Challenges and Potential in Increasing Lutein Content in Microalgae
Abstract
:1. Introduction
2. Lutein Sequestration Versus Structural Function
2.1. Estimation Of Lutein Storage Capacity in LHCs
2.2. Estimated Storage Capacity of Lutein in Lipid Droplets
2.3. Limits of Structural Lutein Content due to Its Function
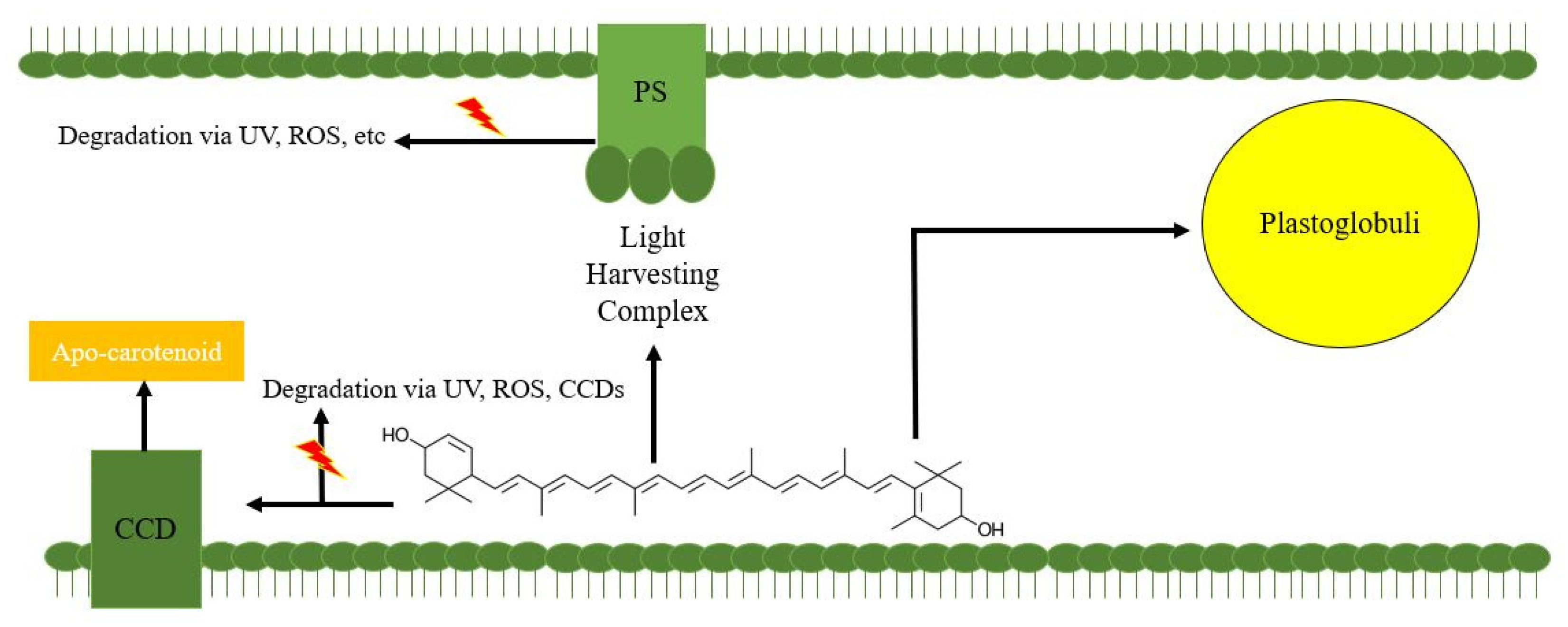
3. Limitation of Lutein Sequestration in Algal Oil Droplets
3.1. Lutein Degradation without Proper Sequestration
3.2. Lack of Lutein Esterification Mechanism
3.3. Lipid Droplet Access Limitation
4. Strategies to Overcome These Barriers
4.1. Lutein Esterification Gene and Mechanisms
4.2. Suppression of the Gene Expression of CCDs in Microalgae
4.3. Investigation of the Mechanisms of βC-Plastoglobuli Biogenesis in Dunaliella spp. and the Carotenoid Trafficking Mechanisms in Haematococcus spp. and C. zofingiensis
4.4. Future Perspectives
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dall’Osto, L.; Lico, C.; Alric, J.; Giuliano, G.; Havaux, M.; Bassi, R. Lutein is needed for efficient chlorophyll triplet quenching in the major LHCII antenna complex of higher plants and effective photoprotection in vivo under strong light. BMC Plant Biol. 2006, 6, 32. [Google Scholar]
- Takaichi, S. Carotenoids in Algae: Distributions, Biosyntheses and Functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef]
- Liu, L.; Shao, Z.; Zhang, M.; Wang, Q. Regulation of Carotenoid Metabolism in Tomato. Mol. Plant 2015, 8, 28–39. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Sevilla, J.M.; Fernández, F.G.A.; Grima, E.M. Biotechnological production of lutein and its applications. Appl. Microbiol. Biotechnol. 2010, 86, 27–40. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Morton, D.W. Cosmeceuticals Derived from Bioactive Substances Found in Marine Algae. Oceanography 2013, 1, 2. [Google Scholar]
- Carpentier, S.; Knaus, M.; Suh, M. Associations between Lutein, Zeaxanthin, and Age-Related Macular Degeneration: An Overview. Crit. Rev. Food Sci. Nutr. 2009, 49, 313–326. [Google Scholar] [CrossRef]
- Bone, R.A.; Landrum, J.T.; Dixon, Z.; Chen, Y.; Llerena, C.M. Lutein and Zeaxanthin in the Eyes, Serum and Diet of Human Subjects. Exp. Eye Res. 2000, 71, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, H.E.; Eperjesi, F.; Eperjesi, H.E.B. Effect of lutein and antioxidant dietary supplementation on contrast sensitivity in age-related macular disease: A randomized controlled trial. Eur. J. Clin. Nutr. 2007, 61, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Erdman, J.J.W.; Smith, J.W.; Kuchan, M.J.; Mohn, E.S.; Johnson, E.J.; Rubakhin, S.S.; Wang, L.; Sweedler, J.V.; Neuringer, M. Lutein and Brain Function. Foods 2015, 4, 547–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishino, H.; Murakoshi, M.; Tokuda, H.; Satomi, Y. Cancer prevention by carotenoids. Arch. Biochem. Biophys. 2009, 483, 165–168. [Google Scholar] [CrossRef]
- Kritchevsky, S.B. β-Carotene, Carotenoids and the Prevention of Coronary Heart Disease. J. Nutr. 1999, 129, 5–8. [Google Scholar] [CrossRef] [Green Version]
- Ribaya-Mercado, J.D.; Blumberg, J.B. Lutein and Zeaxanthin and Their Potential Roles in Disease Prevention. J. Am. Coll. Nutr. 2004, 23, 567S–587S. [Google Scholar] [CrossRef]
- Lin, J.-H.; Lee, D.-J.; Chang, J.-S. Lutein production from biomass: Marigold flowers versus microalgae. Bioresour. Technol. 2015, 184, 421–428. [Google Scholar] [CrossRef]
- Vasudevan, P.; Kashyap, S.; Sharma, S. Tagetes: A multipurpose plant. Bioresour. Technol. 1997, 62, 29–35. [Google Scholar] [CrossRef]
- Cui, Z.-H.; Sun, H.-J.; Gao, C.-L.; Zhang, J.; Zhang, L.-J. Research on Extraction Technology of Lutein from Marigold. Liaoning Chem. Ind. 2008, 4, 7. [Google Scholar]
- Shi, X.-M.; Chen, F. High-yield production of lutein by the green microalga Chlorella protothecoidesin heterotrophic fed-batch culture. Biotechnol. Prog. 2002, 18, 723–727. [Google Scholar]
- Ho, S.-H.; Chan, M.-C.; Liu, C.-C.; Chen, C.-Y.; Lee, W.-L.; Lee, D.-J.; Chang, J.-S. Enhancing lutein productivity of an indigenous microalga Scenedesmus obliquus FSP-3 using light-related strategies. Bioresour. Technol. 2014, 152, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, J.A.; Moreno, J.; Rodríguez, H.; Vargas, M.A.; Rivas, J.; Guerrero, M.G. Carotenoid Content of Chlorophycean Microalgae: Factors Determining Lutein Accumulation in Muriellopsis Sp.(Chlorophyta). J. Biotechnol. 2000, 76, 51–59. [Google Scholar] [CrossRef]
- Ye, Z.-W.; Jiang, J.-G.; Wu, G.-H. Biosynthesis and regulation of carotenoids in Dunaliella: Progresses and prospects. Biotechnol. Adv. 2008, 26, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Li, J.; Ma, R.; Ho, S.-H.; Shi, X.; Liu, L.; Chen, J. Bioprocess operation strategies with mixotrophy/photoinduction to enhance lutein production of microalga Chlorella sorokiniana FZU60. Bioresour. Technol. 2019, 290, 121798. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Li, J.; Ho, S.-H.; Ma, R.; Shi, X.; Liu, L.; Chen, J. Pilot-scale cultivation of Chlorella sorokiniana FZU60 with a mixotrophy/photoautotrophy two-stage strategy for efficient lutein production. Bioresour. Technol. 2020, 314, 123767. [Google Scholar] [CrossRef]
- Heo, J.; Shin, D.-S.; Cho, K.; Cho, D.-H.; Lee, Y.J.; Kim, H.-S. Indigenous microalga Parachlorella sp. JD-076 as a potential source for lutein production: Optimization of lutein productivity via regulation of light intensity and carbon source. Algal Res. 2018, 33, 1–7. [Google Scholar] [CrossRef]
- Xie, Y.; Zhao, X.; Chen, J.; Yang, X.; Ho, S.-H.; Wang, B.; Chang, J.-S.; Shen, Y. Enhancing cell growth and lutein productivity of Desmodesmus sp. F51 by optimal utilization of inorganic carbon sources and ammonium salt. Bioresour. Technol. 2017, 244, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-H.; Chen, C.-Y.; Chang, J.-S. Lutein production with wild-type and mutant strains of Chlorella sorokiniana MB-1 under mixotrophic growth. J. Taiwan Inst. Chem. Eng. 2017, 79, 66–73. [Google Scholar] [CrossRef]
- Gan, S.Y.; Maggs, C.A. Random mutagenesis and precise gene editing technologies: Applications in algal crop improvement and functional genomics. Eur. J. Phycol. 2017, 52, 466–481. [Google Scholar] [CrossRef]
- Cordero, B.F.; Obraztsova, I.; Couso, I.; Leon, R.; Vargas, M.A.; Rodriguez, H. Enhancement of Lutein Production in Chlorella sorokiniana (Chorophyta) by Improvement of Culture Conditions and Random Mutagenesis. Mar. Drugs 2011, 9, 1607–1624. [Google Scholar] [CrossRef] [Green Version]
- Del Campo, J.A.; Moreno, J.; Rivas, J.; Guerrero, M.G. Accumulation of astaxanthin and lutein in Chlorella zofingiensis (Chlorophyta). Appl. Microbiol. Biotechnol. 2004, 64, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Lin, Y.; He, M.; Gong, Y.; Huang, J. Induced High-Yield Production of Zeaxanthin, Lutein, and β-Carotene by a Mutant of Chlorella zofingiensis. J. Agric. Food Chem. 2018, 66, 891–897. [Google Scholar] [CrossRef]
- Couso, I.; Vila, M.; Rodriguez, H.; Vargas, M.A.; León, R. Overexpression of an exogenous phytoene synthase gene in the unicellular alga Chlamydomonas reinhardtii leads to an increase in the content of carotenoids. Biotechnol. Prog. 2011, 27, 54–60. [Google Scholar] [CrossRef]
- Cordero, B.F.; Couso, I.; León, R.; Rodríguez, H.; Vargas, M.Á. Enhancement of Carotenoids Biosynthesis in Chlamydomonas Reinhardtii by Nuclear Transformation Using a Phytoene Synthase Gene Isolated from Chlorella Zofingiensis. Appl. Microbiol. Biotechnol. 2011, 91, 341–351. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Sun, Z.; Gerken, H.; Huang, J.; Jiang, Y.; Chen, F. Genetic engineering of the green alga Chlorella zofingiensis: A modified norflurazon-resistant phytoene desaturase gene as a dominant selectable marker. Appl. Microbiol. Biotechnol. 2014, 98, 5069–5079. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Uraguchi, Y.; Sanda, S.; Nakagawa, S.; Sawayama, S. Overexpression of Dnaj-Like Chaperone Enhances Carotenoid Synthesis in Chlamydomonas Reinhardtii. Appl. Biochem. Biotechnol. 2018, 184, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Saini, D.K.; Chakdar, H.; Pabbi, S.; Shukla, P. Enhancing production of microalgal biopigments through metabolic and genetic engineering. Crit. Rev. Food Sci. Nutr. 2020, 60, 391–405. [Google Scholar] [CrossRef]
- Patel, V.K.; Soni, N.; Prasad, V.; Sapre, A.; Dasgupta, S.; Bhadra, B. CRISPR–Cas9 System for Genome Engineering of Photosynthetic Microalgae. Mol. Biotechnol. 2019, 61, 541–561. [Google Scholar] [CrossRef] [PubMed]
- Mosey, M.; Douchi, D.; Knoshaug, E.P.; Laurens, L.M. Methodological review of genetic engineering approaches for non-model algae. Algal Res. 2021, 54, 102221. [Google Scholar] [CrossRef]
- Baek, K.; Yu, J.; Jeong, J.; Sim, S.J.; Bae, S.; Jin, E. Photoautotrophic production of macular pigment in a Chlamydomonas reinhardtii strain generated by using DNA-free CRISPR-Cas9 RNP-mediated mutagenesis. Biotechnol. Bioeng. 2017, 115, 719–728. [Google Scholar] [CrossRef]
- Polle, J.E.; Barry, K.; Cushman, J.; Schmutz, J.; Tran, D.; Hathwaik, L.T.; Yim, W.C.; Jenkins, J.; McKie-Krisberg, Z.; Prochnik, S.; et al. Draft Nuclear Genome Sequence of the Halophilic and Beta-Carotene-Accumulating Green Alga Dunaliella Salina Strain Ccap19/18. Genome Announc. 2017, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, T.H.; Zhou, F.; Liu, C.J.; Zhuang, Z.; Lu, S. The Dnaj-Like Zinc Finger Domain Protein Orange Localizes to the Nucleus in Etiolated Cotyledons of Arabidopsis Thaliana. Protoplasma 2016, 253, 1599–1604. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.S.; Jung, Y.J.; Kim, S.H.; Ji, C.Y.; Wang, Z.; Jeong, J.C.; Lee, H.-S.; Lee, S.Y.; Kwak, S.-S. Orange protein has a role in phytoene synthase stabilization in sweetpotato. Sci. Rep. 2016, 6, 33563. [Google Scholar] [CrossRef]
- Chayut, N.; Yuan, H.; Ohali, S.; Meir, A.; Sa’ar, U.; Tzuri, G.; Zheng, Y.; Mazourek, M.; Gepstein, S.; Zhou, X. Distinct Mechanisms of the Orange Protein in Controlling Carotenoid Flux. Plant Physiol. 2017, 173, 376–389. [Google Scholar] [CrossRef]
- Pourkarimi, S.; Hallajisani, A.; Alizadehdakhel, A.; Nouralishahi, A.; Golzary, A. Factors affecting production of beta-carotene from Dunaliella salina microalgae. Biocatal. Agric. Biotechnol. 2020, 29, 101771. [Google Scholar] [CrossRef]
- Mobin, S.; Alam, F. Some Promising Microalgal Species for Commercial Applications: A review. Energy Procedia 2017, 110, 510–517. [Google Scholar] [CrossRef]
- Kumar, S.; Gill, B.S.; Verma, A.; Verma, M.L.; Kushwaha, R. Biotechnological production of high-valued algal astaxanthin and lutein under different growth conditions. In Biotechnological Production of Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2020; pp. 191–220. [Google Scholar]
- Gong, M.; Bassi, A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid Metabolism in Plants. Mol. Plant 2015, 8, 68–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, M.-H.; Zhu, J.; Jiang, J.-G. Carotenoids biosynthesis and cleavage related genes from bacteria to plants. Crit. Rev. Food Sci. Nutr. 2018, 58, 2314–2333. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.H.; Strack, D. Carotenoids and their cleavage products: Biosynthesis and functions. Nat. Prod. Rep. 2011, 28, 663–692. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Yuan, H.; Cao, H.; Yazdani, M.; Tadmor, Y.; Li, L. Carotenoid Metabolism in Plants: The Role of Plastids. Mol. Plant 2018, 11, 58–74. [Google Scholar] [CrossRef] [Green Version]
- Cazzonelli, C.I.; Pogson, B.J. Source to sink: Regulation of carotenoid biosynthesis in plants. Trends Plant Sci. 2010, 15, 266–274. [Google Scholar] [CrossRef]
- Varela, J.C.; Pereira, H.; Vila, M.; León, R. Production of Carotenoids by Microalgae: Achievements and Challenges. Photosynth. Res. 2015, 125, 423–436. [Google Scholar] [CrossRef]
- Crepin, A.; Caffarri, S. Functions and Evolution of Lhcb Isoforms Composing LHCII, the Major Light Harvesting Complex of Photosystem II of Green Eukaryotic Organisms. Curr. Protein Pept. Sci. 2018, 19, 699–713. [Google Scholar] [CrossRef]
- Deineka, V.I.; Sorokopudov, V.N.; Deineka, L.A.; Tret’Yakov, M.Y. Flowers of marigold (Tagetes) species as a source of xanthophylls. Pharm. Chem. J. 2007, 41, 540–542. [Google Scholar] [CrossRef]
- Hadden, W.L.; Watkins, R.H.; Levy, L.W.; Regalado, E.; Rivadeneira, D.M.; Van Breemen, R.B.; Schwartz, S.J. Carotenoid composition of marigold (Tagetes erecta) flower extract used as nutritional supplement. J. Agric. Food Chem. 1999, 47, 4189–4194. [Google Scholar] [CrossRef] [PubMed]
- Vanegas-Espinoza, P.E.; Ramos-Viveros, V.; Jiménez-Aparicio, A.R.; López-Villegas, O.; Heredia-Mira, F.J.; Meléndez-Martínez, A.J.; Quintero-Gutiérrez, A.G.; Paredes-López, O.; Del Villar-Martínez, A.A.; Villar-Martínez, A.A. Plastid analysis of pigmented undifferentiated cells of marigold Tagetes erecta L. by transmission electron microscopy. Vitr. Cell. Dev. Biol. Anim. 2011, 47, 596–603. [Google Scholar] [CrossRef]
- Pagliano, C.; Saracco, G.; Barber, J. Structural, functional and auxiliary proteins of photosystem II. Photosynth. Res. 2013, 116, 167–188. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Cao, P.; Su, X.; Liu, Z.; Li, M. Structural analysis and comparison of light-harvesting complexes I and II. Biochim. Biophys. Acta (BBA) Bioenerg. 2020, 1861, 148038. [Google Scholar] [CrossRef]
- Nelson, N.; Junge, W. Structure and Energy Transfer in Photosystems of Oxygenic Photosynthesis. Annu. Rev. Biochem. 2015, 84, 659–683. [Google Scholar] [CrossRef]
- Dekker, J.P.; Boekema, E.J. Supramolecular organization of thylakoid membrane proteins in green plants. Biochim. Biophys. Acta (BBA) Bioenerg. 2005, 1706, 12–39. [Google Scholar] [CrossRef] [Green Version]
- Ben-Amotz, A.; Lers, A.; Avron, M. Stereoisomers of β-Carotene and Phytoene in the Alga Dunaliella bardawil. Plant Physiol. 1988, 86, 1286–1291. [Google Scholar] [CrossRef] [Green Version]
- Ben-Amotz, A.; Shaish, A.; Avron, M. Mode of Action of the Massively Accumulated β-Carotene of Dunaliella bardawil in Protecting the Alga against Damage by Excess Irradiation. Plant Physiol. 1989, 91, 1040–1043. [Google Scholar] [CrossRef] [Green Version]
- Shah, M.R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-Producing Green Microalga Haematococcus pluvialis: From Single Cell to High Value Commercial Products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Villar-Martínez, A.A.; García-Saucedo, P.A.; Carabez-Trejo, A.; Cruz-Hernández, A.; Paredes-Lópeza, O. Carotenogenic gene expression and ultrastructural changes during development in marigold. J. Plant Physiol. 2005, 162, 1046–1056. [Google Scholar] [CrossRef] [PubMed]
- Amar, I.; Aserin, A.A.; Garti, N. Solubilization Patterns of Lutein and Lutein Esters in Food Grade Nonionic Microemulsions. J. Agric. Food Chem. 2003, 51, 4775–4781. [Google Scholar] [CrossRef]
- Becker, E. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Sajjadi, B.; Chen, W.-Y.; Raman, A.A.A.; Ibrahim, S. Microalgae lipid and biomass for biofuel production: A comprehensive review on lipid enhancement strategies and their effects on fatty acid composition. Renew. Sustain. Energy Rev. 2018, 97, 200–232. [Google Scholar] [CrossRef]
- Moehs, C.P.; Tian, L.; Osteryoung, K.W.; DellaPenna, D. Analysis of carotenoid biosynthetic gene expression during marigold petal development. Plant Mol. Biol. 2001, 45, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Davidi, L.; Levin, Y.; Ben-Dor, S.; Pick, U. Proteome Analysis of Cytoplasmatic and Plastidic β-Carotene Lipid Droplets in Dunaliella bardawil. Plant Physiol. 2014, 167, 60–79. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Wang, B.; Han, D.; Sommerfeld, M.; Lu, Y.; Chen, F.; Hu, Q. Molecular mechanisms of the coordination between astaxanthin and fatty acid biosynthesis inHaematococcus pluvialis(Chlorophyceae). Plant J. 2014, 81, 95–107. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, D.; Zhang, Y.; Chen, F. Glucose triggers cell structure changes and regulates astaxanthin biosynthesis in Chromochloris zofingiensis. Algal Res. 2019, 39, 101455. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, D.; Mao, X.; Liu, J.; Chen, F. The crosstalk between astaxanthin, fatty acids and reactive oxygen species in heterotrophic Chlorella zofingiensis. Algal Res. 2016, 19, 178–183. [Google Scholar] [CrossRef]
- Tan, C.; Xue, J.; Abbas, S.; Feng, B.; Zhang, X.; Xia, S. Liposome as a Delivery System for Carotenoids: Comparative Antioxidant Activity of Carotenoids As Measured by Ferric Reducing Antioxidant Power, DPPH Assay and Lipid Peroxidation. J. Agric. Food Chem. 2014, 62, 6726–6735. [Google Scholar] [CrossRef]
- Pintea, A.; Diehl, H.A.; Momeu, C.; Aberle, L.; Socaciu, C. Incorporation of carotenoid esters into liposomes. Biophys. Chem. 2005, 118, 7–14. [Google Scholar] [CrossRef]
- Kloer, D.P.; Ruch, S.; Al-Babili, S.; Beyer, P.; Schulz, G.E. The Structure of a Retinal-Forming Carotenoid Oxygenase. Science 2005, 308, 267–269. [Google Scholar] [CrossRef] [Green Version]
- Sui, X.; Kiser, P.D.; Von Lintig, J.; Palczewski, K. Structural basis of carotenoid cleavage: From bacteria to mammals. Arch. Biochem. Biophys. 2013, 539, 203–213. [Google Scholar] [CrossRef] [Green Version]
- Joyard, J.; Ferro, M.; Masselon, C.; Seigneurin-Berny, D.; Salvi, D.; Garin, J.; Rolland, N. Chloroplast Proteomics and the Compartmentation of Plastidial Isoprenoid Biosynthetic Pathways. Mol. Plant 2009, 2, 1154–1180. [Google Scholar] [CrossRef]
- Ytterberg, A.J.; Peltier, J.-B.; Van Wijk, K.J. Protein Profiling of Plastoglobules in Chloroplasts and Chromoplasts. A Surprising Site for Differential Accumulation of Metabolic Enzymes. Plant Physiol. 2006, 140, 984–997. [Google Scholar] [CrossRef] [Green Version]
- Vidi, P.A.; Kanwischer, M.; Baginsky, S.; Austin, J.R.; Csucs, G.; Dörmann, P.; Kessler, F.; Bréhélin, C. Tocopherol Cyclase (Vte1) Localization and Vitamin E Accumulation in Chloroplast Plastoglobule Lipoprotein Particles. J. Biol. Chem. 2006, 281, 11225–11234. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-Q.; Yang, Y.; Fei, Z.; Yuan, H.; Fish, T.; Thannhauser, T.W.; Mazourek, M.; Kochian, L.V.; Wang, X.; Li, L. Proteomic analysis of chromoplasts from six crop species reveals insights into chromoplast function and development. J. Exp. Bot. 2013, 64, 949–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, Y.; Pan, Z.; Ding, Y.; Zhu, A.; Cao, H.; Xu, Q.; Deng, X. A proteomic analysis of the chromoplasts isolated from sweet orange fruits [Citrus sinensis (L.) Osbeck]. J. Exp. Bot. 2011, 62, 5297–5309. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Geßner, G.; Luff, M.; Heiland, I.; Wagner, V.; Kaminski, M.; Geimer, S.; Eitzinger, N.; Reißenweber, T.; Voytsekh, O.; et al. Proteomic Analysis of the Eyespot of Chlamydomonas reinhardtii Provides Novel Insights into Its Components and Tactic Movements. Plant Cell 2006, 18, 1908–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercadante, A.Z.; Rodrigues, D.B.; Petry, F.C.; Mariutti, L.R.B. Carotenoid esters in foods—A review and practical directions on analysis and occurrence. Food Res. Int. 2017, 99, 830–850. [Google Scholar] [CrossRef] [PubMed]
- Watkins, J.L.; Li, M.; McQuinn, R.P.; Chan, K.X.; McFarlane, H.E.; Ermakova, M.; Furbank, R.T.; Mares, D.; Dong, C.; Chalmers, K.J.; et al. A GDSL Esterase/Lipase Catalyzes the Esterification of Lutein in Bread Wheat. Plant Cell 2019, 31, 3092–3112. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, Y.; Ding, W.; Mao, X.; Li, Y.; Gerken, H.; Liu, J. Astaxanthin Is Ketolated from Zeaxanthin Independent of Fatty Acid Synthesis in Chromochloris zofingiensis. Plant Physiol. 2020, 183, 883–897. [Google Scholar] [CrossRef]
- Breithaupt, D.E.; Bamedi, A. Carotenoids and Carotenoid Esters in Potatoes (Solanum tuberosumL.): New Insights into an Ancient Vegetable. J. Agric. Food Chem. 2002, 50, 7175–7181. [Google Scholar] [CrossRef]
- Jacob, K.; Garcia-Alonso, F.J.; Ros, G.; Periago, M.J. Stability of carotenoids, phenolic compounds, ascorbic acid and antioxidant capacity of tomatoes during thermal processing. Arch. Latinoam. Nutr. 2010, 60, 192. [Google Scholar] [PubMed]
- Schaub, P.; Rodriguez-Franco, M.; Cazzonelli, C.I.; Alvarez, D.; Wüst, F.; Welsch, R. Establishment of an Arabidopsis callus system to study the interrelations of biosynthesis, degradation and accumulation of carotenoids. PLoS ONE 2018, 13, e0192158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subagio, A.; Wakaki, H.; Morita, N. Stability of Lutein and Its Myristate Esters. Biosci. Biotechnol. Biochem. 1999, 63, 1784–1786. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Xie, B.; Fan, G.; Ma, S.; Zhu, X.; Pan, S. Effect of esterification with fatty acid of β-cryptoxanthin on its thermal stability and antioxidant activity by chemiluminescence method. Food Chem. 2010, 122, 602–609. [Google Scholar] [CrossRef]
- Ariizumi, T.; Kishimoto, S.; Kakami, R.; Maoka, T.; Hirakawa, H.; Suzuki, Y.; Ozeki, Y.; Shirasawa, K.; Bernillon, S.; Okabe, Y.; et al. Identification of the carotenoid modifying genePALE YELLOW PETAL 1as an essential factor in xanthophyll esterification and yellow flower pigmentation in tomato (Solanum lycopersicum). Plant J. 2014, 79, 453–465. [Google Scholar] [CrossRef]
- Kreimer, G. The green algal eyespot apparatus: A primordial visual system and more? Curr. Genet. 2008, 55, 19–43. [Google Scholar] [CrossRef]
- Goold, H.; Beisson, F.; Peltier, G.; Li-Beisson, Y. Microalgal lipid droplets: Composition, diversity, biogenesis and functions. Plant Cell Rep. 2015, 34, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Pick, U.; Zarka, A.; Boussiba, S.; Davidi, L. A hypothesis about the origin of carotenoid lipid droplets in the green algae Dunaliella and Haematococcus. Planta 2018, 249, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Grünewald, K.; Hirschberg, J.; Hagen, C. Ketocarotenoid Biosynthesis Outside of Plastids in the Unicellular Green Alga Haematococcus pluvialis. J. Biol. Chem. 2001, 276, 6023–6029. [Google Scholar] [CrossRef] [Green Version]
- Zacarías-García, J.; Lux, P.E.; Carle, R.; Schweiggert, R.M.; Steingass, C.B.; Zacarías, L.; Rodrigo, M.J. Characterization of the Pale Yellow Petal/Xanthophyll Esterase Gene Family in Citrus as Candidates for Carotenoid Esterification in Fruits. Food Chem. 2021, 342, 128322. [Google Scholar] [CrossRef]
- Kishimoto, S.; Oda-Yamamizo, C.; Ohmiya, A. Heterologous expression of xanthophyll esterase genes affects carotenoid accumulation in petunia corollas. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Hai, N.T.L.; Masuda, J.-I.; Miyajima, I.; Thien, N.Q.; Mojtahedi, N.; Hiramatsu, M.; Kim, J.-H.; Okubo, H. Involvement of Carotenoid Cleavage Dioxygenase 4 Gene in Tepal Color Change in Lilium brownii var. colchesteri. J. Jpn. Soc. Hortic. Sci. 2012, 81, 366–373. [Google Scholar] [CrossRef] [Green Version]
- Ohmiya, A.; Kishimoto, S.; Aida, R.; Yoshioka, S.; Sumitomo, K. Carotenoid Cleavage Dioxygenase (CmCCD4a) Contributes to White Color Formation in Chrysanthemum Petals. Plant Physiol. 2006, 142, 1193–1201. [Google Scholar] [CrossRef] [Green Version]
- Auldridge, M.E.; Block, A.; Vogel, J.T.; Dabney-Smith, C.; Mila, I.; Bouzayen, M.; Magallanes-Lundback, M.; DellaPenna, D.; McCarty, D.R.; Klee, H.J. Characterization of three members of the Arabidopsis carotenoid cleavage dioxygenase family demonstrates the divergent roles of this multifunctional enzyme family. Plant J. 2006, 45, 982–993. [Google Scholar] [CrossRef]
- Brandi, F.; Bar, E.; Mourgues, F.; Horváth, G.; Turcsi, E.; Giuliano, G.; Liverani, A.; Tartarini, S.; Lewinsohn, E.; Rosati, C. Study of ’Redhaven’ peach and its white-fleshed mutant suggests a key role of CCD4 carotenoid dioxygenase in carotenoid and norisoprenoid volatile metabolism. BMC Plant Biol. 2011, 11, 24. [Google Scholar] [CrossRef] [Green Version]
- Campbell, R.; Ducreux, L.J.; Morris, W.L.; Morris, J.A.; Suttle, J.C.; Ramsay, G.; Bryan, G.J.; Hedley, P.E.; Taylor, M.A. The Metabolic and Developmental Roles of Carotenoid Cleavage Dioxygenase 4 from Potato (Solanum Tuberosum L). Plant Physiol. 2010, 154, 656–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Jorge, S.; Ha, S.-H.; Magallanes-Lundback, M.; Gilliland, L.U.; Zhou, A.; Lipka, A.E.; Nguyen, Y.-N.; Angelovici, R.; Lin, H.; Cepela, J.; et al. Carotenoid Cleavage Dioxygenase4 Is a Negative Regulator of β-Carotene Content in Arabidopsis Seeds. Plant Cell 2013, 25, 4812–4826. [Google Scholar] [CrossRef] [Green Version]
- Yamamizo, C.; Kishimoto, S.; Ohmiya, A. Carotenoid composition and carotenogenic gene expression during Ipomoea petal development. J. Exp. Bot. 2009, 61, 709–719. [Google Scholar] [CrossRef] [Green Version]
- Kato, M.; Matsumoto, H.; Ikoma, Y.; Okuda, H.; Yano, M. The role of carotenoid cleavage dioxygenases in the regulation of carotenoid profiles during maturation in citrus fruit. J. Exp. Bot. 2006, 57, 2153–2164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthäus, F.; Ketelhot, M.; Gatter, M.; Barth, G. Production of Lycopene in the Non-Carotenoid-Producing Yeast Yarrowia lipolytica. Appl. Environ. Microbiol. 2013, 80, 1660–1669. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Tong, Y.; Zhu, L.; Ge, M.; Jiang, Y.; Chen, D.; Yang, S. Production of β-carotene by expressing a heterologous multifunctional carotene synthase in Yarrowia lipolytica. Biotechnol. Lett. 2017, 39, 921–927. [Google Scholar] [CrossRef]
- Tramontin, L.R.R.; Kildegaard, K.R.; Sudarsan, S.; Borodina, I. Enhancement of Astaxanthin Biosynthesis in Oleaginous Yeast Yarrowia lipolytica via Microalgal Pathway. Microorganisms 2019, 7, 472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.-K.; Wang, D.-N.; Chen, J.; Liu, Z.-J.; Wei, L.-J.; Hua, Q. Metabolic engineering of β-carotene biosynthesis in Yarrowia lipolytica. Biotechnol. Lett. 2020, 42, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Abghari, A.; Chen, S. Yarrowia lipolytica as an Oleaginous Cell Factory Platform for Production of Fatty Acid-Based Biofuel and Bioproducts. Front. Energy Res. 2014, 2, 21. [Google Scholar] [CrossRef]
| Microalgal Strains | Lutein Content (mg/g) | Lutein Productivity (mg/L/d) | References |
|---|---|---|---|
| C. sorokiniana FZU60 | 9.57 | 11.57 | [20] |
| Parachlorella sp. JD-076 | 11.8 | 25 | [22] |
| Desmodesmus sp. F51 | 5.56 | 5.22 | [23] |
| Scenedesmus obliquus FSP-3 | 4.52 | 4.15 | [17] |
| C. sorokiniana MB-1 | 5.86 | 2.39 | [24] |
| Microalgal Strains | Strategy | Lutein Yield (mg/g) | References |
|---|---|---|---|
| C. sorokiniana MB-1-M12 | Random mutagenesis | 7.52 | [24] |
| C. sorokiniana mutant DMR-5&8 | Random mutagenesis | 7.0 | [16] |
| C.zofingiensis mutant CZ-bkt1 | Dysfunction of BKT1 by chemical mutation | 13.81 | [28] |
| Chlamydomonas reinhardtii | Overexpressing PSY gene from Dunaliella salina | 2.2-fold increase of lutein content | [29] |
| C. reinhardtii | Overexpressing PSY gene from C. zofingiensis | 2.2-fold increase of lutein content | [30] |
| C. zofingiensis | Overexpressing PDS gene | Total carotenoid content increased by 32.1% | [31] |
| C. reinhardtii | Overexpressing OR gene from A. thaliana | 1.9-fold increase of lutein content | [32] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Xiong, X.; Chen, S. Challenges and Potential in Increasing Lutein Content in Microalgae. Microorganisms 2021, 9, 1068. https://doi.org/10.3390/microorganisms9051068
Xie Y, Xiong X, Chen S. Challenges and Potential in Increasing Lutein Content in Microalgae. Microorganisms. 2021; 9(5):1068. https://doi.org/10.3390/microorganisms9051068
Chicago/Turabian StyleXie, Yuxiao, Xiaochao Xiong, and Shulin Chen. 2021. "Challenges and Potential in Increasing Lutein Content in Microalgae" Microorganisms 9, no. 5: 1068. https://doi.org/10.3390/microorganisms9051068





