Abstract
Discoveries of novel myxobacteria have started to unveil the potentially vast phylogenetic diversity within the family Myxococcaceae and have brought about an updated approach to myxobacterial classification. While traditional approaches focused on morphology, 16S gene sequences, and biochemistry, modern methods including comparative genomics have provided a more thorough assessment of myxobacterial taxonomy. Herein, we utilize long-read genome sequencing for two myxobacteria previously classified as Archangium primigenium and Chondrococcus macrosporus, as well as four environmental myxobacteria newly isolated for this study. Average nucleotide identity and digital DNA–DNA hybridization scores from comparative genomics suggest previously classified as A. primigenium to instead be a novel member of the genus Melittangium, C. macrosporus to be a potentially novel member of the genus Corallococcus with high similarity to Corallococcus exercitus, and the four isolated myxobacteria to include another novel Corallococcus species, a novel Pyxidicoccus species, a strain of Corallococcus exiguus, and a potentially novel Myxococcus species with high similarity to Myxococcus stipitatus. We assess the biosynthetic potential of each sequenced myxobacterium and suggest that genus-level conservation of biosynthetic pathways support our preliminary taxonomic assignment. Altogether, we suggest that long-read genome sequencing benefits the classification of myxobacteria and improves determination of biosynthetic potential for prioritization of natural product discovery.
1. Introduction
Over the last decade, 34 novel species of myxobacteria have been described including representatives from 10 newly described genera within the order Myxococcales (Table S1) [1,2,3,4,5,6,7,8,9,10,11,12,13,14]. Prevalent in soils and marine sediments, predatory and cellulolytic myxobacteria contribute to nutrient cycling within microbial food webs. Perhaps most-studied for their cooperative lifestyles, myxobacteria have been an excellent resource for investigations concerning developmental multicellularity and two-component signaling, swarming motilities and predatory features, and the discovery of biologically active metabolites [15,16,17,18,19,20,21,22,23]. Each of these areas of interest have benefited from the increased utility and accessibility of next-generation sequencing (NGS) technologies. The driving force behind the recent surge in efforts to discover novel species of myxobacteria can also be attributed to advances in sequencing technologies. Genome sequencing of myxobacteria has demonstrated that they possess large genomes replete with biosynthetic gene clusters, and myxobacteria have recently been deemed a “gifted” taxon for the production of specialized metabolites with drug-like properties [24,25,26,27,28,29]. These efforts, combined with a thorough metabolic survey of over 2000 strains within the order Myxococcales, concluded that the odds of novel metabolite discovery increase when exploring novel genera of myxobacteria [30]. Motivated by these observations, we sought to isolate novel myxobacteria from lesser-studied North American soils.
Recently, comparative genomic analyses have been utilized to provide efficient preliminary classification of novel myxobacteria, and we considered that such an approach would expedite prioritization of strains for future metabolic studies [3,8,11,31,32,33,34,35,36,37]. While traditional myxobacterial classification efforts relied on morphology, biochemistry, and the conservation of 16S gene sequences, updated methods including genome-based taxonomy have provided excellent preliminary taxonomic classification of myxobacterial isolates [38,39,40]. Considering that genome sequencing would also afford the biosynthetic potential of any isolated myxobacteria, we sought to employ long-read sequencing to generate high-quality draft genomes hoping to avoid fragmented, partial biosynthetic pathways. For example, of the 11 currently sequenced myxobacteria from the genus Corallococcus, 68% of the 621 total putative biosynthetic gene clusters (BGCs) predicted by the analysis platform AntiSMASH are positioned on a contig edge and are potentially incomplete (Table S2). In fact, the only two Corallococcus genomes sequenced with long-read techniques (Corallococcus coralloides DSM 2259T and C. coralloides strain B035) each included 34 predicted BGCs with none located on a contig edge [41,42]. Ideally, larger contigs generated from long-read sequencing might benefit the comparative genomic analyses and provide a more complete assessment of biosynthetic potential.
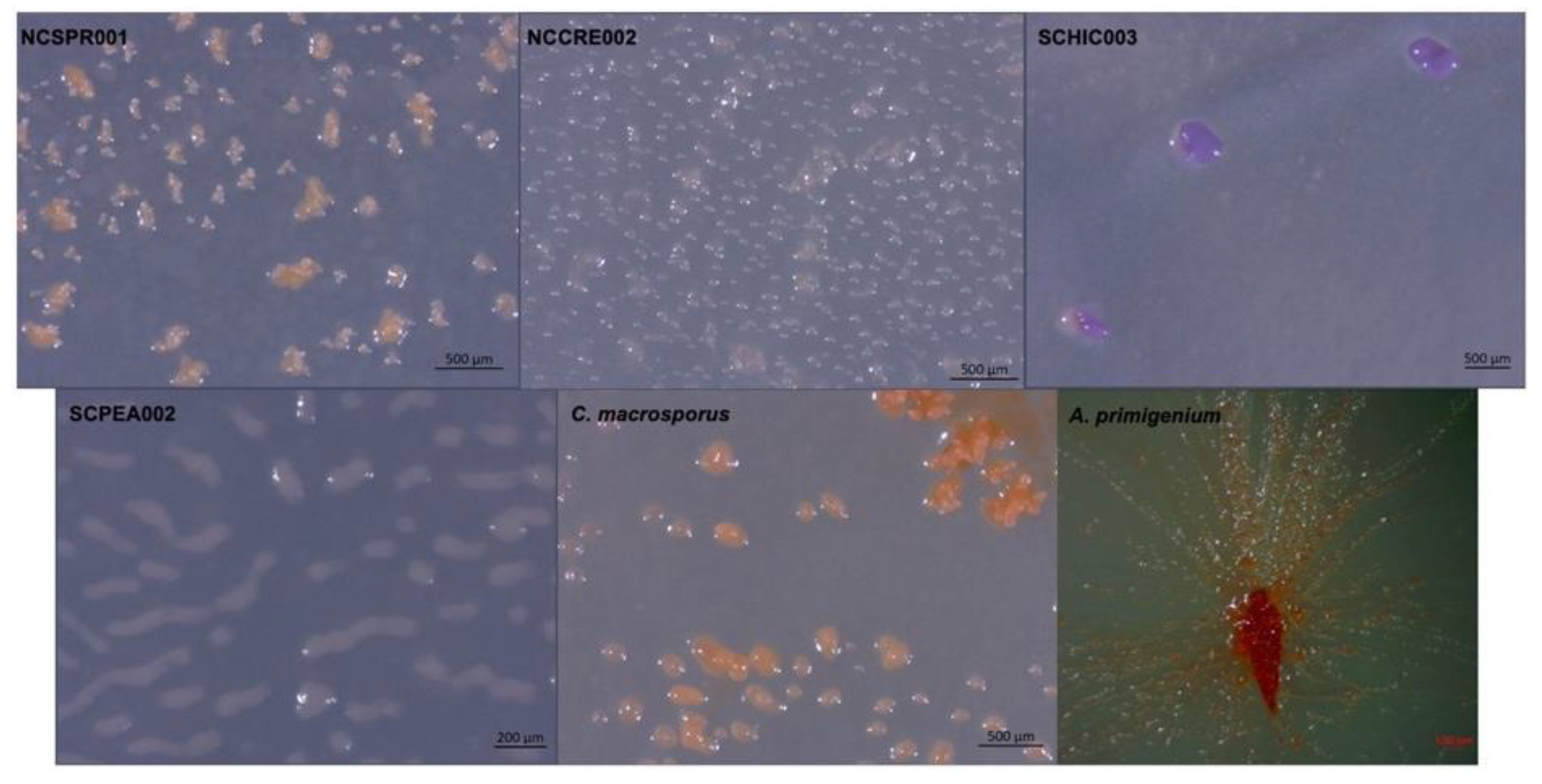
In addition to four environmental isolates of putative myxobacteria included in this study, we acquired two previously characterized myxobacteria from the American Type Culture Collection (ATCC): Archangium primigenium ATCC 29,037 and Chondrococcus macrosporus ATCC 29039. Previously miscategorized as Polyangium primigenium, the original morphological descriptions for A. primigenium were remarkably apt for the strain acquired from the ATCC and cultivated in our lab, including obvious fruiting body formation and carotene-like pigmentation (Figure 1) [43,44]. The original description of A. primigenium fruiting bodies initially piqued our interest in the strain as members of the genus Archangium typically do not or very rarely form defined fruiting bodies when cultivated with standard laboratory conditions [45,46]. Archangium species have previously been referred to as “degenerate forms’’ of myxobacteria due to diminished fruiting bodies with no sporangioles or absent fruiting body formation [46]. Comparatively, little historical data is available for C. macrosporus ATCC 29039. The strain was deposited at the ATCC by distinguished taxonomist Professor V. B. D. Skerman and was subsequently included in a methodology study focused on isolating myxobacteria from soils [47,48,49]. The decision to change the genus Chondrococcus to instead be Corallococcus has been validated with many novel Corallococcus species being described afterwards [8,40,50]. However, we were curious to determine the status of C. macrosporus ATCC 29039. Considering the proposed reassignment of Corallococcus macrosporus DSM 14697T to the genus Myxococcus, it was unclear if C. macrosporus ATCC 29,039 should also be reassigned. Both characterized using traditional approaches that heavily relied on morphology, we sought to determine how genomic comparisons might impact the current taxonomic assignments of these available myxobacteria.
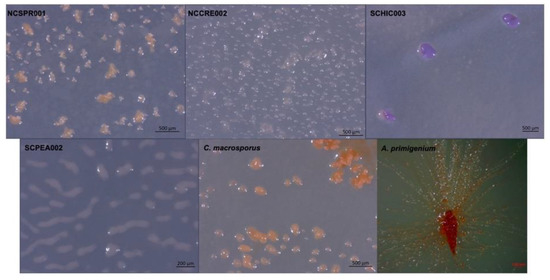
Figure 1.
Myxobacterial fruiting bodies from strains NCSPR001, NCCRE002, SCHIC003, SCPEA002, and the strains C. macrosporus ATCC 29,039 and A. primigenium ATCC 29037.
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
A. primigenium and C. macrosporus were procured from the ATCC as strain numbers ATCC 29037 and ATCC 29039, respectively. The remaining strains were isolated from soil as described later. All strains were cultured either on VY/2 or VY/4 agar plates (5 or 2.5 g/L baker’s yeast, 1.5 g/L CaCl2·2H2O, 0.5 mg/L vitamin B12, 15 g/L agar, pH 7.2). Swarming and fruiting bodies on agar plates were observed under a Zeiss discovery V12 stereo microscope and photographed using a Zeiss axiocam105.
2.2. Isolation of Environmental Myxobacteria
Soil samples, collected in Asheville, NC and Tryon, SC, were taken from the base of trees and dried in open air before storage. Detailed location data are provided as Supplemental Information (Table S3). Myxobacteria were isolated using a slightly modified Coli-spot method [51]. A 1 mg/mL solution of cycloheximide/nystatin was used to wet the soil sample to a paste-like consistency before inoculation onto an Escherichia coli baited WAT agar plate (1 g/L CaCl2·2H2O, 15 g/L agar, 20 mM HEPES). To prepare the baiting plate, a lawn of E. coli was grown overnight on tryptone soya broth (TSB) with agar (1.5%), and the cells were scraped and suspended in 2 mL of sterile deionized water. Four hundred μL of the E. coli suspension was spread over the surface of a WAT agar plate to create a bait circle of approximately 2 inches in diameter and let dry. Once the E. coli was dried, a pea sized amount of soil paste was placed at the center of the bait circle. Plates were incubated at 25 °C for up to a month, and degradation of the E. coli was monitored over time. Visible degrading swarms were seen after a few days, and swarm edges or fruiting bodies were passaged onto VY/4 media for purification. Purification was accomplished by repeated swarm edge transfer.
2.3. Genomic DNA Isolation, Sequencing, Assembly, and Annotation
Genomic DNA for NGS was obtained from actively growing bacteria on VY/2 or VY/4 plates using NucleoBond high molecular weight DNA kit (Macherey-Nagel, Bethlehem, PA, USA). The quantity and quality of the extraction were checked by Nanodrop (Thermo Scientific NanoDrop One) and followed by Qubit quantification using Qubit®® dsDNA HS Assay Kit (ThermoFisher Scientific, Suwanee, GA, USA).
Sequencing for all samples was performed on a Pacific Biosciences single-molecule real-time (SMRT) sequencing platform at the MR DNA facility (Shallowater, TX, USA). The SMRTbell libraries for the sample were prepared using the SMRTbell Express Template Prep Kit 2.0 (Pacific Biosciences, Menlo Park, CA, USA) following the manufacturer’s user guide. Following library preparation, the final concentration of each library was measured using the Qubit® dsDNA HS Assay Kit (ThermoFisher Scientific, Suwanee, GA, USA), and the average library sizes were determined using the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). Each library pool was then sequenced using the 10-h movie time on the PacBio Sequel (Pacific Biosciences, Menlo Park, CA, USA). De Novo Assembly of each genome was accomplished using the PacBio SMRT Analysis Hierarchical Genome Assembly Process (HGAP). Genome annotation was done using Rapid Annotation using Subsystem Technology (RAST) with further annotation requested by the NCBI Prokaryotic Genome Annotation Pipeline [52]. Sequencing data have been deposited in NCBI under the accession numbers JADWYI000000000.1, JAFIMU000000000, JAFIMS000000000, JAFIMT000000000, CP071090, and CP071091 for strains A. primigenium, C. macrosporus, NCSPR001, NCCRE002, SCPEA002, and SCHIC003, respectively.
2.4. Comparative Genomic Studies
The genome sequence data were uploaded to the Type (Strain) Genome Server (TYGS), a free bioinformatics platform available under https://tygs.dsmz.de (accessed 10 January 2021), for a whole genome-based taxonomic analysis. TYGS was used to calculate the dDDH values and construct minimum evolution trees using the Genome BLAST Distance Phylogeny approach (GBDP) [53,54]. GBDP trees were visualized using MEGA-X [55]. The average nucleotide identity (ANI) was calculated using the ANI/AAI-Matrix calculator [56,57].
2.5. BIG-SCAPE Analysis
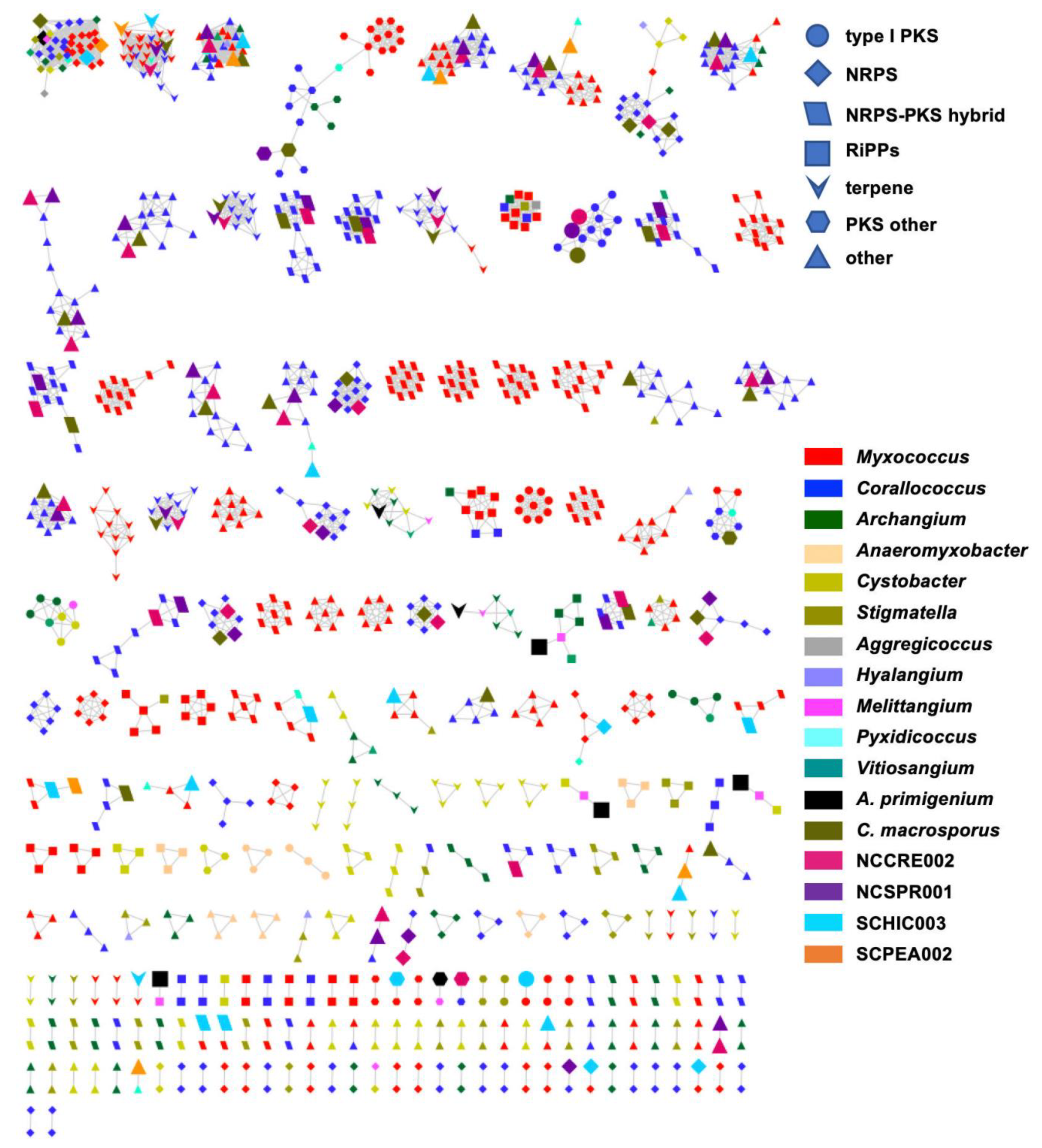
Genome data for all myxobacteria belonging to the Cystobacterineae suborder were downloaded from the NCBI database. A list of all myxobacteria used in this analysis are listed in List S1. These genomes in addition to genomes of A. primigenium, C. macrosporus, and the environmental isolates were analyzed by the AntiSMASH platform (version 5 available at https://docs.antismash.secondarymetabolites.org; accessed 1 February 2021) to assess specialized metabolite gene clusters using the “relaxed” strictness setting [58,59]. A total of 1826 predicted BGCs (.gbk files) were then processed locally using the BiG-SCAPE program (version 20181005, available at https://git.wageningenur.nl/medema-group/BiG-SCAPE; accessed 1 February 2021), with the MiBIG database (version 2.0 available at https://mibig.secondarymetabolites.org; accessed 1 February 2021) as reference [60,61]. BiG-SCAPE analysis was supplemented with Pfam database version 33.1 [62]. The singleton parameter in BiG-SCAPE was selected to ensure that BGCs with distances lower than the default cutoff distance of 0.3 were included in the corresponding output data. The hybrids-off parameter was selected to prevent hybrid BGC redundancy. Generated network files separated by BiG-SCAPE class were combined for visualization using Cytoscape version 3.8.2 (http://www.cytoscape.org; accessed 1 February 2021) [63]. Annotations associated with each BGC were included in Cytoscape networks by importing curated tables generated by BiG-SCAPE.
3. Results
3.1. Comparative Genomics and Taxonomic Assessment of Archangium Primigenium, Chondrococcus Macrosporus, and Environmental Isolates
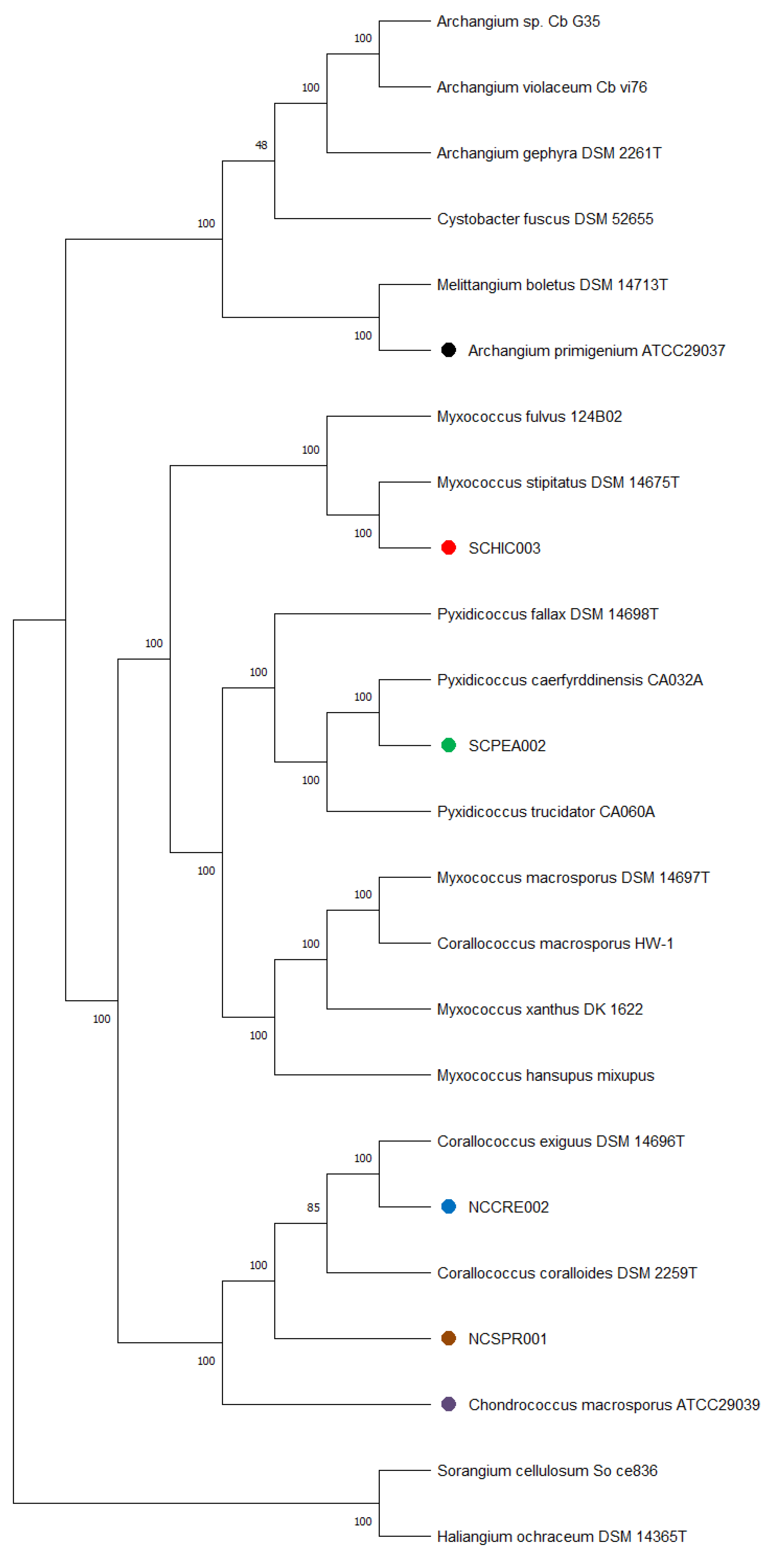
Genome sequencing provided high quality draft genomes for each of the six investigated myxobacteria, as indicated by the summary of general features in Table 1. The total genome sizes ranged from ~9.5–13 Mb, and the %GC content varied around ~69–71%. Of the six genomes, both environmental strains SCHIC003 and SCPEA002 were assembled on a single contig. Overall, the assemblies for each genome provided much lower total contig counts (1–17) than recently sequenced myxobacterial genomes [3,8]. Interestingly, a minimum evolution of phylogenetic trees generated from the whole genome sequence data clustered A. primigenium with Melittangium boletus DSM 14713T and not with the three currently sequenced strains from the genus Archangium (Figure 2, Figures S1 and S2). Accordingly, ANI and dDDH values supported the placement of A. primigenium in the genus Melittangium (Table 2) as a novel species with both values well below the established cutoffs for classification of distinct species (<95% ANI; <70% dDDH) [31,34,35,37,64]. These data suggest A. primigenium is currently misclassified as a member of the genus Archangium and should instead be placed in the genus Melittangium.

Table 1.
Genome properties and general features of myxobacteria under investigation in this study.
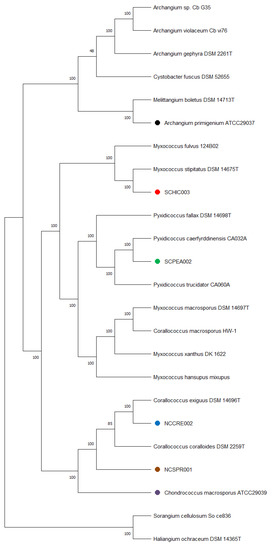
Figure 2.
Minimum evolution tree from the whole genomes of different myxobacteria including the six strains under investigation in this study using the GBDP approach. The numbers in bold above branches are GBDP pseudo-bootstrap support values > 60% from 100 replications, with an average branch support of 100.0%. Branch pseudo-bootstraps less than 50% are not shown. The numbers below branches are branch lengths scaled in terms of GBDP distance formula d5. The tree was rooted at the midpoint.

Table 2.
16S rRNA identity, ANI, and dDDH values for pairwise comparisons between A. primigenium with the most similar fully sequenced myxobacteria.
The calculated ANI and dDDH values for the sequenced C. marcosporus strain acquired from the ATCC support the original assignment to the genus Chondrococcus, now Corallococcus [31,50]. As opposed to the recently reclassified Myxococcus macrosporus DSM 14697T, previously Corallococcus macrosporus, the minimum evolution phylogenetic tree suggested C. macrosporus ATCC 29039 to be a member or the genus Corallococcus most similar to Corallococcus exercitus DSM 108849T (Figure 2, Figures S1 and S3) [50]. The isolated strains NCCRE002 and NCSPR001 were also determined to be members of the genus Corallococcus (Figure 2, Figures S1 and S3). Comparative genome analyses implied that strain NCCRE002 is an isolate of Corallococcus exiguus DSM 14696T. However, the ANI and GBDP trees suggested that strain NCSPR001 is a novel member of the genus Corallococcus most similar to Corallococcus coralloides DSM 2259T (Table 3).

Table 3.
Differentiation chart comparing C. macrosporus ATCC 29039, NCCRE002, and NCSPR001 draft genome data with sequenced members of the genus Corallococcus. The top half uses total genome comparison methods (ANI and dDDH) while the bottom half uses 16S rRNA sequence for pairwise comparison. Orange shading represents species that would be designated as the same using the designated method. Blue shading represents unique species using the designated method, <98.65% 16S identity%, or < 95%/70% for ANI/dDDH.
The isolated SCHIC003 and SCPEA002 strains were initially determined to be members of the genus Myxococcus. However, inclusion of sequenced representatives from the genus Pyxidicoccus (considered to be synonymous with Myxococcus) [3] in our comparative analysis grouped strain SCPEA002 within the Pyxidicoccus clade (Figure 2, Figures S1 and S4). Most similar to Pyxidicoccus caerfyrddinensis CA032AT, dDDH and ANI analysis suggested the SCPEA002 strain to be a novel member of the genus Pyxidicoccus (Table 4). Similarly, comparative genome analysis determined that strain SCHIC003 is likely be a novel member of the genus Myxococcus, albeit highly similar to Myxococcus stipitatus DSM 14675T with ANI and dDDH values just below the cutoffs for species differentiation [31,37,64] (Table 4 and Figure 2, Figures S1 and S3).

Table 4.
Differentiation chart comparing SCPEA002 and SCHIC003 draft genome data with sequenced members of the genera Myxococcus and Pyxidicoccus. The top half uses total genome comparison methods (ANI and dDDH) while the bottom half uses 16S rRNA sequence for pairwise comparison. Orange shading represents species that would be designated as the same using the designated method. Blue shading represents unique species using the designated method, <98.65% 16S identity%, or < 95%/70% for ANI/dDDH.
3.2. Biosynthetic Potential and Genus Level Correlations
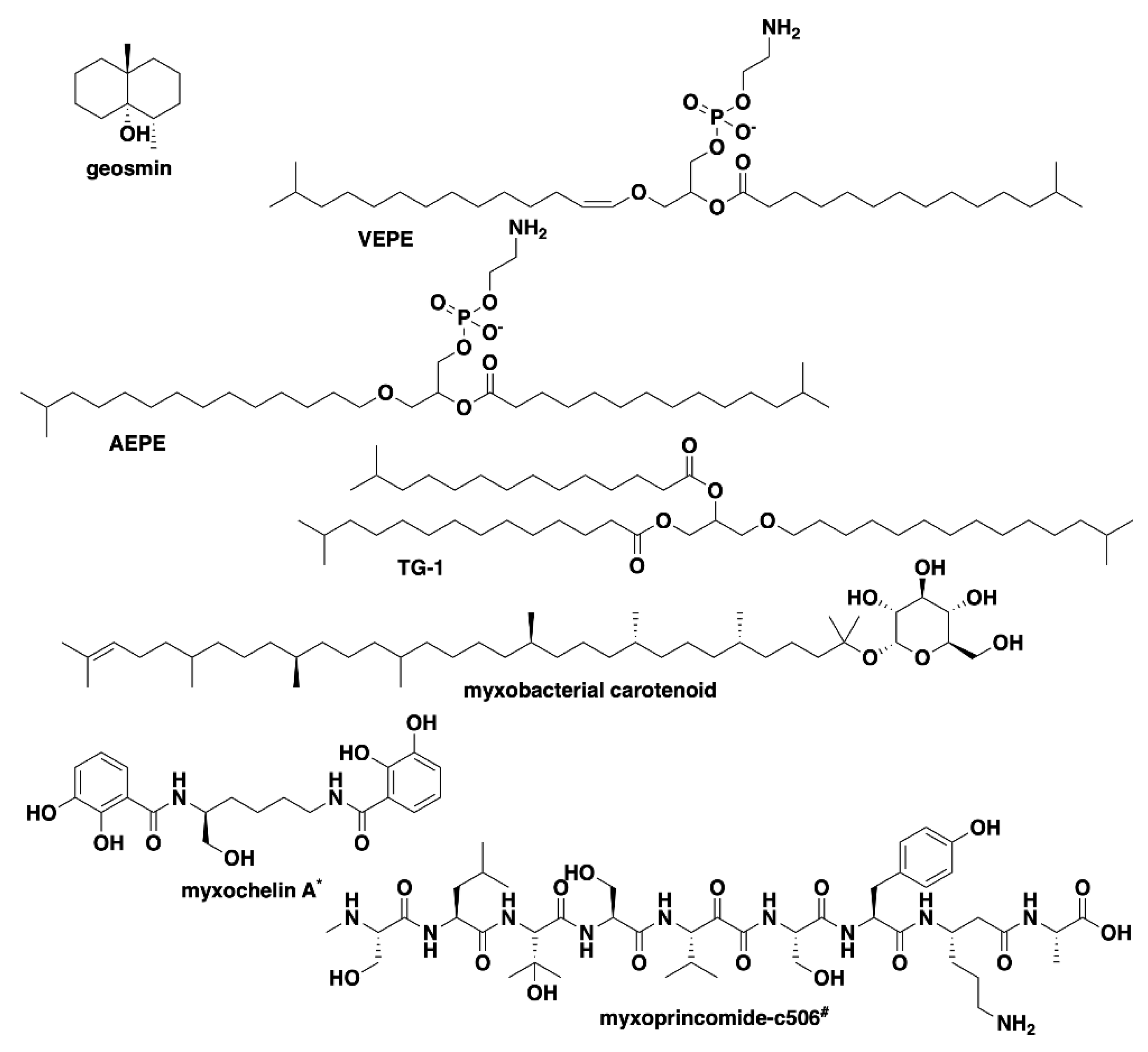
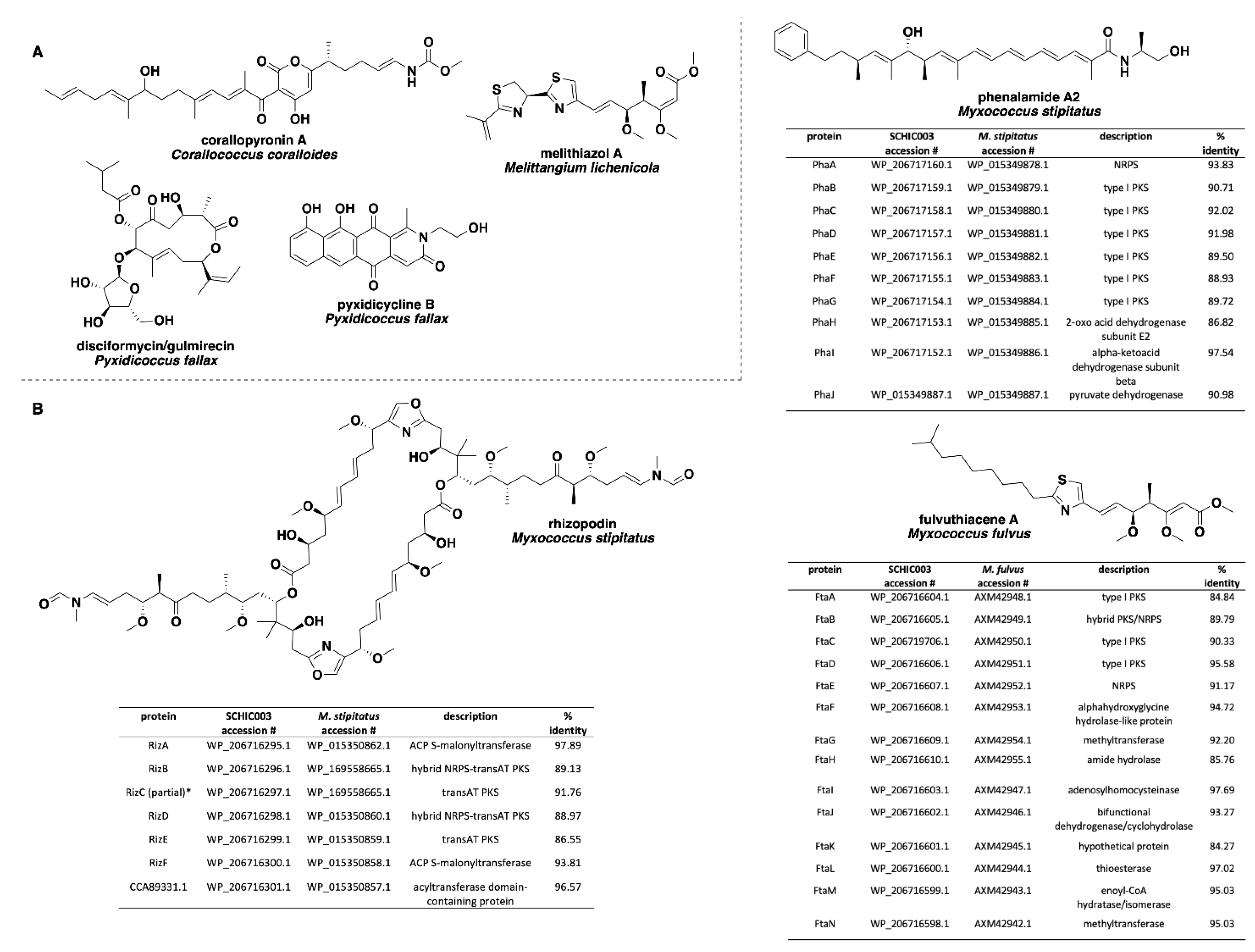
Analysis of our draft genomes using the biosynthetic pathway prediction platform AntiSMASH revealed a range of 29–42 total predicted BGCs with C. macrosporus including the highest total of BGCs. However, the draft genome for C. macrosporus also included the highest total of four partial BGCs positioned on the edges of contigs. No BGCs occurring on contig edges were observed from A. primigenium, NCSPR001, or SCPEA002. All of the sequenced strains included highly similar (≥75% similarity score) biosynthetic pathways for the signaling terpene geosmin [65,66], the signaling lipids VEPE/AEPE/TG-1 [67,68], and carotenoids [69,70,71,72] (Figure 3). Excluding SCHIC003, each genome included a BGC highly homologous to the pathway associated with the myxobacterial siderophore myxochelin [73,74]. Pathways somewhat similar (similarity scores of 66%) to the myxoprincomide-c506 BGC were observed in every genome except the A. primigenium genome [75]. Clusters with ≥75% similarity to pathways from M. stipitatus DSM 14675T associated with the metabolites rhizopodin [76,77] and phenalamide A2 [78] were observed in the SCHIC003 draft genome as well as clusters also present in the M. stipitatus DSM 14675T genome deposited in the AntiSMASH database [79], including the dkxanthene [80], fulvuthiacene [81], and violacein [82,83,84] BGCs (Figure 4). Considering previously characterized BGCs from each genus associated with the six investigated myxobacteria, the corallopyronin BGC from C. coralloides B035 [85,86] was absent from all three of the putative Corallococcus strains, the melithiazol BGC from Melittangium lichenicola Me I46 [87] was not present in A. primigenium, and neither the disciformycin/gulmirecin BGC [88,89] or the pyxidicycline BGC [90] from Pyxidicoccus fallax were present in SCPEA002.
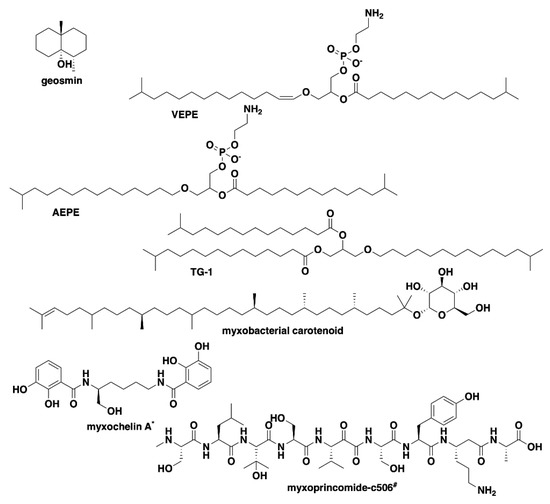
Figure 3.
Common specialized metabolites from myxobacteria associated with characterized BGCs present in the six investigated strains of myxobacteria. * Myxochelin BGC not present in SCHIC003 genome data. # Myxoprincomide BGC not present in A. primigenium genome data.
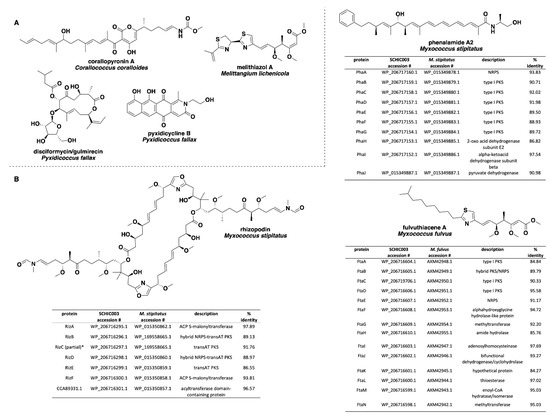
Figure 4.
(A) Specialized metabolites produced by members of the genera Corallococcus, Melittangium, and Pyxidicoccus with no associated BGCs observed in any of the six investigated myxobacterial strains. (B) Comparisons of the rhizopodin, phenalamide A2, and fulvuthiacene BGCs from SCHIC003 genome data and the characterized pathways from M. stipitatus and M. fulvus. All SCHIC003 gene products, excluding RizC, had coverages ≥99% with the indicated homolog. * RizC located on a contig edge and is incomplete in SCHIC003 genome data.
Utilizing the BiG-SCAPE platform to render BGC sequence similarity networks, we sought to determine the extent of homology between BGCs from our six sequenced myxobacteria and BGCs from all currently sequenced members of the suborder Cystobacterineae [91]. The resulting sequence similarity network included 1080 BGCs connected by 3046 edges (not including self-looped nodes/singletons) and depicted genus-level homologies across all BGCs from the newly sequenced myxobacteria corroborating our suggested taxonomic assignments (Figure 5 and Table 5). For example, BGCs from the three newly sequenced samples C. macrosporus, NCSPR001, and NCCRE002 were almost exclusively clustered with BGCs from members of the genus Corallococcus, and BGCs from SCHIC003 and SCPEA002 samples clustered with the genera Myxococcus and Pyxidicoccus (Figure 5). However, SCPEA002 BGCs do not cluster as frequently with Pyxidicoccus BGCs as they do Myxococcus BGCs, and the majority (76.5%) were not clustered with any BGC within the network (Table 5). This is likely due to the highly fragmented nature of available Pyxidicoccus genomes resulting in many incomplete or partial BGCs. Therefore, few Pyxidicoccus pathways appear in the similarity network, and the percentage of unique pathways associated with SCPEA002 is likely overestimated. Regardless, the limited number of SCPEA002 BGCs clustered with BGCs from Myxococcus/Pyxidicoccus genomes indicates a potential to discover novel metabolites despite placement in the highly scrutinized clade. The only clustered groups with numerous edges formed between BGCs from the genera Myxococcus and Corallococcus included characterized biosynthetic pathways for ubiquitous signaling lipids VEPE/AEPE/TG-1, carotenoids, and the siderophore myxochelin as well as two uncharacterized BGCs predicted to produce ribosomally synthesized and post-translationally modified peptides (RiPPs).
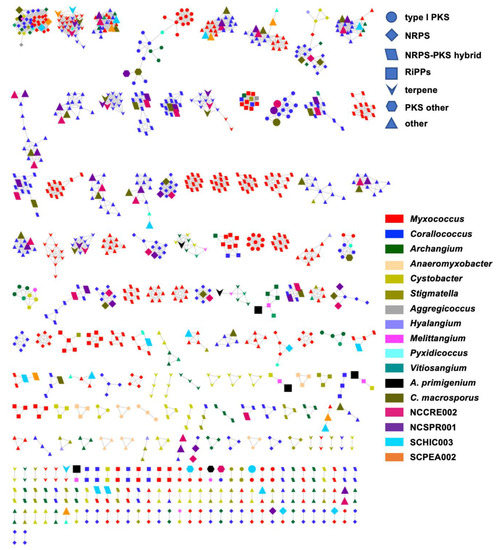
Figure 5.
BiG-SCAPE BGC sequence similarity networks (c = 0.3) as visualized with Cytoscape. The network is generated from A. primigenium, C. macrosporus, NCCRE002, NCSPR001, SCHIC003, SCPEA002, and all myxobacteria belonging to the Cystobacterineae suborder with genomes deposited in NCBI. Each node represents one BGC identified by AntiSMASH 5.0, where the colors and shapes of the nodes represent different genera and AntiSMASH-predicted classes, respectively. Nodes representing BGCs from newly sequenced myxobacteria included in this study are enlarged. BGCs included as singletons in the original BiG-SCAPE analysis removed.

Table 5.
Overview of BiG-SCAPE BGC sequence similarity networks of the six strains under investigation in this study.
Interestingly, a total of 23 A. primigenium BGCs (out of 32 BGCs) appear as singletons in the network with no homology to any of the included BGCs from Cystobacterineae. In fact, aside from the VEPE/AEPE/TG-1 cluster and a terpene cluster that included members of the genera Archangium and Cystobacter, all remaining BGCs from A. primigenium had connecting edges to BGCs from Melittangium boletus DSM 14713T. Out of 21 edges formed by A. primigenium in the network, four edges were formed with four species of Corallococcus (a total of 11 Corallococcus species in the network), four edges were formed with all species of Cystobacter (three species in the network), six edges were formed with all species of Archangium (three species in the network), and seven edges were formed with the only Melittangium species in the network, M. boletus DSM 14713T. Overall, these data corroborate our preliminary taxonomic assignments and suggest that the prioritization of A. primigenium for subsequent discovery efforts is most likely to yield novel metabolites.
4. Discussion
As novel myxobacteria continue to be isolated and explored for natural product discovery, efficient approaches for approximate taxonomic placement will assist the prioritization of lesser studied genera. Utilizing long-read genome sequencing and comparative genomic analyses, we determine preliminary taxonomic placement for four myxobacteria isolated from North American soils and two myxobacteria deposited at the ATCC. This approach indicated that previously classified A. primigenium ATCC 29037 is instead a novel member of the genus Melittangium, and that three of our four environmental isolates included potentially novel members of the genera Corallococcus, Myxococcus, and Pyxidicoccus. Previously classified Chondorococcus macrosporus ATCC 29039 was also determined to be a potentially novel member of the genus Corallococcus, with high similarity to C. exercitus DSM 108849T and phylogenetically distinct from M. macrosporus DSM 14697T previously assigned to the genus Corallococcus. Subsequent bioinformatic analysis of biosynthetic pathways included in the newly sequenced genomes corroborated our preliminary taxonomic placements for each sample. Ultimately, this process identified A. primigenium to be a member of the lesser studied genus Melittangium and indicated that it should be prioritized for continued natural product discovery efforts. Of the environmental isolates, BGCs from SCPEA002 were determined to include the least amount of overlap with BGCs from other Myxococcus/Pyxidicoccus species. While environmental isolates SCHIC002 and NCSPR001 were also identified as novel members of the genera Myxococcus and Corallococcus, respectively, the apparent overlap in BGCs from thoroughly explored myxobacteria determined from sequence similarity network analysis suggests a limited potential for discovery of novel specialized metabolites. Overall, comparative genomic techniques including the assessment of biosynthetic potential enabled a phylogenetic approximation and suggested prioritization of A. primigenium for natural product discovery efforts from a sample set of six newly sequenced myxobacteria.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/microorganisms9071376/s1. Supplemental Table S1: Novel myxobacteria described in literature spanning 2011-2021; Table S2: Comparison of biosynthetic gene clusters (BGCs) located on contig edges from previously sequenced members of the genus Corallococcus, Table S3: Locations of soil samples used for environmental strains isolation, List S1: A list of all myxobacteria and their accession numbers used in BiG-SCAPE; Figure S1: Minimum evolution tree from the 16S rRNA of the 6 strains under investigation in this study and all myxobacteria Type strains deposited in DSMZ; Figure S2: Minimum evolution tree from the whole genomes of A. primigenium and different members of the family Archangiaceae using the GBDP approach; Figure S3: Minimum evolution tree from the whole genomes of C. macrosporus ATCC 29039, NCCRE002, NCSPR001 and different members of genus Corallococcus using the GBDP approach; Figure S4: Minimum evolution tree from the whole genomes of SCHIC003, SCPEA002, and different members of Myxococcus and Pyxidicoccus using the GBDP approach.
Author Contributions
Conceptualization, supervision, and administration D.C.S.; formal analysis and data curation A.A., H.A., S.E.D., and D.C.S.; methodology, validation, and writing A.A., H.A., and D.C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by funds from the National Institute of Allergy and Infectious Diseases (1 R15 AI137996-01A1) and the National Institute of General Medical Sciences (1 P20 GM130460-01A1).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Sequencing data have been deposited in NCBI under the accession numbers JADWYI000000000.1, JAFIMU000000000, JAFIMS000000000, JAFIMT000000000, CP071090, and CP071091 for strains A. primigenium, C. macrosporus, NCSPR001, NCCRE002, SCPEA002, and SCHIC003, respectively.
Acknowledgments
The authors would like to acknowledge the University of Mississippi School of Pharmacy for startup support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Awal, R.P.; Garcia, R.; Gemperlein, K.; Wink, J.; Kunwar, B.; Parajuli, N.; Muller, R. Vitiosangium cumulatum gen. nov., sp. nov. and Vitiosangium subalbum sp. nov., soil myxobacteria, and emended descriptions of the genera Archangium and Angiococcus, and of the family Cystobacteraceae. Int. J. Syst. Evol. Microbiol. 2017, 67, 1422–1430. [Google Scholar] [CrossRef]
- Awal, R.P.; Garcia, R.; Muller, R. Racemicystis crocea gen. nov., sp. nov., a soil myxobacterium in the family Polyangiaceae. Int. J. Syst. Evol. Microbiol. 2016, 66, 2389–2395. [Google Scholar] [CrossRef] [Green Version]
- Chambers, J.; Sparks, N.; Sydney, N.; Livingstone, P.G.; Cookson, A.R.; Whitworth, D.E. Comparative Genomics and Pan-Genomics of the Myxococcaceae, including a Description of Five Novel Species: Myxococcus eversor sp. nov., Myxococcus llanfairpwllgwyngyllgogerychwyrndrobwllllantysiliogogogochensis sp. nov., Myxococcus vastator sp. nov., Pyxidicoccus caerfyrddinensis sp. nov., and Pyxidicoccus trucidator sp. nov. Genome Biol. Evol. 2020, 12, 2289–2302. [Google Scholar] [CrossRef]
- Garcia, R.; Gemperlein, K.; Muller, R. Minicystis rosea gen. nov., sp. nov., a polyunsaturated fatty acid-rich and steroid-producing soil myxobacterium. Int. J. Syst. Evol. Microbiol. 2014, 64, 3733–3742. [Google Scholar] [CrossRef]
- Garcia, R.; Muller, R. Simulacricoccus ruber gen. nov., sp. nov., a microaerotolerant, non-fruiting, myxospore-forming soil myxobacterium and emended description of the family Myxococcaceae. Int. J. Syst. Evol. Microbiol. 2018, 68, 3101–3110. [Google Scholar] [CrossRef]
- Garcia, R.; Stadler, M.; Gemperlein, K.; Muller, R. Aetherobacter fasciculatus gen. nov., sp. nov. and Aetherobacter rufus sp. nov., novel myxobacteria with promising biotechnological applications. Int. J. Syst. Evol. Microbiol. 2016, 66, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, T.; Jojima, Y.; Hayakawa, A.; Fujii, T.; Yamanaka, S.; Fudou, R. Pseudenhygromyxa salsuginis gen. nov., sp. nov., a myxobacterium isolated from an estuarine marsh. Int. J. Syst. Evol. Microbiol. 2013, 63, 1360–1369. [Google Scholar] [CrossRef]
- Livingstone, P.G.; Ingleby, O.; Girdwood, S.; Cookson, A.R.; Morphew, R.M.; Whitworth, D.E. Predatory Organisms with Untapped Biosynthetic Potential: Descriptions of Novel Corallococcus Species C. aberystwythensis sp. nov., C. carmarthensis sp. nov., C. exercitus sp. nov., C. interemptor sp. nov., C. llansteffanensis sp. nov., C. praedator sp. nov., C. sicarius sp. nov., and C. terminator sp. nov. Appl. Environ. Microbiol. 2020, 86. [Google Scholar] [CrossRef]
- Mohr, K.I.; Garcia, R.O.; Gerth, K.; Irschik, H.; Muller, R. Sandaracinus amylolyticus gen. nov., sp. nov., a starch-degrading soil myxobacterium, and description of Sandaracinaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2012, 62, 1191–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohr, K.I.; Moradi, A.; Glaeser, S.P.; Kampfer, P.; Gemperlein, K.; Nubel, U.; Schumann, P.; Muller, R.; Wink, J. Nannocystis konarekensis sp. nov., a novel myxobacterium from an Iranian desert. Int. J. Syst. Evol. Microbiol. 2018, 68, 721–729. [Google Scholar] [CrossRef]
- Mohr, K.I.; Wolf, C.; Nubel, U.; Szafranska, A.K.; Steglich, M.; Hennessen, F.; Gemperlein, K.; Kampfer, P.; Martin, K.; Muller, R.; et al. A polyphasic approach leads to seven new species of the cellulose-decomposing genus Sorangium, Sorangium ambruticinum sp. nov., Sorangium arenae sp. nov., Sorangium bulgaricum sp. nov., Sorangium dawidii sp. nov., Sorangium kenyense sp. nov., Sorangium orientale sp. nov. and Sorangium reichenbachii sp. nov. Int. J. Syst. Evol. Microbiol. 2018, 68, 3576–3586. [Google Scholar] [CrossRef] [PubMed]
- Moradi, A.; Ebrahimipour, G.H.; Mohr, K.I.; Kampfer, P.; Glaeser, S.P.; Hennessen, F.; Gemperlein, K.; Awal, R.P.; Wolf, C.; Muller, R.; et al. Racemicystis persica sp. nov., a myxobacterium from soil. Int. J. Syst. Evol. Microbiol. 2017, 67, 472–478. [Google Scholar] [CrossRef]
- Sood, S.; Awal, R.P.; Wink, J.; Mohr, K.I.; Rohde, M.; Stadler, M.; Kampfer, P.; Glaeser, S.P.; Schumann, P.; Garcia, R.; et al. Aggregicoccus edonensis gen. nov., sp. nov., an unusually aggregating myxobacterium isolated from a soil sample. Int. J. Syst. Evol. Microbiol. 2015, 65, 745–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, E.; Muramatsu, H.; Nagai, K. Vulgatibacter incomptus gen. nov., sp. nov. and Labilithrix luteola gen. nov., sp. nov., two myxobacteria isolated from soil in Yakushima Island, and the description of Vulgatibacteraceae fam. nov., Labilitrichaceae fam. nov. and Anaeromyxobacteraceae fam. nov. Int. J. Syst. Evol. Microbiol. 2014, 64, 3360–3368. [Google Scholar] [CrossRef]
- Bader, C.D.; Panter, F.; Muller, R. In depth natural product discovery–Myxobacterial strains that provided multiple secondary metabolites. Biotechnol. Adv. 2020, 39, 107480. [Google Scholar] [CrossRef] [PubMed]
- Bretl, D.J.; Kirby, J.R. Molecular Mechanisms of Signaling in Myxococcus xanthus Development. J. Mol. Biol. 2016, 428, 3805–3830. [Google Scholar] [CrossRef] [PubMed]
- Mercier, R.; Mignot, T. Regulations governing the multicellular lifestyle of Myxococcus xanthus. Curr. Opin. Microbiol. 2016, 34, 104–110. [Google Scholar] [CrossRef]
- Mohr, K.I. Diversity of Myxobacteria-We Only See the Tip of the Iceberg. Microorganisms 2018, 6, 84. [Google Scholar] [CrossRef] [Green Version]
- Pathak, D.T.; Wei, X.; Wall, D. Myxobacterial tools for social interactions. Res. Microbiol. 2012, 163, 579–591. [Google Scholar] [CrossRef] [Green Version]
- Petters, S.; Gross, V.; Sollinger, A.; Pichler, M.; Reinhard, A.; Bengtsson, M.M.; Urich, T. The soil microbial food web revisited: Predatory myxobacteria as keystone taxa? ISME J. 2021. [Google Scholar] [CrossRef]
- Sah, G.P.; Wall, D. Kin recognition and outer membrane exchange (OME) in myxobacteria. Curr. Opin. Microbiol. 2020, 56, 81–88. [Google Scholar] [CrossRef]
- Thiery, S.; Kaimer, C. The Predation Strategy of Myxococcus xanthus. Front. Microbiol. 2020, 11, 2. [Google Scholar] [CrossRef]
- Whitworth, D.E. Genome-wide analysis of myxobacterial two-component systems: Genome relatedness and evolutionary changes. BMC Genom. 2015, 16, 780. [Google Scholar] [CrossRef] [Green Version]
- Baltz, R.H. Molecular beacons to identify gifted microbes for genome mining. J. Antibiot. 2017, 70, 639–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baltz, R.H. Gifted microbes for genome mining and natural product discovery. J. Ind. Microbiol. Biotechnol. 2017, 44, 573–588. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Fayad, A.A.; Muller, R. Natural products from myxobacteria: Novel metabolites and bioactivities. Nat. Prod. Rep. 2017, 34, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Landwehr, W.; Wolf, C.; Wink, J. Actinobacteria and Myxobacteria-Two of the Most Important Bacterial Resources for Novel Antibiotics. Curr. Top. Microbiol. Immunol. 2016, 398, 273–302. [Google Scholar] [CrossRef] [PubMed]
- Weissman, K.J.; Muller, R. Myxobacterial secondary metabolites: Bioactivities and modes-of-action. Nat. Prod. Rep. 2010, 27, 1276–1295. [Google Scholar] [CrossRef]
- Wenzel, S.C.; Muller, R. Myxobacteria--‘microbial factories’ for the production of bioactive secondary metabolites. Mol. Biosyst. 2009, 5, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, T.; Krug, D.; Bozkurt, N.; Duddela, S.; Jansen, R.; Garcia, R.; Gerth, K.; Steinmetz, H.; Muller, R. Correlating chemical diversity with taxonomic distance for discovery of natural products in myxobacteria. Nat. Commun. 2018, 9, 803. [Google Scholar] [CrossRef]
- Chun, J.; Oren, A.; Ventosa, A.; Christensen, H.; Arahal, D.R.; da Costa, M.S.; Rooney, A.P.; Yi, H.; Xu, X.W.; De Meyer, S.; et al. Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int. J. Syst. Evol. Microbiol. 2018, 68, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Rainey, F.A. Integrating genomics into the taxonomy and systematics of the Bacteria and Archaea. Int. J. Syst. Evol. Microbiol. 2014, 64, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Garcia, R.; Gerth, K.; Stadler, M.; Dogma, I.J., Jr.; Muller, R. Expanded phylogeny of myxobacteria and evidence for cultivation of the ‘unculturables’. Mol. Phylogenet. Evol. 2010, 57, 878–887. [Google Scholar] [CrossRef]
- Goris, J.; Konstantinidis, K.T.; Klappenbach, J.A.; Coenye, T.; Vandamme, P.; Tiedje, J.M. DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int. J. Syst. Evol. Microbiol. 2007, 57, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Oh, H.S.; Park, S.C.; Chun, J. Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int. J. Syst. Evol. Microbiol. 2014, 64, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, P.G.; Morphew, R.M.; Whitworth, D.E. Genome Sequencing and Pan-Genome Analysis of 23 Corallococcus spp. Strains Reveal Unexpected Diversity, With Particular Plasticity of Predatory Gene Sets. Front. Microbiol. 2018, 9, 3187. [Google Scholar] [CrossRef] [Green Version]
- Sangal, V.; Goodfellow, M.; Jones, A.L.; Schwalbe, E.C.; Blom, J.; Hoskisson, P.A.; Sutcliffe, I.C. Next-generation systematics: An innovative approach to resolve the structure of complex prokaryotic taxa. Sci. Rep. 2016, 6, 38392. [Google Scholar] [CrossRef] [Green Version]
- Shimkets, L.; Woese, C.R. A phylogenetic analysis of the myxobacteria: Basis for their classification. Proc. Natl. Acad. Sci. USA 1992, 89, 9459–9463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sproer, C.; Reichenbach, H.; Stackebrandt, E. The correlation between morphological and phylogenetic classification of myxobacteria. Int. J. Syst. Evol. Microbiol. 1999, 49 Pt 3, 1255–1262. [Google Scholar] [CrossRef]
- Stackebrandt, E.; Pauker, O.; Steiner, U.; Schumann, P.; Straubler, B.; Heibei, S.; Lang, E. Taxonomic characterization of members of the genus Corallococcus: Molecular divergence versus phenotypic coherency. Syst. Appl. Microbiol. 2007, 30, 109–118. [Google Scholar] [CrossRef]
- Bouhired, S.; Rupp, O.; Blom, J.; Schaberle, T.F.; Schiefer, A.; Kehraus, S.; Pfarr, K.; Goesmann, A.; Hoerauf, A.; Konig, G. Complete Genome Sequence of the Corallopyronin A-Producing Myxobacterium Corallococcus coralloides B035. Microbiol. Resour. Announc. 2019, 8, e00050-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huntley, S.; Zhang, Y.; Treuner-Lange, A.; Kneip, S.; Sensen, C.W.; Sogaard-Andersen, L. Complete genome sequence of the fruiting myxobacterium Corallococcus coralloides DSM 2259. J. Bacteriol. 2012, 194, 3012–3013. [Google Scholar] [CrossRef] [Green Version]
- McDonald, J.C. Studies on the genus Archangium (Myxobacterales). II. The effect of temperature and carbohydrates on some physiological processes. Mycologia 1967, 59, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.C. Studies on the genus Archangium (Myxobacterales) I. Morphology. Mycologia 1965, 57, 737–747. [Google Scholar] [CrossRef]
- Shimkets, L.J.; Dworkin, M.; Reichenbach, H. The Myxobacteria. In The Prokaryotes; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006. [Google Scholar] [CrossRef]
- McCurdy, H.D. Studies on the Taxonomy of the Myxobacterales. Int. J. Syst. Bacteriol. 1971, 21, 50–54. [Google Scholar] [CrossRef]
- Starr, M.P.; Skerman, V.B. Bacterial diversity: The natural history of selected morphologically unusual bacteria. Annu. Rev. Microbiol. 1965, 19, 407–454. [Google Scholar] [CrossRef] [PubMed]
- Sly, L.I. Taxonomic Note: V. B. D. Skerman (1921–1993), a Reforming Force in Bacterial Systematics and Nomenclature. Int. J. Syst. Evol. Microbiol. 1995, 45, 412–413. [Google Scholar] [CrossRef]
- Karwowski, J.P.; Sunga, G.N.; Kadam, S.; McAlpine, J.B. A method for the selective isolation of Myxococcus directly from soil. J. Ind. Microbiol. 1996, 16, 230–236. [Google Scholar] [CrossRef]
- Lang, E.; Stackebrandt, E. Emended descriptions of the genera Myxococcus and Corallococcus, typification of the species Myxococcus stipitatus and Myxococcus macrosporus and a proposal that they be represented by neotype strains. Request for an Opinion. Int. J. Syst. Evol. Microbiol. 2009, 59, 2122–2128. [Google Scholar] [CrossRef]
- Dawid, W. Biology and global distribution of myxobacteria in soils. FEMS Microbiol. Rev. 2000, 24, 403–427. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef] [Green Version]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [Green Version]
- Meier-Kolthoff, J.P.; Goker, M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Ouk Kim, Y.; Park, S.C.; Chun, J. OrthoANI: An improved algorithm and software for calculating average nucleotide identity. Int. J. Syst. Evol. Microbiol. 2016, 66, 1100–1103. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-R, L.M.; Konstantinidis, K.T. The enveomics collection: A toolbox for specialized analyses of microbial genomes and metagenomes. PeerJ 2016, 4, e1900v1. [Google Scholar]
- Medema, M.H.; Blin, K.; Cimermancic, P.; de Jager, V.; Zakrzewski, P.; Fischbach, M.A.; Weber, T.; Takano, E.; Breitling, R. antiSMASH: Rapid identification, annotation and analysis of secondary metabolite biosynthesis gene clusters in bacterial and fungal genome sequences. Nucleic Acids Res. 2011, 39, W339–W346. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Steinke, K.; Villebro, R.; Ziemert, N.; Lee, S.Y.; Medema, M.H.; Weber, T. antiSMASH 5.0: Updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 2019, 47, W81–W87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kautsar, S.A.; Blin, K.; Shaw, S.; Navarro-Munoz, J.C.; Terlouw, B.R.; van der Hooft, J.J.J.; van Santen, J.A.; Tracanna, V.; Suarez Duran, H.G.; Pascal Andreu, V.; et al. MIBiG 2.0: A repository for biosynthetic gene clusters of known function. Nucleic Acids Res. 2020, 48, D454–D458. [Google Scholar] [CrossRef] [Green Version]
- Navarro-Munoz, J.C.; Selem-Mojica, N.; Mullowney, M.W.; Kautsar, S.A.; Tryon, J.H.; Parkinson, E.I.; De Los Santos, E.L.C.; Yeong, M.; Cruz-Morales, P.; Abubucker, S.; et al. A computational framework to explore large-scale biosynthetic diversity. Nat. Chem. Biol. 2020, 16, 60–68. [Google Scholar] [CrossRef]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.; Rossello-Mora, R. Shifting the genomic gold standard for the prokaryotic species definition. Proc. Natl. Acad. Sci. USA 2009, 106, 19126–19131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bentley, R.; Meganathan, R. Geosmin and methylisoborneol biosynthesis in streptomycetes. Evidence for an isoprenoid pathway and its absence in non-differentiating isolates. FEBS Lett. 1981, 125, 220–222. [Google Scholar] [CrossRef] [Green Version]
- Dickschat, J.S.; Bode, H.B.; Mahmud, T.; Muller, R.; Schulz, S. A novel type of geosmin biosynthesis in myxobacteria. J. Org. Chem. 2005, 70, 5174–5182. [Google Scholar] [CrossRef]
- Bhat, S.; Ahrendt, T.; Dauth, C.; Bode, H.B.; Shimkets, L.J. Two lipid signals guide fruiting body development of Myxococcus xanthus. mBio 2014, 5, e00939-13. [Google Scholar] [CrossRef] [Green Version]
- Lorenzen, W.; Bozhuyuk, K.A.; Cortina, N.S.; Bode, H.B. A comprehensive insight into the lipid composition of Myxococcus xanthus by UPLC-ESI-MS. J. Lipid Res. 2014, 55, 2620–2633. [Google Scholar] [CrossRef] [Green Version]
- Botella, J.A.; Murillo, F.J.; Ruiz-Vazquez, R. A cluster of structural and regulatory genes for light-induced carotenogenesis in Myxococcus xanthus. Eur. J. Biochem. 1995, 233, 238–248. [Google Scholar] [CrossRef]
- Cervantes, M.; Murillo, F.J. Role for vitamin B(12) in light induction of gene expression in the bacterium Myxococcus xanthus. J. Bacteriol. 2002, 184, 2215–2224. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Rubio, J.J.; Elias-Arnanz, M.; Padmanabhan, S.; Murillo, F.J. A repressor-antirepressor pair links two loci controlling light-induced carotenogenesis in Myxococcus xanthus. J. Biol. Chem. 2002, 277, 7262–7270. [Google Scholar] [CrossRef] [Green Version]
- Perez-Marin, M.C.; Padmanabhan, S.; Polanco, M.C.; Murillo, F.J.; Elias-Arnanz, M. Vitamin B12 partners the CarH repressor to downregulate a photoinducible promoter in Myxococcus xanthus. Mol. Microbiol. 2008, 67, 804–819. [Google Scholar] [CrossRef] [PubMed]
- Gaitatzis, N.; Kunze, B.; Muller, R. In vitro reconstitution of the myxochelin biosynthetic machinery of Stigmatella aurantiaca Sg a15: Biochemical characterization of a reductive release mechanism from nonribosomal peptide synthetases. Proc. Natl. Acad. Sci. USA 2001, 98, 11136–11141. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Weissman, K.J.; Muller, R. Myxochelin biosynthesis: Direct evidence for two- and four-electron reduction of a carrier protein-bound thioester. J. Am. Chem. Soc. 2008, 130, 7554–7555. [Google Scholar] [CrossRef]
- Cortina, N.S.; Krug, D.; Plaza, A.; Revermann, O.; Muller, R. Myxoprincomide: A natural product from Myxococcus xanthus discovered by comprehensive analysis of the secondary metabolome. Angew. Chem. Int. Ed. 2012, 51, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Sasse, F.; Steinmetz, H.; Hofle, G.; Reichenbach, H. Rhizopodin, a new compound from Myxococcus stipitatus (myxobacteria) causes formation of rhizopodia-like structures in animal cell cultures. Production, isolation, physico-chemical and biological properties. J. Antibiot. 1993, 46, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Pistorius, D.; Muller, R. Discovery of the rhizopodin biosynthetic gene cluster in Stigmatella aurantiaca Sg a15 by genome mining. Chembiochem 2012, 13, 416–426. [Google Scholar] [CrossRef]
- Park, S.; Hyun, H.; Lee, J.S.; Cho, K. Identification of the Phenalamide Biosynthetic Gene Cluster in Myxococcus stipitatus DSM 14675. J. Microbiol. Biotechnol. 2016, 26, 1636–1642. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Kautsar, S.A.; Medema, M.H.; Weber, T. The antiSMASH database version 3: Increased taxonomic coverage and new query features for modular enzymes. Nucleic Acids Res. 2021, 49, D639–D643. [Google Scholar] [CrossRef]
- Meiser, P.; Weissman, K.J.; Bode, H.B.; Krug, D.; Dickschat, J.S.; Sandmann, A.; Muller, R. DKxanthene biosynthesis--understanding the basis for diversity-oriented synthesis in myxobacterial secondary metabolism. Chem. Biol. 2008, 15, 771–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panter, F.; Krug, D.; Muller, R. Novel Methoxymethacrylate Natural Products Uncovered by Statistics-Based Mining of the Myxococcus fulvus Secondary Metabolome. ACS Chem. Biol. 2019, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Momen, A.Z.; Hoshino, T. Biosynthesis of violacein: Intact incorporation of the tryptophan molecule on the oxindole side, with intramolecular rearrangement of the indole ring on the 5-hydroxyindole side. Biosci. Biotechnol. Biochem. 2000, 64, 539–549. [Google Scholar] [CrossRef]
- Brady, S.F.; Chao, C.J.; Handelsman, J.; Clardy, J. Cloning and heterologous expression of a natural product biosynthetic gene cluster from eDNA. Org. Lett. 2001, 3, 1981–1984. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, T. Violacein and related tryptophan metabolites produced by Chromobacterium violaceum: Biosynthetic mechanism and pathway for construction of violacein core. Appl. Microbiol. Biotechnol. 2011, 91, 1463–1475. [Google Scholar] [CrossRef]
- Erol, O.; Schaberle, T.F.; Schmitz, A.; Rachid, S.; Gurgui, C.; El Omari, M.; Lohr, F.; Kehraus, S.; Piel, J.; Muller, R.; et al. Biosynthesis of the myxobacterial antibiotic corallopyronin A. Chembiochem 2010, 11, 1253–1265. [Google Scholar] [CrossRef] [PubMed]
- Pogorevc, D.; Panter, F.; Schillinger, C.; Jansen, R.; Wenzel, S.C.; Muller, R. Production optimization and biosynthesis revision of corallopyronin A, a potent anti-filarial antibiotic. Metab. Eng. 2019, 55, 201–211. [Google Scholar] [CrossRef]
- Weinig, S.; Hecht, H.J.; Mahmud, T.; Muller, R. Melithiazol biosynthesis: Further insights into myxobacterial PKS/NRPS systems and evidence for a new subclass of methyl transferases. Chem. Biol. 2003, 10, 939–952. [Google Scholar] [CrossRef] [Green Version]
- Schieferdecker, S.; Konig, S.; Weigel, C.; Dahse, H.M.; Werz, O.; Nett, M. Structure and biosynthetic assembly of gulmirecins, macrolide antibiotics from the predatory bacterium Pyxidicoccus fallax. Chemistry 2014, 20, 15933–15940. [Google Scholar] [CrossRef] [PubMed]
- Surup, F.; Viehrig, K.; Mohr, K.I.; Herrmann, J.; Jansen, R.; Muller, R. Disciformycins A and B: 12-membered macrolide glycoside antibiotics from the myxobacterium Pyxidicoccus fallax active against multiresistant staphylococci. Angew. Chem. Int. Ed. 2014, 53, 13588–13591. [Google Scholar] [CrossRef]
- Panter, F.; Krug, D.; Baumann, S.; Muller, R. Self-resistance guided genome mining uncovers new topoisomerase inhibitors from myxobacteria. Chem. Sci. 2018, 9, 4898–4908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregory, K.; Salvador, L.A.; Akbar, S.; Adaikpoh, B.I.; Stevens, D.C. Survey of Biosynthetic Gene Clusters from Sequenced Myxobacteria Reveals Unexplored Biosynthetic Potential. Microorganisms 2019, 7, 781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).