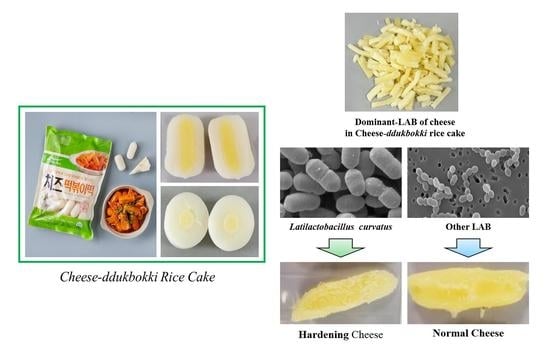
Hardening Properties of Cheeses by Latilactobacillus curvatus PD1 Isolated from Hardened Cheese-Ddukbokki Rice Cake
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of Lactic Acid Bacteria Strains from Cheese and Cheese-Ddukbokki Rice Cake
2.2. Growth Properties of Latilactobacillus curvatus at Different Temperature Conditions
2.3. Preparation of Cheese-Ddukbokki Rice Cake Containing Different Dominant Lactic Acid Bacteria
2.4. pH, Water Content and Meltability Test of Cheese-Ddukbokki Rice Cake
2.5. Microbial Analysis of Cheese-Ddukbokki Rice Cake
2.6. Proteolytic Activity of Lactic Acid Bacteria and Cheese-Ddukbokki Rice Cake
3. Results and Discussion
3.1. Isolation and Identification of Lactic Acid Bacteria Strains
3.2. Growth Properties of Latilactobacillus curvatus at Different Temperature
3.3. pH, Water Content, and Meltability Test of Cheese-Ddukbokki Rice Cake
3.4. Microbial Analysis of Cheese-Ddukbokki Rice Cake
3.5. Proteolytic Activity of Lactic Acid Bacteria and Cheese-Ddukbokki Rice Cake
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beresford, T.P.; Fitzsimons, N.A.; Brennan, N.L.; Cogan, T.M. Recent advances in cheese microbiology. Int. Dairy J. 2001, 11, 259–274. [Google Scholar] [CrossRef]
- Sandine, W.E.; Elliker, P.R. Microbially induced flavours and fermented foods flavour in fermented dairy products. J. Agric. Food Chem. 1970, 18, 557–562. [Google Scholar] [CrossRef]
- Fox, P.F. Cheese: An overview. In Cheese: Chemistry, Physics and Microbiology, 2nd ed.; Fox, P.F., Ed.; Springer: Boston, MA, USA, 1993; pp. 1–36. [Google Scholar]
- Lee, J.; Yoon, Y. Microbiological safety concerns with dairy products from farmstead plants. J. Milk Sci. Biotechnol. 2017, 35, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Food Information Statistics System Home Page. Available online: https://www.atfis.or.kr/article/M001050000/list.do (accessed on 27 November 2015).
- Eliot, S.C.; Vuillemard, J.-C.; Emond, J.-P. Stability of shredded mozzarella cheese under modified atmospheres. J. Food Sci. 1998, 63, 1075–1080. [Google Scholar] [CrossRef]
- Cheon, H.S.; Cho, W.-I.; Lee, S.J.; Chung, M.-S.; Choi, J.-B. Acidic and steaming treatments of tteokbokki rice cake to improve its microbial and textural properties. Korean J. Food Sci. Technol. 2017, 49, 502–506. [Google Scholar]
- Lawrence, R.C.; Creamer, L.K.; Gilles, J. Texture development during cheese ripening. J. Dairy Sci. 1987, 70, 1748–1760. [Google Scholar] [CrossRef]
- Fox, P.F. Proteolysis in cheese during ripening. J. Dairy Sci. 1996, 72, 1379–1400. [Google Scholar] [CrossRef]
- Law, J.; Haandrikman, A. Proteolytic enzymes of lactic acid bacteria. Int. Dairy J. 1997, 7, 1–11. [Google Scholar] [CrossRef]
- Christensen, J.E.; Dudley, E.G.; Pederson, J.A.; Steele, J.L. Peptidases and amino acid catabolism in lactic acid bacteria. Antoine van Leeuwenhoek 1999, 76, 217–246. [Google Scholar] [CrossRef]
- Sousaa, M.J.; Ardo, Y.; McSweeney, P.L.H. Advances in the study of proteolysis during cheese ripening. Int. Dairy J. 2001, 11, 327–345. [Google Scholar] [CrossRef]
- Yun, J.J.; Barbano, D.M.; Kiely, L.J.; Kindstedt, P.S. Mozzarella cheese: Impact of rod:coccus ratio on composition, proteolysis, and functional properties. J. Dairy Sci. 1995, 78, 751–760. [Google Scholar] [CrossRef]
- Pax, A.P.; Ong, L.; Kentish, S.E.; Gras, S.L. Effects of shredding on the functionality, microstructure and proteolysis of low moisture mozzarella cheese. Int. Dairy J. 2021, 117, 104979. [Google Scholar] [CrossRef]
- Bergamini, C.V.; Hynes, E.R.; Palma, S.B.; Sabbag, N.G.; Zalazar, C.A. Proteolytic activity of three probiotic strains in semi-hard cheese as single and mixed cultures: Lactobacillus acidophilus, Lactobacillus paracasei and Bifidobacterium lactis. Int. Dairy J. 2009, 19, 467–475. [Google Scholar] [CrossRef]
- Williams, A.G.; Banks, J.M. Proteolytic and other hydrolytic enzyme activities in non-starter lactic acid bacteria (NSLAB) isolated from cheddar cheese manufactured in the United Kingdom. Int. Dairy J. 1997, 7, 763–774. [Google Scholar] [CrossRef]
- Porcellato, D.; Johnson, M.E.; Houck, K.; Skeie, S.B.; Mills, D.A.; Kalanetra, K.M.; Steele, J.L. Potential of Lactobacillus curvatus LFC1 to produce slits in Cheddar cheese. Food Microbiol. 2015, 49, 65–73. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Mikelsone, A.; Ciprovica, I. Analysis of cheese microflora. Fac. Food Technol. 2011, 2010, 96–102. [Google Scholar]
- De Marques, J.L.; Funck, G.D.; da Dannenberg, G.S.; dos Cruxen, C.E.S.; Halal, S.L.M.E.; Dias, A.R.G.; Fiorentini, A.M.; da Silva, W.P. Bacteriocin-like substances of Lactobacillus curvatus P99: Characterization and application in biodegradable films for control of Listeria monocytogenes in cheese. Food Microbiol. 2017, 63, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Ahmadova, A.; Todorov, S.D.; Hadji-Sfaxi, I.; Choiset, Y.; Rabesona, H.; Messaoudi, S.; Kuliyev, A.; de Franco, B.D.G.M.; Chobert, J.-M.; Haertlé, T. Antimicrobial and antifungal activities of Lactobacillus curvatus strain isolated from homemade Azerbaijani cheese. Anaerobe 2013, 20, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wu, H.; Gao, L.; Jia, H.; Zhang, Y.; Cui, Z.; Li, Y. Effects of Lactobacillus curvatus and Leuconostoc mesenteroides on Suan Cai Fermentation in Northeast China. J. Microbiol. Biotechnol. 2016, 26, 2148–2158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funck, G.D.; de Marques, J.L.; dos Cruxen, C.E.S.; Sehn, C.P.; Haubert, L.; da Dannenberg, G.S.; Klajn, V.M.; Silva, W.P.; Fiorentini, Â.M. Probiotic potential of Lactobacillus curvatus P99 and viability in fermented oat dairy beverage. J. Food Process Preserv. 2019, 43, 1–11. [Google Scholar] [CrossRef]
- Lee, K.W.; Kim, G.S.; Baek, A.H.; Hwang, H.S.; Kwon, D.Y.; Kim, S.G.; Lee, S.Y. Isolation and characterization of Kimchi starters Leuconostoc mesenteroides PBio03 and Leuconostoc mesenteroides PBio104 for manufacture of commercial Kimchi. J. Microbiol. Biotechnol. 2020, 30, 1060–1066. [Google Scholar] [CrossRef]
- Jeon, H.; Lee, S.; Kim, S.; Kim, Y. Quality characteristics of modified Doenjang and traditional Doenjang. J. Korean Soc. Food Sci. Nutr. 2016, 45, 1001–1009. [Google Scholar] [CrossRef] [Green Version]
- Cupp-Enyard, C. Sigma’s non-specific protease activity assay—Casein as a substrate. J. Vis. Exp. 2008, 19, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Mikelsone, A.; Ciprovica, I. Diversity of non-starter lactic acid bacteria in Latvian semi-hard cheeses. Res. Rural Dev. 2009, 15, 103–107. [Google Scholar]
- Choi, M.-S.; Kim, D.-M.; Oh, K.-H. Studies on the enhanced physiological activities of mixed lactic acid bacteria isolated. Korean Soc. Biotechnol. Bioeng. J. 2015, 30, 245–252. [Google Scholar]
- Casaburi, A.; Martino, V.D.; Ferranti, P.; Picariello, L.; Villani, F. Technological properties and bacteriocins production by Lactobacillus curvatus 54M16 and its use as starter culture for fermented sausage manufacture. Food Control 2016, 59, 31–45. [Google Scholar] [CrossRef]
- Kindstedt, P.S.; Zielinski, A.; Almena-Aliste, M.; Ge, C. A post-manufacture method to evaluate the effect of pH on Mozzarella cheese characteristics. Aust. J. Dairy Technol. 2001, 56, 202–207. [Google Scholar]
- Choi, C.; Lim, H.-W.; Chon, J.-W.; Kim, D.-H.; Song, K.-Y.; Kim, S.-Y.; Kim, H.; Seo, K.-H. Sensory evaluation of various gouda cheeses produced from raw milk. J. Milk Sci. Biotechnol. 2018, 36, 95–105. [Google Scholar] [CrossRef]
- Mozzi, F. Lactic acid bacteria. In Encyclopedia of Food and Health, 1st ed.; Caballero, B., Finglas, P., Toldra, F., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; pp. 501–508. [Google Scholar]
- Vihavainen, E.; Lundstrom, H.-S.; Susiluoto, T.; Koort, J.; Paulin, L.; Auvinen, P.; Bjorkroth, K.J. Role of broiler carcasses and processing plant air in contamination of modified-atmosphere-packaged broiler products with psychrotrophic lactic acid bacteria. Appl. Environ. Microbiol. 2006, 73, 1136–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorgensen, L.V.; Huss, H.H.; Dalgaard, P. The effect of biogenic amine production by single bacterial cultures and metabiosis on cold-smoked salmon. J. Appl. Microbiol. 2000, 89, 920–934. [Google Scholar] [CrossRef]
- Pachlová, V.; Buňková, L.; Flasarová, R.; Salek, R.-N.; Dlabajová, A.; Butor, I.; Buňka, F. Biogenic amine production by nonstarter strains of Lactobacillus curvatus and Lactobacillus paracasei in the model system of Dutch-type cheese. LWT 2018, 97, 730–735. [Google Scholar] [CrossRef]
- Hugenholtz, J.; Kleerebezem, M. Metabolic engineering of lactic acid bacteria: Overview of the approaches and results of pathway rerouting involved in food fermentations. Curr. Opin. Biotechnol. 1999, 10, 492–497. [Google Scholar] [CrossRef]
- Savijoki, K.; Ingmer, H.; Varmanen, P. Proteolytic systems of lactic acid bacteria. Appl. Microbiol. Biotechnol. 2006, 71, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, N.; Soda, M.E.; Shafei, H.E.; Olson, N. Cell-wall associated peptide hydrolase and esterase activities in several cheese-related bacteria. Food Chem. 1993, 48, 19–23. [Google Scholar] [CrossRef]
- So, M.H. Influences of proteolytic ability of lactic acid bacteria on acid production and precipitates occurrence in liquid yogurt preparation. Korean J. Appl. Microbiol. Bioeng. 1984, 12, 285–291. [Google Scholar]









| Sample | Cheese’s Expired Dates | Identification of LAB | Colony Numbers out of 30 | Hardening of Cheese | Sour Strength |
|---|---|---|---|---|---|
| Cheese-ddukbokki | 13 November 2020 | Latilactobacillus curvatus | 30 (100%) | Strong | Strong |
| 13 November 2020 | Latilactobacillus curvatus | 30 (100%) | Strong | Strong | |
| 21 November 2020 | Lacticaseibacillus paracasei Lacticaseibacillus casei Latilactobacillus curvatus | 9 (30.00%) 9 (30.00%) 12 (40.00%) | Weak | Weak | |
| 21 November 2020 | Leuconostocmesenteroides Lacticaseibacillus casei | 22 (73.33%) 8 (26.67%) | Non-hardening | None | |
| 6 November 2020 | Leuconostoc mesenteroides | 30 (100%) | Non-hardening | None | |
| 26 November 2020 | Leuconostoc mesenteroides | 30 (100%) | Non-hardening | None | |
| 2 December 2020 | Leuconostoc mesenteroides | 30 (100%) | Non-hardening | None |
| Sample | Manufacture Dates | Identification of LAB | Colony Numbers out of 30 |
|---|---|---|---|
| Pizza Shredded Ⅱ mix-5 cheese | 20.08.14 | Latilactobacillus curvatus | 15 (50.00%) |
| Leuconostoc pseudomesenteroides | 9 (30.00%) | ||
| Lacticaseibacillus paracasei | 3 (10.00%) | ||
| Lacticaseibacillus casei | 3 (10.00%) | ||
| 20.08.22 | Latilactobacillus curvatus | 30 (100%) | |
| 20.08.27 | Latilactobacillus curvatus | 30 (100%) | |
| 20.09.03 | Leuconostoc mesenteroides | 30 (100%) | |
| 20.09.18 | Leuconostoc mesenteroides | 21 (70.00%) | |
| Latilactobacillus curvatus | 9 (30.00%) | ||
| 20.09.24 | Leuconostoc mesenteroides | 18 (60.00%) | |
| Latilactobacillus curvatus | 12 (40.00%) | ||
| 20.10.02 | Latilactobacillus curvatus | 30 (100%) | |
| 20.10.08 | Leuconostoc mesenteroides | 21 (70.00%) | |
| Latilactobacillus curvatus | 9 (30.00%) | ||
| 20.10.15 | Latilactobacillus curvatus | 15 (50.00%) | |
| Latilactobacillus sakei | 15 (50.00%) | ||
| 20.10.26 | Latilactobacillus curvatus | 30 (100%) | |
| 20.11.05 | Leuconostoc mesenteroides | 12 (40.00%) | |
| Levilactobacillus brevis | 6 (20.00%) | ||
| Lacticaseibacillus casei | 12 (40.00%) | ||
| 20.12.11 | Leuconostoc mesenteroides | 15 (50%) | |
| Lacticaseibacillus casei | 15 (50%) |
| Storage Time (Day) | Con-CD 1 | LC-CD 2 | LM-CD 3 |
|---|---|---|---|
| 0 | Melted | Melted | Melted |
| 10 | Melted | Melted | Melt |
| 20 | Melted | Melted | Melted |
| 30 | Melted | Non-melting | Melted |
| 40 | Melted | Non-melting | Melted |
| 50 | Non-melting | Non-melting | Melted |
| Sample | Storage Time(Day) | Identification of LAB | Colony Numbers out of 30 (%) |
|---|---|---|---|
| LC-CD | 0 | Lactobacillus curvatus | 30/30 (100%) |
| 30 | Lactobacillus curvatus | 30/30 (100%) | |
| LM-CD | 0 | Leuconostoc mesenteroides | 30/30 (100%) |
| 30 | Leuconostocmesenteroides Lactobacillus curvatus | 24/30 (80%) 6/30 (20%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-A.; Kim, G.-S.; Choi, S.-M.; Kim, M.-S.; Kwon, D.-Y.; Kim, S.-G.; Lee, S.-Y.; Lee, K.-W. Hardening Properties of Cheeses by Latilactobacillus curvatus PD1 Isolated from Hardened Cheese-Ddukbokki Rice Cake. Microorganisms 2021, 9, 1044. https://doi.org/10.3390/microorganisms9051044
Kim J-A, Kim G-S, Choi S-M, Kim M-S, Kwon D-Y, Kim S-G, Lee S-Y, Lee K-W. Hardening Properties of Cheeses by Latilactobacillus curvatus PD1 Isolated from Hardened Cheese-Ddukbokki Rice Cake. Microorganisms. 2021; 9(5):1044. https://doi.org/10.3390/microorganisms9051044
Chicago/Turabian StyleKim, Jeong-A., Geun-Su Kim, Se-Mi Choi, Myeong-Seon Kim, Do-Young Kwon, Sang-Gu Kim, Sang-Yun Lee, and Kang-Wook Lee. 2021. "Hardening Properties of Cheeses by Latilactobacillus curvatus PD1 Isolated from Hardened Cheese-Ddukbokki Rice Cake" Microorganisms 9, no. 5: 1044. https://doi.org/10.3390/microorganisms9051044
APA StyleKim, J.-A., Kim, G.-S., Choi, S.-M., Kim, M.-S., Kwon, D.-Y., Kim, S.-G., Lee, S.-Y., & Lee, K.-W. (2021). Hardening Properties of Cheeses by Latilactobacillus curvatus PD1 Isolated from Hardened Cheese-Ddukbokki Rice Cake. Microorganisms, 9(5), 1044. https://doi.org/10.3390/microorganisms9051044