Oral Lactobacillus Species in Systemic Sclerosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. DNA Extraction and Quantification of Lactobacillus
2.2. Statistical Analysis
3. Results
3.1. Patients and Healthy Subjects
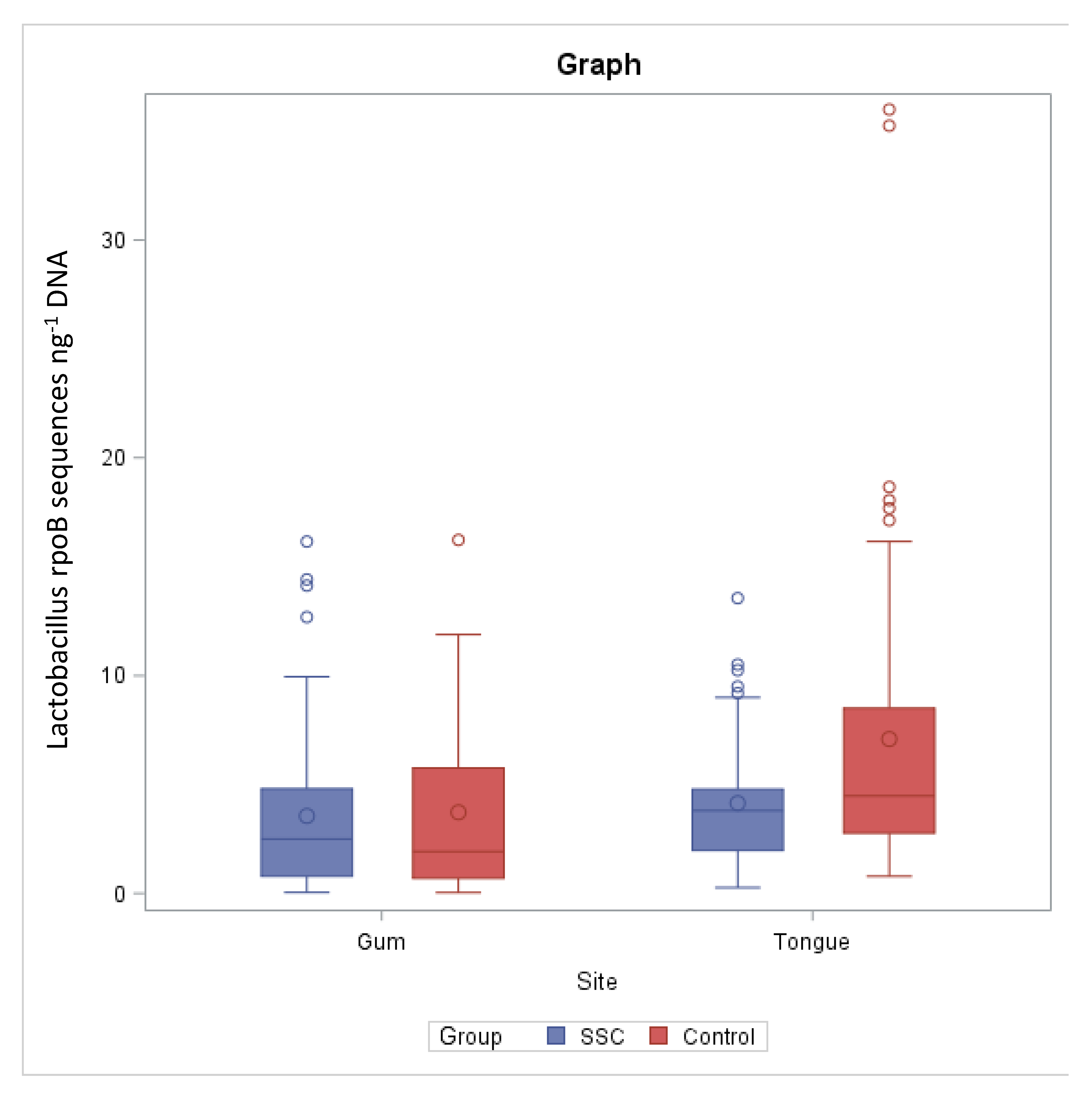
3.2. Lactobacillus rpoB Quantification in Tongue Samples
3.3. Lactobacillus rpoB Quantification in Gum Samples
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Konig, M.F. The microbiome in autoimmune rheumatic disease. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101473. [Google Scholar] [CrossRef] [PubMed]
- De Luca, F.; Shoenfeld, Y. The microbiome in autoimmune diseases. Clin. Exp. Immunol. 2019, 195, 74–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varga, J.; Trojanowska, M.; Kuwana, M. Pathogenesis of systemic sclerosis: Recent insights of molecular and cellular mechanisms and therapeutic opportunities. J. Scleroderma Relat. Disord. 2017, 2, 137–152. [Google Scholar] [CrossRef]
- SaveriaFioretto, B.; Rosa, I.; Romano, E.; Wang, Y.; Guiducci, S.; Zhang, G.; Manetti, M.; Matucci-Cerinic, M. The contribution of epigenetics to the pathogenesis and gender dimorphism of systemic sclerosis: A comprehensive overview. Ther. Adv. Musculoskelet. Dis. 2020, 12, 1–24. [Google Scholar] [CrossRef]
- Nikitakis, N.G.; Papaioannou, W.; Sakkas, L.I.; Kousvelari, E. The autoimmunity-oral microbiome connection. Oral Dis. 2017, 23, 828–839. [Google Scholar] [CrossRef] [PubMed]
- Scofield, R.H. Rheumatic disease and the microbiome. Int. J. Rheum. Dis. 2014, 17, 489–492. [Google Scholar] [CrossRef] [Green Version]
- Yeoh, N.; Burton, J.P.; Suppiah, P.; Reid, G.; Stebbings, S. The role of the microbiome in rheumatic diseases. Curr. Rheumatol. Rep. 2013, 15, 314. [Google Scholar] [CrossRef]
- Bellocchi, C.; Volkmann, E.R. Update on the Gastrointestinal Microbiome in Systemic Sclerosis. Curr. Rheumatol. Rep. 2018, 20, 49. [Google Scholar] [CrossRef]
- Morrisroe, K.; Murray, B.; Tracy, F.; Mandana, N. Small intestinal bacterial overgrowth in systemic sclerosis. J. Scleroderma Relat. Disord. 2019, 5, 33–39. [Google Scholar] [CrossRef]
- Andréasson, K.; Alrawi, Z.; Persson, A.; Jönsson, G.; Marsal, J. Intestinal dysbiosis is common in systemic sclerosis and associated with gastrointestinal and extraintestinal features of disease. Arthritis Res.Ther. 2016, 18, 278. [Google Scholar] [CrossRef] [Green Version]
- Volkmann, E.R.; Chang, Y.L.; Barroso, N.; Furst, D.E.; Clements, P.J.; Gorn, A.H.; Roth, B.E.; Conklin, J.L.; Getzug, T.; Borneman, J.; et al. Systemic sclerosis is associated with a unique colonic microbial consortium. Arthritis Rheumatol. 2016, 68, 1483–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkmann, E.R.; Hoffmann-Vold, A.M.; Chang, Y.L.; Jacobs, J.P.; Tillisch, K.; Mayer, E.A.; Clements, P.J.; Hov, J.R.; Kummen, M.; Midtvedt, Ø.; et al. Systemic sclerosis is associated with specific alterations in gastrointestinal microbiota in two independent cohorts. BMJ Open Gastroenterol. 2017, 4, e000134. [Google Scholar] [CrossRef]
- Patrone, V.; Puglisi, E.; Cardinali, M.; Schnitzler, T.S.; Svegliati, S.; Festa, A.; Gabrielli, A.; Morelli, L. Gut microbiota profile in systemic sclerosis patients with and without clinical evidence of gastrointestinal involvement. Sci. Rep. 2017, 7, 14874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natalello, G.; Bosello, S.L.; Paroni Sterbini, F.; Posteraro, B.; De Lorenzis, E.; Canestrari, G.B.; Gigante, L.; Verardi, L.; Ferraccioli, G.; Sanguinetti, M.; et al. Gut microbiota analysis in systemic sclerosis according to disease characteristics and nutritional status. Clin. Exp. Rheumatol. 2020, 38 (Suppl. 125), S73–S84. [Google Scholar]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Zhong, D.; Wu, C.; Zeng, X.; Wang, Q. The role of gut microbiota in the pathogenesis of rheumatic diseases. Clin. Rheumatol. 2018, 37, 25–34. [Google Scholar] [CrossRef]
- Linares, D.M.; Gómez, C.; Renes, E.; Fresno, J.M.; Tornadijo, M.E.; Ross, R.P.; Stanton, C. Lactic Acid Bacteria and Bifidobacteria with Potential to Design Natural Biofunctional Health-Promoting Dairy Foods. Front. Microbiol. 2017, 8, 846. [Google Scholar] [CrossRef]
- Brusca, S.B.; Abramson, S.B.; Scher, J.U. Microbiome and mucosal inflammation as extra-articular triggers for rheumatoid arthritis and autoimmunity. Curr. Opin. Rheumatol. 2014, 26, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, E.J.; Tyrrell, K.L.; Citron, D.M. Lactobacillus species: Taxonomic complexity and controversial susceptibilities. Clin. Infect. Dis. 2015, 60 (Suppl. 2), S98–S107. [Google Scholar] [CrossRef] [Green Version]
- Matsuzaki, T.; Nagata, Y.; Kado, S.; Uchida, K.; Kato, I.; Hashimoto, S.; Yokokura, T. Prevention of onset in an insulin-dependent diabetes mellitus model, NOD mice, by oral feeding of Lactobacillus casei. APMIS 1997, 105, 643–649. [Google Scholar] [CrossRef]
- Kano, H.; Kaneko, T.; Kaminogawa, S. Oral intake of Lactobacillus delbrueckii subsp. bulgaricus OLL1073R-1 prevents collagen-induced arthritis in mice. J. Food Prot. 2002, 65, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Baharav, E.; Mor, F.; Halpern, M.; Weinberger, A. Lactobacillus GG bacteria ameliorate arthritis in Lewis rats. J. Nutr. 2004, 134, 1964–1969. [Google Scholar] [CrossRef] [PubMed]
- So, J.S.; Lee, C.G.; Kwon, H.K.; Yi, H.J.; Chae, C.S.; Park, J.A.; Hwang, K.C.; Im, S.H. Lactobacillus casei potentiates induction of oral tolerance in experimental arthritis. Mol. Immunol. 2008, 46, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Lavasani, S.; Dzhambazov, B.; Nouri, M.; Fåk, F.; Buske, S.; Molin, G.; Thorlacius, H.; Alenfall, J.; Jeppsson, B.; Weström, B. A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PLoS ONE 2010, 5, e9009. [Google Scholar] [CrossRef]
- Kwon, H.K.; Kim, G.C.; Kim, Y.; Hwang, W.; Jash, A.; Sahoo, A.; Kim, J.-E.; Nam, J.H.; Im, S.-H. Amelioration of experimental autoimmune encephalomyelitis by probioticmixture is mediated by a shift in T helper cell immune response. Clin. Immunol. 2013, 146, 217–227. [Google Scholar] [CrossRef]
- Johnson, B.M.; Gaudreau, M.C.; Al-Gadban, M.M.; Gudi, R.; Vasu, C. Impact of dietary deviation on disease progression and gut microbiome composition in lupus-prone SNF1 mice. Clin. Exp. Immunol. 2015, 181, 323–337. [Google Scholar] [CrossRef] [Green Version]
- Walter, J. Ecological role of lactobacilli in the gastrointestinal tract: Implications for fundamental and biomedical research. Appl. Environ. Microbiol. 2008, 74, 4985–4996. [Google Scholar] [CrossRef] [Green Version]
- Sharma, D.; Sandhya, P.; Vellarikkal, S.K.; Surin, A.K.; Jayarajan, R.; Verma, A.; Kumar, A.; Ravi, R.; Danda, D.; Sivasubbu, S.; et al. Saliva microbiome in primary Sjögren’s syndrome reveals distinct set of disease-associated microbes. Oral Dis. 2020, 26, 295–301. [Google Scholar] [CrossRef]
- Liu, X.; Zou, Q.; Zeng, B.; Fang, Y.; Wei, H. Analysis of fecal Lactobacillus community structure in patients with early rheumatoid arthritis. Curr. Microbiol. 2013, 67, 170–176. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Jia, H.; Feng, Q.; Wang, D.; Liang, D.; Wu, X.; Li, J.; Tang, L.; Li, Y.; et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 2015, 21, 895–905. [Google Scholar] [CrossRef]
- Walker, M.Y.; Pratap, S.; Southerland, J.H.; Farmer-Dixon, C.M.; Lakshmyya, K.; Gangula, P.R. Role of Oral and Gut Microbiome in Nitric Oxide-Mediated ColonMotility. Nitric Oxide 2018, 73, 81–88. [Google Scholar] [CrossRef]
- Martu, I.; Goriuc, A.; Martu, M.A.; Vata, I.; Baciu, R.; Mocanu, R.; Surdu, A.E.; Popa, C.; Luchian, I. Identification of bacteria involved in periodontal disease using molecular biology techniques. Rev. Chim. 2017, 68, 2407–2412. [Google Scholar] [CrossRef]
- Martu, M.A.; Solomon, S.M.; Sufaru, I.G.; Jelihovschi, I.; Martu, S.; Rezus, E.; Surdu, A.E.; Onea, R.M.; Grecu, G.P.; Foia, L. Study on the prevalence of periodontopathogenic bacteria in serum and subgingival bacterial plaque in patients with rheumatoid arthritis. Rev. Chim. 2017, 68, 1946–1949. [Google Scholar] [CrossRef]
- Luisi, M.L.E.; Lucarini, L.; Biffi, B.; Rafanelli, E.; Pietramellara, G.; Durante, M.C.; Vidali, S.; Provensi, G.; Madiai, S.; Gheri, C.F.; et al. Effect of Mediterranean Diet Enriched in High 482 Quality Extra Virgin Olive Oil on Oxidative Stress, Inflammation and Gut Microbiota in Obese and Normal Weight Adult Subjects. Front.Pharmacol. 2019, 10, 1366. [Google Scholar] [CrossRef] [Green Version]
- Teodorescu, A.C.; Teslaru, S.; Solomon, S.M.; Zetu, L.; Luchian, I.; Sioustis, I.-A.; Martu, M.A.; Valiliu, B.; Martu, S. Assessment of Bacterial Associations Involved in Periodontal Disease Using Crevicular Fluid. Rev. Chim. 2019, 70, 2145–2149. [Google Scholar] [CrossRef]
- Solomon, S.M.; Bataiosu, M.; Popescu, D.M.; Rauten, A.M.; Gheorghe, D.N.; Petrescu, R.A.; Maftei, G.A.; Maglaviceanu, C.F. Biochemical Assesment of SalivaryParameters in Young Patients with Dental Lesions. Rev. Chim. 2019, 70, 4095–4097. [Google Scholar] [CrossRef]
- Jeong, Y.; Kim, J.W.; You, H.J.; Park, S.J.; Lee, J.; Ju, J.H.; Park, M.S.; Jin, H.; Cho, M.L.; Kwon, B.; et al. Gut microbial composition and function are altered in patients with earlyRheumatoid Arthritis. J. Clin. Med. 2019, 8, 693. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Chen, Q.; Lin, P.; Xu, R.; He, D.; Ji, W.; Bian, Y.; Shen, Y.; Li, Q.; Liu, C.; et al. Characteristics of Gut Microbiota in Patients With Rheumatoid Arthritis in Shanghai, China. Front. Cell. Infect. Microbiol. 2019, 9, 369. [Google Scholar] [CrossRef] [Green Version]
- Scher, J.U.; Sczesnak, A.; Longman, R.S.; Segata, N.; Ubeda, C.; Bielski, B.; Rostron, T.; Cerundolo, V.; Pamer, E.G.; Abramson, S.B.; et al. Expansion of intestinal Prevotellacopri correlates with enhanced susceptibility to arthritis. eLife 2013, 2, e01202. [Google Scholar] [CrossRef]
- Alpizar-Rodriguez, D.; Lesker, T.R.; Gronow, A.; Gilbert, B.; Raemy, E.; Lamacchia, C.; Gabay, C.; Finckh, A.; Strowig, T. Prevotellacopri in individuals at risk for rheumatoid arthritis. Ann. Rheum. Dis. 2019, 78, 590–593. [Google Scholar] [CrossRef]
- Moreno, J. Prevotellacopri and the microbial pathogenesis of rheumatoid arthritis. Reumatol. Clin. 2015, 11, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Volkmann, E.R.; Hoffmann-Vold, A.M. Gastrointestinal tract microbiota modifications in systemic sclerosis. Eur. J. Rheumatol. 2020, 7 (Suppl. 3), S228–S236. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, W.; Cai, Q.Y.; Shrubsole, M.J.; Pei, Z.; Brucker, R.; Steinwandel, M.D.; Bordenstein, S.R.; Li, Z.; Blot, W.J.; et al. Cigarette smoking and oral microbiota in low-income and African-American populations. J. Epidemiol. Commun. Health 2019, 73, 1108–1115. [Google Scholar] [CrossRef]
- Picchianti-Diamanti, A.; Panebianco, C.; Salemi, S.; Sorgi, M.L.; Di Rosa, R.; Tropea, A.; Sgrulletti, M.; Salerno, G.; Terracciano, F.; D’Amelio, R.; et al. Analysis of gut microbiota in Rheumatoid Arthritis patients: Disease-related dysbiosis and modifications induced by Etanercept. Int. J. Mol. Sci. 2018, 19, 2938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dissick, A.; Redman, R.S.; Jones, M.; Rangan, B.V.; Reimold, A.; Griffiths, G.R.; Mikuls, T.R.; Amdur, R.L.; Richards, J.S.; Kerr, G.S. Association of periodontitis with Rheumatoid Arthritis: A pilot study. J. Periodontol. 2010, 81, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Pischon, N.; Pischon, T.; Kröger, J.; Gülmez, E.; Kleber, B.M.; Bernimoulin, J.P.; Landau, H.; Brinkmann, P.G.; Schlattmann, P.; Zernicke, J.; et al. Association among rheumatoid arthritis, oral hygiene, andperiodontitis. J. Periodontol. 2008, 79, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Abou-Raya, A.; Abou-Raya, S.; Abu-Elkheir, H. Periodontal disease and rheumatoid arthritis: Is there a link? Scand. J. Rheumatol. 2005, 34, 408–410. [Google Scholar] [CrossRef]
- Bingham, C.O., III; Giles, J.T.; Jones, P.E.; Bathon, J.M.; Bartlett, S.J. High prevalence of moderate to severe periodontal disease in rheumatoid arthritis patients. Arthritis Rheum. 2007, 56, S420. [Google Scholar]
- Eriksson, K.; Guozhong, F.; Lundmark, A.; Benchimol, D.; Lee, L.; Hu, Y.O.O.; Kats, A.; Saevarsdottir, S.; Anca, I.C.; Klinge, B.; et al. Periodontal health and oral microbiota in patients with Rheumatoid Arthritis. J. Clin. Med. 2019, 8, 630. [Google Scholar] [CrossRef] [Green Version]
- Scher, J.U.; Ubeda, C.; Equinda, M.; Khanin, R.; Buischi, Y.; Viale, A.; Lipuma, L.; Attur, M.; Pillinger, M.H.; Weissmann, G.; et al. Periodontal disease and the oral microbiota in new-onset rheumatoid arthritis. Arthritis Rheum. 2012, 64, 3083–3094. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.; Do, T.; Mankia, K.; Meade, J.; Hunt, L.; Clerehugh, V.; Speirs, A.; Tugnait, A.; Emery, P.; Devine, D. Dysbiosis in the oral microbiomes of anti-CCP positive individuals at risk of developing rheumatoid arthritis. Ann. Rheum. Dis. 2021, 80, 162–168. [Google Scholar] [CrossRef]
- Volkmann, E.R. Intestinalmicrobiome in scleroderma: Recent progress. Curr. Opin. Rheumatol. 2017, 29, 553–560. [Google Scholar]
- Dal Bello, F.; Hertel, C. Oral cavity as natural reservoir for intestinal lactobacilli. Syst. Appl. Microbiol. 2006, 29, 69–76. [Google Scholar] [CrossRef]
- Badet, C.; Thebaud, N.B. Ecology of lactobacilli in the oral cavity: A review of literature. Open Microbiol. J. 2008, 2, 38–48. [Google Scholar]
- Turroni, F.; Ventura, M.; Buttó, L.F.; Duranti, S.; O’Toole, P.W.; Motherway, M.O.; van Sinderen, D. Molecular dialogue between the human gut microbiota and the host: A Lactobacillus and Bifidobacterium perspective. Cell. Mol. Life Sci. 2014, 71, 183–203. [Google Scholar] [CrossRef]
- Rodríguez Mínguez, E.; Arqués, J.L.; Rodríguez, R.; Peirotén, A.; Landete, J.M.; Medina, M. Antimicrobial properties of probiotic strains isolated from breastfed infants. J. Funct. Foods 2012, 4, 542–551. [Google Scholar]
- Maldonado Galdeano, C.; Perdigón, G. The Probiotic Bacterium Lactobacillus casei Induces Activation of the Gut Mucosal Immune System through Innate Immunity. Clin. Vaccine Immunol. 2006, 13, 219–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishikawa, T.; Maeda, Y.; Nii, T.; Motooka, D.; Matsumoto, Y.; Matsushita, M.; Matsuoka, H.; Yoshimura, M.; Kawada, S.; Teshigawara, S.; et al. Metagenome-wide association study of gut microbiome revealed novel aetiology of rheumatoid arthritis in the Japanese population. Ann. Rheum. Dis. 2020, 79, 103–111. [Google Scholar] [CrossRef]
- Johnson, M.E.; Franks, J.M.; Cai, G.; Mehta, B.K.; Wood, T.A.; Archambault, K.; Pioli, P.A.; Simms, R.W.; Orzechowski, N.; Arron, S.; et al. Microbiome dysbiosis is associated with disease duration and increased inflammatory gene expression in systemic sclerosis skin. Arthritis Res. Ther. 2019, 21, 49. [Google Scholar] [CrossRef] [Green Version]
- Reuter, G. The Lactobacillus and Bifidobacterium microflora of the human intestine: Composition and succession. Curr. Issues Intest. Microbiol. 2001, 2, 43–53. [Google Scholar]
- Ragab, G.; Atkinson, T.P.; Stoll, M. The Microbiome in Rheumatic Diseases and Infection; Springer International Publishing: New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Showalter, K.; Hoffmann, A.; DeCredico, N.; Thakrar, A.; Arroyo, E.; Goldberg, I.; Hinchcliff, M. Complementary therapies for patients with systemic sclerosis. J. Scleroderma Relat. Disord. 2019, 4, 187–199. [Google Scholar] [CrossRef]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shreiner, A.B.; Murray, C.; Denton, C.; Khanna, D. Gastrointestinal manifestations of systemic sclerosis. J. Scleroderma Relat. Disord. 2016, 1, 247–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, A.-M.; Volkmann, E.R. Gastrointestinal involvement in sistemic sclerosis. Effects on morbidity and mortality and new therapeutic approaches. J. Scleroderma Relat. Disord. 2019, 4, 33–39. [Google Scholar]
- Carlson, R.V.; Boyd, K.M.; Webb, D.J. The revision of the Declarationof Helsinki: Past, present and future. Br. J. Clin. Pharmacol. 2004, 57, 695–713. [Google Scholar] [CrossRef]
| Species | Quantity/Function | Reference |
|---|---|---|
| Lactobacillus casei | Prevention of diabetes mellitus in NOD mice | [20] |
| Lactobacillus delbrueckii subsp. bulgaricus | Prevention of collagen-induced arthritis in mice | [21] |
| Lactobacillus GG | Oral intake improves arthritis in Lewis rats | [22] |
| Lactobacillus casei | Potentiated induction of oral tolerance in EAA | [23] |
| Lactobacillus paracasei/Lactobacillus plantarum | Improvement of EAE, by Treg  and Th1/Th17 and Th1/Th17  | [24,25] |
| Lactobacillus spp. |  in the gut of lupus-prone mice. Restoring normal number reverse SLE in the gut of lupus-prone mice. Restoring normal number reverse SLE | [26] |
| Lactobacillus spp. | Increase in saliva of primary Sjögren’ssyndrome patients | [28] |
| Lactobacillus spp./Lactobacillus salivarius |  in RA patients’ gut, possible link with disease onset/progression in RA patients’ gut, possible link with disease onset/progression | [29,30] |
| Characteristic | SScPatients (n = 29) | Healthy Subjects (n = 23) |
|---|---|---|
| Age (years) Mean ± SD Range | 62 ± 12.43 43–80 | 57.6 ± 8.47NS 44–72 |
| Female gender | 29 | 23 |
| Smoking current former never | 7 9 13 | 7NS 5NS 11NS |
| LcSSc | 13 | NA |
| Disease duration (years) | 12 | NA |
| ANA positivity | 13 (100%) | NA |
| ACA positivity | 13 (100%) | NA |
| DcSSc | 15 | NA |
| Disease duration (years) | 14 | NA |
| ANApositivity | 15 (100%) | NA |
| Anti-scl70 positivity | 15 (100%) | NA |
| Overlapsyndrome | 1 | NA |
| Disease duration (years) | 22 | NA |
| ANA, anti-scl70, SSA, SSB | Positive for all | NA |
| cDMARDs | 16 (58.6%) | NA |
| I.v.prostanoid | 13 (44.8%) | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melchiorre, D.; Ceccherini, M.T.; Romano, E.; Cometi, L.; El-Aoufy, K.; Bellando-Randone, S.; Roccotelli, A.; Bruni, C.; Moggi-Pignone, A.; Carboni, D.; et al. Oral Lactobacillus Species in Systemic Sclerosis. Microorganisms 2021, 9, 1298. https://doi.org/10.3390/microorganisms9061298
Melchiorre D, Ceccherini MT, Romano E, Cometi L, El-Aoufy K, Bellando-Randone S, Roccotelli A, Bruni C, Moggi-Pignone A, Carboni D, et al. Oral Lactobacillus Species in Systemic Sclerosis. Microorganisms. 2021; 9(6):1298. https://doi.org/10.3390/microorganisms9061298
Chicago/Turabian StyleMelchiorre, Daniela, Maria Teresa Ceccherini, Eloisa Romano, Laura Cometi, Khadija El-Aoufy, Silvia Bellando-Randone, Angela Roccotelli, Cosimo Bruni, Alberto Moggi-Pignone, Davide Carboni, and et al. 2021. "Oral Lactobacillus Species in Systemic Sclerosis" Microorganisms 9, no. 6: 1298. https://doi.org/10.3390/microorganisms9061298
APA StyleMelchiorre, D., Ceccherini, M. T., Romano, E., Cometi, L., El-Aoufy, K., Bellando-Randone, S., Roccotelli, A., Bruni, C., Moggi-Pignone, A., Carboni, D., Guiducci, S., Lepri, G., Tofani, L., Pietramellara, G., & Matucci-Cerinic, M. (2021). Oral Lactobacillus Species in Systemic Sclerosis. Microorganisms, 9(6), 1298. https://doi.org/10.3390/microorganisms9061298










