chemother. 2016, 71, 576–586. [Google Scholar] [CrossRef] [Green Version]
- Gerber, J.S.; Coffin, S.E.; Smathers, S.A.; Zaoutis, T.E. Trends in the Incidence of Methicillin-Resistant Staphylococcus aureus Infection in Children’s Hospitals in the United States. Clin. Infect. Dis. 2009, 49, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Guimaraes, A.O.; Cao, Y.; Hong, K.; Mayba, O.; Peck, M.C.; Gutierrez, J.; Ruffin, F.; Carrasco-Triguero, M.; Dinoso, J.B.; Clemenzi-Allen, A.; et al. A prognostic model of persistent bacteremia and mortality in complicated Staphylococcus aureus bloodstream infection. Clin. Infect. Dis. 2019, 68, 1502–1511. [Google Scholar] [CrossRef] [PubMed]
- Rose, W.E.; Shukla, S.K.; Berti, A.D.; Hayney, M.S.; Henriquez, K.M.; Ranzoni, A.; Cooper, M.A.; Proctor, R.A.; Nizet, V.; Sakoulas, G. Increased Endovascular Staphylococcus aureus Inoculum Is the Link Between Elevated Serum Interleukin 10 Concentrations and Mortality in Patients with Bacteremia. Clin. Infect. Dis. 2017, 64, 1406–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical Practice Guidelines by the Infectious Diseases Society of America for the Treatment of Methicillin-Resistant Staphylococcus aureus Infections in Adults and Children: Executive Summary. Clin. Infect. Dis. 2011, 52, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Claeys, K.C.; Zasowski, E.J.; Casapao, A.M.; Lagnf, A.M.; Nagel, J.L.; Nguyen, C.T.; Hallesy, J.A.; Compton, M.T.; Kaye, K.S.; Levine, D.P.; et al. Daptomycin improves outcomes regardless of vancomycin MIC in a pro-pensity-matched analysis of methicillin-resistant Staphylococcus aureus bloodstream infections. Antimicrob. Agents Chemother. 2016, 60, 5841–5848. [Google Scholar] [CrossRef] [Green Version]
- Moise, P.A.; Culshaw, D.L.; Wong-Beringer, A.; Bensman, J.; Lamp, K.C.; Smith, W.J.; Bauer, K.; Goff, D.A.; Adamson, R.; Leuthner, K.; et al. Comparative effectiveness of vancomycin versus daptomycin for MRSA bacteremia with vancomycin MIC >1 mg/L: A multicenter evaluation. Clin. Ther. 2016, 38, 16–30. [Google Scholar] [CrossRef]
- Fowler, V.G., Jr.; Boucher, H.W.; Corey, G.R.; Abrutyn, E.; Karchmer, A.W.; Rupp, M.E.; Levine, D.P.; Chambers, H.F.; Tally, F.P.; Vigliani, G.A.; et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N. Engl. J. Med. 2006, 355, 653–665. [Google Scholar] [CrossRef] [Green Version]
- Capone, A.; Cafiso, V.; Campanile, F.; Parisi, G.; Mariani, B.; Petrosillo, N.; Stefani, S. In vivo development of daptomycin resistance in vancomycin-susceptible methicillin-resistant Staphylococcus aureus severe infections previously treated with glycopeptides. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 625–631. [Google Scholar] [CrossRef]
- Marty, F.M.; Yeh, W.W.; Wennersten, C.B.; Venkataraman, L.; Albano, E.; Alyea, E.P.; Gold, H.S.; Baden, L.R.; Pillai, S.K. Emergence of a clinical daptomycin-resistant Staphylococcus aureus isolate during treatment of methicillin-resistant Staphylococcus aureus bacteremia and osteomyelitis. J. Clin. Microbiol. 2006, 44, 595–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefani, S.; Campanile, F.; Santagati, M.; Mezzatesta, M.L.; Cafiso, V.; Pacini, G. Insights and clinical perspectives of daptomycin resistance in Staphylococcus aureus: A review of the available evidence. Int. J. Antimicrob. Agents 2015, 46, 278–289. [Google Scholar] [CrossRef]
- Skiest, D.J. Treatment Failure Resulting from Resistance of Staphylococcus aureus to Daptomycin. J. Clin. Microbiol. 2006, 44, 655–656. [Google Scholar] [CrossRef] [Green Version]
- Chan, L.C.; Basuino, L.; Dip, E.C.; Chambers, H.F. Comparative Efficacies of Tedizolid Phosphate, Vancomycin, and Daptomycin in a Rabbit Model of Methicillin-Resistant Staphylococcus aureus Endocarditis. Antimicrob. Agents Chemother. 2015, 59, 3252–3256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, K.D.; Sulaiman, R.M.; Rybak, M.J. Dalbavancin and Oritavancin: An Innovative Approach to the Treatment of Gram-Positive Infections. Pharmacotherapy 2015, 35, 935–948. [Google Scholar] [CrossRef] [PubMed]
- Guskey, M.T.; Tsuji, B.T. A Comparative Review of the Lipoglycopeptides: Oritavancin, Dalbavancin, and Telavancin. Pharmacotherapy 2010, 30, 80–94. [Google Scholar] [CrossRef] [PubMed]
- Long, S.W.; Olsen, R.J.; Mehta, S.C.; Palzkill, T.; Cernoch, P.L.; Perez, K.K.; Musick, W.L.; Rosato, A.E.; Musser, J.M. PBP2a Mutations Causing High-Level Ceftaroline Resistance in Clinical Methicillin-Resistant Staphylococcus aureus Isolates. Antimicrob. Agents Chemother. 2014, 58, 6668–6674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, L.C.; Basuino, L.; Diep, B.; Hamilton, S.; Chatterjee, S.S.; Chambers, H.F. Ceftobiprole- and Ceftaroline-Resistant Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2015, 59, 2960–2963. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.-J.; Xiong, Y.Q.; Boyle-Vavra, S.; Daum, R.S.; Jones, T.; Bayer, A.S. Daptomycin-Oxacillin Combinations in Treatment of Experimental Endocarditis Caused by Daptomycin-Nonsusceptible Strains of Methicillin-Resistant Staphylococcus aureus with Evolving Oxacillin Susceptibility (the “Seesaw Effect”). Antimicrob. Agents Chemother. 2010, 54, 3161–3169. [Google Scholar] [CrossRef] [Green Version]
- Ortwine, J.K.; Werth, B.J.; Sakoulas, G.; Rybak, M.J. Reduced glycopeptide and lipopeptide susceptibility in Staphylococcus aureus and the “seesaw effect”: Taking advantage of the back door left open? Drug Resist. Updates 2013, 16, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Vignaroli, C.; Rinaldi, C.; Varaldo, P.E. Striking “seesaw effect” between daptomycin nonsusceptibility and beta-lactam susceptibility in Staphylococcus haemolyticus. Antimicrob. Agents Chemother. 2011, 55, 2495–2496. [Google Scholar] [CrossRef] [Green Version]
- Patel, J.B.; Jevitt, L.A.; Hageman, J.; McDonald, L.C.; Tenover, F.C. An Association between Reduced Susceptibility to Daptomycin and Reduced Susceptibility to Vancomycin in Staphylococcus aureus. Clin. Infect. Dis. 2006, 42, 1652–1653. [Google Scholar] [CrossRef]
- Fischer, A.; Yang, S.-J.; Bayer, A.S.; Vaezzadeh, A.R.; Herzig, S.; Stenz, L.; Girard, M.; Sakoulas, G.; Scherl, A.; Yeaman, M.R.; et al. Daptomycin resistance mechanisms in clinically derived Staphylococcus aureus strains assessed by a combined transcriptomics and proteomics approach. J. Antimicrob. Chemother. 2011, 66, 1696–1711. [Google Scholar] [CrossRef] [Green Version]
- Bayer, A.S.; Mishra, N.N.; Chen, L.; Kreiswirth, B.N.; Rubio, A.; Yang, S.J. Frequency and distribution of single-nucleotide polymorphisms within mprF in methicillin-resistant Staphylococcus aureus clinical isolates and their role in cross-resistance to daptomycin and host defense antimicrobial peptides. Antimicrob. Agents Chemother. 2015, 59, 4930–4937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayer, A.S.; Mishra, N.N.; Cheung, A.L.; Rubio, A.; Yang, S.J. Dysregulation of mprF and dltABCD expression among dap-tomycin-non-susceptible MRSA clinical isolates. J. Antimicrob. Chemother. 2016, 71, 2100–2104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayer, A.S.; Schneider, T.; Sahl, H.-G. Mechanisms of daptomycin resistance in Staphylococcus aureus: Role of the cell membrane and cell wall. Ann. N. Y. Acad. Sci. 2013, 1277, 139–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ernst, C.M.; Slavetinsky, C.J.; Kuhn, S.; Hauser, J.N.; Nega, M.; Mishra, N.N.; Gekeler, C.; Bayer, A.S.; Peschel, A. Gain-of-Function Mutations in the Phospholipid Flippase MprF Confer Specific Daptomycin Resistance. mBio 2018, 9, e0659-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.J.; Mishra, N.N.; Kang, K.M.; Lee, G.Y.; Park, J.H.; Bayer, A.S. Impact of multiple single-nucleotide polymorphisms within mprF on daptomycin resistance in Staphylococcus aureus. Microb. Drug Resist. 2018, 24, 1075–1081. [Google Scholar] [CrossRef]
- Ernst, C.M.; Staubitz, P.; Mishra, N.N.; Yang, S.-J.; Hornig, G.; Kalbacher, H.; Bayer, A.S.; Kraus, D.; Peschel, A. The Bacterial Defensin Resistance Protein MprF Consists of Separable Domains for Lipid Lysinylation and Antimicrobial Peptide Repulsion. PLOS Pathog. 2009, 5, e1000660. [Google Scholar] [CrossRef] [Green Version]
- Mishra, N.N.; Yang, S.J.; Chen, L.; Muller, C.; Saleh-Mghir, A.; Kuhn, S.; Peschel, A.; Yeaman, M.R.; Nast, C.C.; Kreiswirt, B.N.; et al. Emergence of daptomycin resistance in daptomycin-naïve rabbits with methicil-lin-resistant Staphylococcus aureus prosthetic joint infection is associated with resistance to host defense cationic peptides and mprF polymorphisms. PLoS ONE 2013, 8, e71151. [Google Scholar] [CrossRef]
- Mishra, N.N.; Bayer, A.S.; Weidenmaier, C.; Grau, T.; Wanner, S.; Stefani, S.; Cafiso, V.; Bertuccio, T.; Yeaman, M.R.; Nast, C.C.; et al. Phenotypic and Genotypic Characterization of Daptomycin-Resistant Methicillin-Resistant Staphylococcus aureus Strains: Relative Roles of mprF and dlt Operons. PLoS ONE 2014, 9, e107426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, N.N.; Rubio, A.; Nast, C.C.; Bayer, A.S. Differential adaptations of methicillin-resistant Staphylococcus aureus to serial in vitro passage in daptomycin: Evolution of daptomycin resistance and role of membrane carotenoid content and fluidity. Int. J. Microbiol. 2012, 2012, 683450. [Google Scholar] [CrossRef] [Green Version]
- Mishra, N.N.; Liu, G.Y.; Yeaman, M.R.; Nast, C.C.; Proctor, R.A.; McKinnell, J.; Bayer, A.S. Carotenoid-Related Alteration of Cell Membrane Fluidity Impacts Staphylococcus aureus Susceptibility to Host Defense Peptides. Antimicrob. Agents Chemother. 2011, 55, 526–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, N.N.; Bayer, A.S. Correlation of cell membrane lipid profiles with daptomycin resistance in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2013, 57, 1082–1085. [Google Scholar] [CrossRef] [Green Version]
- Mishra, N.N.; Yang, S.-J.; Sawa, A.; Rubio, A.; Nast, C.C.; Yeaman, M.R.; Bayer, A.S. Analysis of Cell Membrane Characteristics of In vitro-Selected Daptomycin-Resistant Strains of Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2009, 53, 2312–2318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhand, A.; Bayer, A.S.; Pogliano, J.; Yang, S.J.; Bolaris, M.; Nizet, V.; Wang, G.; Sakoulas, G. Use of antistaphylococcal beta-lactams to increase daptomycin activity in eradicating persistent bacteremia due to methicillin-resistant Staphylococcus aureus: Role of enhanced daptomycin binding. Clin. Infect. Dis. 2011, 53, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Berti, A.D.; Theisen, E.; Sauer, J.-D.; Nonejuie, P.; Olson, J.; Pogliano, J.; Sakoulas, G.; Nizet, V.; Proctor, R.A.; Rose, W.E. Penicillin Binding Protein 1 Is Important in the Compensatory Response of Staphylococcus aureus to Daptomycin-Induced Membrane Damage and Is a Potential Target for β-Lactam–Daptomycin Synergy. Antimicrob. Agents Chemother. 2015, 60, 451–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, A.; Wenzel, M.; Strahl, H.; Grein, F.; Saaki, T.N.; Kohl, B.; Siersma, T.; Bandow, J.E.; Sahl, H.-G.; Schneider, T.; et al. Daptomycin inhibits cell envelope synthesis by interfering with fluid membrane mi-crodomains. Proc. Natl. Acad. Sci. USA 2016, 113, E7077–E7086. [Google Scholar] [CrossRef] [Green Version]
- Grein, F.; Müller, A.; Scherer, K.M.; Liu, X.; Ludwig, K.C.; Klöckner, A.; Strach, M.; Sahl, H.-G.; Kubitscheck, U.; Schneider, T. Ca2+-Daptomycin targets cell wall biosynthesis by forming a tripartite complex with undecaprenyl-coupled intermediates and membrane lipids. Nat. Commun. 2020, 11, 1455. [Google Scholar] [CrossRef] [Green Version]
- Jenson, R.E.; Baines, S.L.; Howden, B.P.; Mishra, N.N.; Farah, S.; Lew, C.; Berti, A.D.; Shukla, S.K.; Bayer, A.S.; Rose, W.E. Prolonged exposure to β-lactam antibiotics reestablishes susceptibility of dap-tomycin-nonsusceptible Staphylococcus aureus to daptomycin. Antimicrob. Agents Chemother. 2020, 64, e00890-20. [Google Scholar] [CrossRef]
- Berti, A.D.; Sakoulas, G.; Nizet, V.; Tewhey, R.; Rose, W.E. β-Lactam Antibiotics Targeting PBP1 Selectively Enhance Daptomycin Activity against Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2013, 57, 5005–5012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasser, M. Identification of Bacteria by Gas Chromatography of Cellular Fatty Acids; MIDI Technical Note 101; MIDI Inc.: Newark, NJ, USA, 1990. [Google Scholar]
- Monk, I.R.; Stinear, T.P. From cloning to mutant in 5 days: Rapid allelic exchange in Staphylococcus aureus. Access Microbiol. 2021, 3, 193. [Google Scholar] [CrossRef]
- Zhang, Y.; Werling, U.; Edelmann, W. SLiCE: A novel bacterial cell extract-based DNA cloning method. Nucleic Acids Res. 2012, 40, e55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monk, I.R.; Tree, J.J.; Howden, B.P.; Stinear, T.P.; Foster, T.J. Complete bypass of restriction systems for major Staphylococcus aureus lineages. mBio 2015, 26, e00308-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, N.N.; McKinnell, J.; Yeaman, M.R.; Rubio, A.; Nast, C.C.; Chen, L.; Kreiswirth, B.N.; Bayer, A.S. In vitro Cross-Resistance to Daptomycin and Host Defense Cationic Antimicrobial Peptides in Clinical Methicillin-Resistant Staphylococcus aureus Isolates. Antimicrob. Agents Chemother. 2011, 55, 4012–4018. [Google Scholar] [CrossRef] [Green Version]
- García-Fernández, E.; Koch, G.; Wagner, R.M.; Fekete, A.; Stengel, S.T.; Schneider, J.; Mielich-Süss, B.; Geibel, S.; Markert, S.M.; Stigloher, C.; et al. Membrane Microdomain Disassembly Inhibits MRSA Antibiotic Resistance. Cell 2017, 171, 1354–1367.e20. [Google Scholar] [CrossRef] [PubMed]
- Klein, W.; Weber, M.H.W.; Marahiel, M.A. Cold Shock Response of Bacillus subtilis: Isoleucine-Dependent Switch in the Fatty Acid Branching Pattern for Membrane Adaptation to Low Temperatures. J. Bacteriol. 1999, 181, 5341–5349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaupp, R.; Lei, S.; Reed, J.M.; Peisker, H.; Boyle-Vavra, S.; Bayer, A.S.; Bischoff, M.; Herrmann, M.; Daum, R.S.; Powers, R.; et al. Staphylococcus aureus metabolic adaptations during the transition from a daptomycin susceptibility phenotype to a daptomycin nonsusceptibility phenotype. Antimicrob. Agents Chemother. 2015, 59, 4226–4238. [Google Scholar] [CrossRef] [Green Version]
| Strain Set a | Strain Name | Strain Description | DAP MIC b (µg/mL) | LOX MIC c (µg/mL) | SNPs in mprF d |
|---|---|---|---|---|---|
| I | C24 | DAP-S | 0.5 | 8 | WT |
| C25 | DAP-R | 2 | 4 | S295L | |
| C25-LOX | DAP-resensitized (LOX passaged) | <0.125 | 8 | S295L + L84 (Translocase domain) | |
| C25, mprF DM | Secondary mprF mutation (L84 e) introduced into C25 | 0.125 | 16 | S295L + L84 e | |
| II | D592 | DAP-S | 0.5 | 512 | WT |
| D712 | DAP-R | 2 | 512 | L341S | |
| D712-LOX | DAP-resensitized (LOX passaged) | 0.5 | 1024 | L341S + S136L (Translocase domain) | |
| D712, mprF DM | Secondary mprF mutation (S136L) introduced into D712 | 0.5 | 1024 | L341S + S136L | |
| III | J01 | DAP-S | 0.5 | 16 | WT |
| J03 | DAP-R | 2–4 | 2 | T345I | |
| J03-LOX | DAP-resensitized (LOX passaged) | 0.125 | 32 | T345I + R788L Synthase domain | |
| J03, mprF DM | Secondary mprF mutation (R788L) introduced into J03 | 0.125 | 16 | T345I + R788L |
| Strains | Total LPG | PG | CL |
|---|---|---|---|
| C24 | 12 ± 3 | 80 ± 6 | 8 ± 5 |
| C25 | 25 ± 5 a | 70 ± 5 a | 6 ± 3 |
| C25-LOX | 5 ± 1 b | 94 ± 1 b | 2 ± 1 b |
| C25, mprF DM | 11 ± 2 c | 83 ± 4 c | 6 ± 6 |
| D592 | 20 ± 3 | 77 ± 3 | 2 ± 3 |
| D712 | 23 ± 2 a | 74 ± 4 a | 3 ± 2 |
| D712-LOX | 16 ± 4 b | 81 ± 5 b | 3 ± 2 |
| D712, mprF DM | 21 ± 2 | 75 ± 2 | 4 ± 1 |
| J01 | 22 ± 2 | 70 ± 2 | 8 ± 2 |
| J03 | 31 ± 7 a | 66 ± 6 a | 3 ± 1 a |
| J03-LOX | 20 ± 1 b | 78 ± 3 b | 3 ± 2 b |
| J03, mprF DM | 16 ± 3 c | 78 ± 4 c | 6 ± 1 c |
| Strains | % Cytochrome C Unbound |
|---|---|
| C24 | 53 ± 1 |
| C25 | 62 ± 0 a |
| C25-LOX | 54 ± 0 b |
| C25, mprF DM | 45 ± 0 c |
| D592 | 56 ± 0 |
| D712 | 85 ± 3 a |
| D712-LOX | 46 ± 1 b |
| D712, mprF DM | 57 ± 1 |
| J01 | 58 ± 0 |
| J03 | 48 ± 0 a |
| J03-LOX | 55 ± 0 b |
| J03, mprF DM | 44 ± 0 c |
| Strain Set a | Iso FA | Anteiso FA | SFA |
|---|---|---|---|
| C24 | 30 ± 0.1 | 41 ± 0.12 | 25 ± 0.03 |
| C25 | 27 ± 0.01 a | 44 ± 0.02 a | 22 ± 0.03 a |
| C25-LOX | 25 ± 0.07 b,c | 41 ± 0.1 b,c | 29 ± 0.3 b,c |
| D592 | 24 ± 0.6 | 40 ± 0.03 | 31 ± 0.4 |
| D712 | 25 ± 0.01 a | 40 ± 0.03 | 26 ± 0.2 a |
| D712-LOX | 25 ± 0.04 b | 45 ± 0.2 b,c | 31 ± 0.2 c |
| J01 | 31 ± 0.03 | 40 ± 0.03 | 25 ± 0.02 |
| J03 | 29 ± 0.1 a | 45 ± 0.2 a | 22 ± 0.11 a |
| J03-LOX | 27 ± 0.1 b,c | 43 ± 0.11 b,c | 26 ± 0.04 b,c |
| Strains | CM Fluidity (PI Value) | Carotenoids (OD450nm) |
|---|---|---|
| C24 | 0.389 ± 0.01 | 0.685 ± 0.04 |
| C25 | 0.370 ± 0.01 a | 0.261 ± 0.03 a |
| C25-LOX | 0.413 ± 0.00 b | 1.129 ± 0.24 b |
| C25, mprF DM | 0.368 ± 0.00 | 0.627 ± 0.02 c |
| D592 | 0.408 ± 0.01 | 1.338 ± 0.01 |
| D712 | 0.372 ± 0.01 a | 0.878 ± 0.01 a |
| D712-LOX | 0.389 ± 0.00 b | 1.037 ± 0.02 b |
| C25, mprF DM | 0.395 ± 0.00 c | 0.971 ± 0.03 c |
| J01 | 0.381± 0.01 | 1.121 ± 0.04 |
| J03 | 0.359 ± 0.01 | 0.697 ± 0.12 a |
| J03-LOX | 0.395 ± 0.01 b | 1.518 ± 0.27 b |
| C25, mprF DM | 0.430 ± 0.03 c | 1.545 ± 0.37 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, N.N.; Bayer, A.S.; Baines, S.L.; Hayes, A.S.; Howden, B.P.; Lapitan, C.K.; Lew, C.; Rose, W.E. Cell Membrane Adaptations Mediate β-Lactam-Induced Resensitization of Daptomycin-Resistant (DAP-R) Staphylococcus aureus In Vitro. Microorganisms 2021, 9, 1028. https://doi.org/10.3390/microorganisms9051028
Mishra NN, Bayer AS, Baines SL, Hayes AS, Howden BP, Lapitan CK, Lew C, Rose WE. Cell Membrane Adaptations Mediate β-Lactam-Induced Resensitization of Daptomycin-Resistant (DAP-R) Staphylococcus aureus In Vitro. Microorganisms. 2021; 9(5):1028. https://doi.org/10.3390/microorganisms9051028
Chicago/Turabian StyleMishra, Nagendra N., Arnold S. Bayer, Sarah L. Baines, Ashleigh S. Hayes, Benjamin P. Howden, Christian K. Lapitan, Cassandra Lew, and Warren E. Rose. 2021. "Cell Membrane Adaptations Mediate β-Lactam-Induced Resensitization of Daptomycin-Resistant (DAP-R) Staphylococcus aureus In Vitro" Microorganisms 9, no. 5: 1028. https://doi.org/10.3390/microorganisms9051028
APA StyleMishra, N. N., Bayer, A. S., Baines, S. L., Hayes, A. S., Howden, B. P., Lapitan, C. K., Lew, C., & Rose, W. E. (2021). Cell Membrane Adaptations Mediate β-Lactam-Induced Resensitization of Daptomycin-Resistant (DAP-R) Staphylococcus aureus In Vitro. Microorganisms, 9(5), 1028. https://doi.org/10.3390/microorganisms9051028






