DMI-Fungicide Resistance in Venturia nashicola, the Causal Agent of Asian Pear Scab—How Reliable Are Mycelial Growth Tests in Culture?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Isolation and Fungicides
2.2. Mycelial Growth Tests on Culture Medium
2.3. Conidia Formation in Culture
2.4. Inoculation of Potted Pear Trees
2.5. DNA Extraction, Polymerase Chain Reaction (PCR) Amplification and Sequence Analysis of the CYP51 Gene
3. Results
3.1. Baseline Sensitivity to Difenoconazole and Hexaconazole in Culture
3.2. Demonstration of Fenarimol Resistance in Planta and in Culture
3.3. Inconsistency of Fenarimol Sensitivity Between in Planta and in Culture Tests
3.4. Possible Source of the Inconsistency between in Planta and in Culture Tests
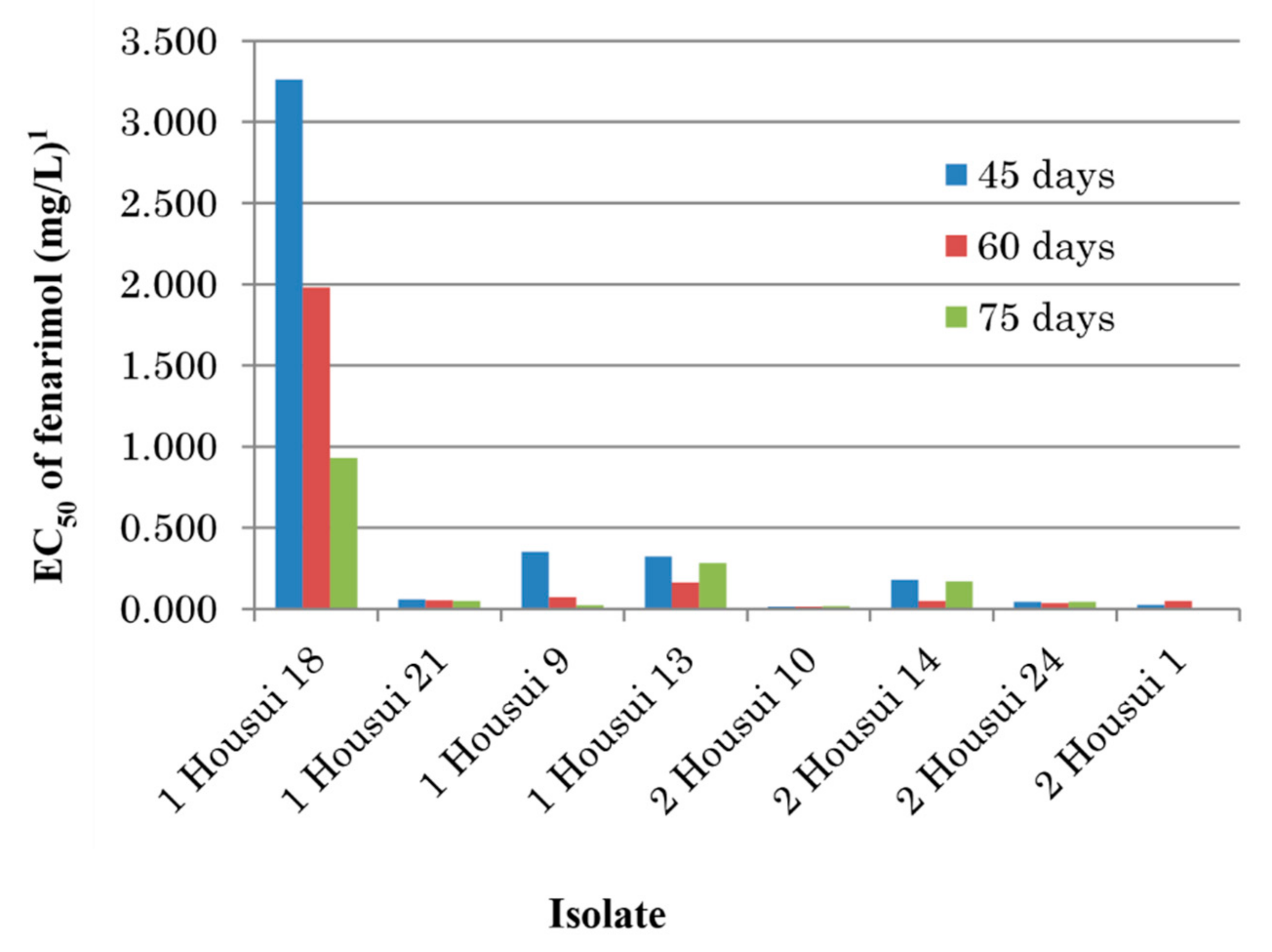
3.5. Change of Cross-Resistance Pattern among DMI Fungicides with Time
3.6. Instability of Resistance
3.7. Sequence Analysis of CYP51 Gene
4. Discussion
5. Conclusion and Future Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abe, K.; Saito, T.; Terai, O.; Sato, Y.; Kotobuki, K. Genotypic difference for the susceptibility of Japanese, Chinese and European pears to Venturia nashicola, the cause of scab on Asian pears. Plant Breed. 2008, 127, 407–412. [Google Scholar] [CrossRef]
- Tanaka, S.; Yamamoto, S. Studies on pear scab. II. Taxonomy of the causal fungus of Japanese pear scab. Ann. Phytopathol. Soc. Jpn. 1964, 29, 128–136. [Google Scholar] [CrossRef]
- Ishii, H.; Yanase, H. Venturia nashicola, the scab fungus of Japanese and Chinese pears: A species distinct from V. pirina. Mycol. Res. 2000, 104, 755–759. [Google Scholar] [CrossRef]
- Ishii, H.; Nishimura, K.; Tanabe, K.; Yamaoka, Y. Pathogenic specialization of Venturia nashicola, causal agent of Asian pear scab, and resistance of pear cultivars Kinchaku and Xiangli. Phytopathology 2020, in press. [Google Scholar] [CrossRef]
- Le Cam, B.; Devaux, M.; Parisi, L. Specific polymerase chain reaction identification of Venturia nashicola using internally transcribed spacer region in the ribosomal DNA. Phytopathology 2001, 91, 900–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EPPO. Venturia nashicola.EPPO Datasheets on Pests Recommended for Regulation. 2020. Available online: https://gd.eppo.int (accessed on 23 June 2021).
- Ishii, H.; Kimura, Y. A new interspecific pear cultivar Yutaka: Highly resistant to the two major diseases scab and black spot on Asian pears. Eur. J. Plant Pathol. 2018, 152, 507–514. [Google Scholar] [CrossRef]
- Saito, T. Advances in Japanese pear breeding in Japan. Breed. Sci. 2016, 66, 46–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishii, H.; Udagawa, H.; Yanase, H.; Yamaguchi, A. Resistance of Venturia nashicola to thiophanate-methyl and benomyl: Build-up and decline of resistance in the field. Plant Pathol. 1985, 34, 363–368. [Google Scholar] [CrossRef]
- Ishii, H. Resistance in Venturia nashicola to benzimidazoles and sterol demethylation inhibitors. In Fungicide Resistance in Crop Protection; Thind, T.S., Ed.; CAB International: Wallingford, UK, 2012; pp. 21–31. [Google Scholar]
- Ziogas, B.N.; Malandrakis, A.A. Sterol biosynthesis inhibitors: C14 demethylation (DMIs). In Fungicide Resistance in Plant Pathogens—Principles and a Guide to Practical Management; Ishii, H., Hollomon, D.W., Eds.; Springer: Tokyo, Japan, 2015; pp. 199–216. [Google Scholar]
- Mehl, A.; Schmitz, H.; Stenzel, K.; Bloomberg, J. DMI fungicides (FRAC Code 3): Sensitivity status of key target pathogens, field versus laboratory resistance, and resistance mechanisms. In Fungicide Resistance in North America, 2nd ed.; Stevenson, K.L., McGrath, M.T., Wyenandt, C.A., Eds.; APS Press: St. Paul, MN, USA, 2019; pp. 51–68. [Google Scholar]
- Umemoto, S. Studies on the ecology and control of Japanese pear scab. Spec. Bull. Chiba Pref. Agric. Exper. Stn. 1993, 22, 1–99, (In Japanese with English summary). [Google Scholar]
- Loeffler, R.S.T.; Butters, J.A.; Hollomon, D.W. The sterol composition of powdery mildews. Phytochemistry 1992, 31, 1561–1563. [Google Scholar] [CrossRef]
- Shirane, N.; Takenaka, H.; Ueda, K.; Hashimoto, Y.; Katoh, K.; Ishii, H. Sterol analysis of DMI-resistant and -sensitive strains of Venturia inaequalis. Phytochemistry 1996, 41, 1301–1308. [Google Scholar] [CrossRef]
- Chapuis, L.; Corio-Costet, M.R.; Malosse, C. Sterol composition of the woody plant pathogenic fungus Eutypa lata. Phytochemistry 1996, 42, 1599–1601. [Google Scholar] [CrossRef]
- Brent, J.K.; Hollomon, D.W. Fungicide Resistance in Crop Pathogens: How Can It Be Managed? FRAC Monograph No. 1 (Second, Revised Edition). 2007. Available online: https://www.frac.info/docs/default-source/publications/monographs/monograph-1 (accessed on 23 June 2021).
- Stanis, V.F.; Jones, A.L. Reduced sensitivity to sterol-inhibiting fungicides in field isolates of Venturia inaequalis. Phytopathology 1985, 75, 1098–1101. [Google Scholar] [CrossRef]
- Thind, T.S.; Clerjeau, M.; Olivier, J.M. First observations on resistance in Venturia inaequalis and Guignardia bidwellii to ergosterol-biosynthesis inhibitors in France. In British Crop Protection Conference. Pests and Diseases; BCPC Publications: Surrey, UK, 1986; Volume 2, pp. 491–498. [Google Scholar]
- Köller, W.; Scheinpflug, H. Fungal resistance to sterol biosynthesis inhibitors: A new challenge. Plant Dis. 1987, 71, 1066–1074. [Google Scholar] [CrossRef] [Green Version]
- Ishii, H. Resistance management in diseases of top fruit in Japan. Pestic. Sci. 1997, 51, 383–386. [Google Scholar] [CrossRef]
- Kikuhara, K.; Ishii, H. Fenarimol resistance in Venturia nashicola, the scab fungus of Japanese pear in Fukuoka Prefecture. Kyushu Plant Prot. Res. 2008, 54, 24–29, (In Japanese with English abstract). [Google Scholar] [CrossRef] [Green Version]
- Ishii, H.; Takeda, H.; Nagamatsu, Y.; Nakashima, H. Sensitivity of the pear scab fungus (Venturia nashicola) to three ergosterol biosynthesis-inhibiting fungicides. Pestic. Sci. 1990, 30, 405–413. [Google Scholar] [CrossRef]
- Tomita, Y.; Ishii, H. Reduced sensitivity to fenarimol in Japanese field strains of Venturia nashicola. Pestic. Sci. 1998, 54, 150–156. [Google Scholar] [CrossRef]
- Cools, H.J.; Ishii, H.; Butters, J.A.; Hollomon, D.W. Cloning and sequence analysis of the eburicol 14α-demethylase encoding gene (CYP51) from the Japanese pear scab fungus Venturia nashicola. J. Phytopathol. 2002, 150, 444–450. [Google Scholar] [CrossRef]
- Ishii, H.; Kikuhara, K. Occurrence of DMI resistance in Venturia nashicola, the scab fungus of Asian pears. In Proceedings of the Abstracts of the 17th Symposium of Research Committee on Fungicide Resistance, the Phytopathological Society of Japan, Utsunomiya, Japan, 31 March 2007; pp. 49–60, (In Japanese with English abstract). [Google Scholar]
- Parker, D.M.; Hilber, U.W.; Bodmer, M.; Smith, F.D.; Yao, C.; Köller, W. Production and transformation of conidia of Venturia inaequalis. Phytopathology 1995, 85, 87–91. [Google Scholar] [CrossRef]
- Ishii, H.; Udagawa, H.; Nishimoto, S.; Tsuda, T.; Nakashima, H. Scab resistance in pear species and cultivars. Acta Phytopathol. Entomol. Hun. 1992, 27, 293–298. [Google Scholar]
- Saitoh, K.; Togashi, K.; Arie, T.; Teraoka, T. A simple method for a mini-preparation of fungal DNA. J. Gen. Plant Pathol. 2006, 72, 348–350. [Google Scholar] [CrossRef]
- Kwon, S.M.; Yeo, M.I.; Choi, S.H.; Kim, G.W.; Jun, K.J.; Uhm, J.Y. Reduced sensitivities of the pear scab fungus (Venturia nashicola) collected in Ulsan and Naju to five ergosterol-biosynthesis-inhibiting fungicides. Res. Plant J. 2010, 16, 48–58, (In Korean with English abstract). [Google Scholar]
- Stammler, G.; Carstensen, M.; Koch, A.; Semar, M.; Strobel, D.; Schlehuber, S. Frequency of different CYP51-haplotypes of Mycosphaerella graminicola and their impact on epoxiconazole-sensitivity and -field efficacy. Crop Prot. 2008, 27, 1448–1456. [Google Scholar] [CrossRef]
- Wyand, R.A.; Brown, J.K.M. Sequence variation in the CYP51 gene of Blumeria graminis associated with resistance to sterol demethylase inhibiting fungicides. Fungal Genet. Biol. 2005, 42, 726–735. [Google Scholar] [CrossRef]
- Rallos, L.E.E.; Baudoin, A.B. Co-occurrence of two allelic variants of CYP51 in Erysiphe necator and their correlation with over-expression for DMI resistance. PLoS ONE 2016, 11, e0148025. [Google Scholar] [CrossRef]
- Chen, F.; Lin, D.; Wang, J.; Li, B.; Duan, H.; Liu, J.; Liu, X. Heterologous expression of the Monilinia fructicola CYP51 (MfCYP51) gene in Pichia pastoris confirms the mode of action of the novel fungicide, SYP-Z048. Front. Microbiol. 2015, 6, 457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, D.A.; McDonald, B.A.; Brunner, P.C. Mutations in the CYP51 gene reduce DMI sensitivity in Parastagonospora nodorum populations in Europe and China. Pest Manag. Sci. 2017, 73, 1503–1510. [Google Scholar] [CrossRef]
- Cools, H.J.; Parker, J.E.; Kelly, D.E.; Lucas, J.A.; Fraaije, B.A.; Kelly, S.L. Heterologous expression of mutated eburicol 14α-demethylase (CYP51) proteins of Mycosphaerella graminicola to assess effects on azole fungicide sensitivity and intrinsic protein function. Appl. Environ. Microbiol. 2010, 76, 2866–2872. [Google Scholar] [CrossRef] [Green Version]
- Spanner, R.; Taliadoros, D.; Richards, J.; Rivera-Varas, V.; Neubauer, J.; Natwick, M.; Hamilton, O.; Vaghefi, N.; Pethybridge, S.; Secor, G.A.; et al. Genome-wide association studies reveal the complex genetic architecture of DMI fungicide resistance in Cercospora beticola. bioRxiv 2020. [Google Scholar] [CrossRef]
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 2018, 360, 739–742. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.M.; Gao, L.Q.; Yang, J.R. Are insensitivities of Venturia inaequalis to myclobutanil and fenbuconazole correlated? Crop Protect. 2010, 29, 183–189. [Google Scholar] [CrossRef]
- Villani, S.M.; Hulvey, J.; Hily, J.M.; Cox, K.D. Overexpression of the CYP51A1 gene and repeated elements are associated with differential sensitivity to DMI fungicides in Venturia inaequalis. Phytopathology 2016, 106, 562–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cools, H.J.; Hawkins, N.J.; Fraaije, B. Constraints on the evolution of azole resistance in plant pathogenic fungi. Plant Pathol. 2013, 62 (Suppl. 1), 36–42. [Google Scholar] [CrossRef] [Green Version]
- Cordero-Limon, L.; Shaw, M.W.; Passey, T.A.J.; Robinson, J.D.; Xu, X. Cross-resistance between myclobutanil and tebuconazole and the genetic basis of tebuconazole resistance in Venturia inaequalis. Pest Manag. Sci. 2021, 77, 844–850. [Google Scholar] [CrossRef]
- Kikuhara, K.; Adachi, T.; Saito, N.; Iiyama, K.; Matsumoto, M.; Furuya, N. Development of resistance to DMI fungicides in Venturia nashicola, the causal agent of the Japanese pear scab, in Fukuoka Prefecture, Japan. Kyushu Plant Prot. Res. 2018, 64, 1–6, (In Japanese with English abstract). [Google Scholar] [CrossRef] [Green Version]
- Kikuhara, K.; Hashimoto, F.; Matsumoto, M.; Iiyama, K.; Furuya, N. The relationship between an increase in Japanese pear rust and decreased efficacy of DMI fungicides: A retrospective cohort study in Yame region, Fukuoka Prefecture, Japan. Jpn. J. Phytopathol. 2018, 84, 98–104, (In Japanese with English abstract). [Google Scholar] [CrossRef]
- Köller, W.; Wilcox, W.F.; Barnard, J.; Jones, A.L.; Braun, P.G. Detection and quantification of resistance of Venturia inaequalis populations to sterol demethylation inhibitors. Phytopathology 1997, 87, 184–190. [Google Scholar] [CrossRef] [Green Version]
- Villani, S.M.; Biggs, A.R.; Cooley, D.R.; Raes, J.J.; Cox, K.D. Prevalence of myclobutanil resistance and difenoconazole insensitivity in populations of Venturia inaequalis. Plant Dis. 2015, 99, 1526–1536. [Google Scholar] [CrossRef] [Green Version]
- Köller, W.; Smith, F.D.; Reynolds, K.L. Phenotypic instability of flusilazole sensitivity in Venturia inaequalis. Plant Pathol. 1991, 40, 608–611. [Google Scholar] [CrossRef]
- Ishii, H.; Homma, F.; Miura, T.; Suzaki, H.; van Raak, M. Resistance of Venturia inaequalis to DMIs—Phenotypic instability and genetic control. In Proceedings of the Abstracts of the 6th International Congress of Plant Pathology, Montreal, ON, Canada, 28 June 1993; p. 92. [Google Scholar]
- Cox, K.D.; Bryson, P.K.; Schnabel, G. Instability of propiconazole resistance and fitness in Monilinia fructicola. Phytopathology 2007, 97, 448–453. [Google Scholar] [CrossRef] [Green Version]
- Zhu, F.; Bryson, P.K.; Schnabel, G. Influence of storage approaches on instability of propiconazole resistance in Monilinia fructicola. Pest Manag. Sci. 2012, 68, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.; Steiner, U.; Dehne, H.W. Differentiation of SBI resistance and parasitic fitness components in German isolates of Venturia inaequalis (Cke.) Wint and interest of induced resistance for antiresistance strategies. Med. Fac. Landbouww. Univ. Gent. 1996, 61, 413–423. [Google Scholar]
- Braun, P.G. Development and decline of a population of Venturia inaequalis resistant to sterol-inhibiting fungicides. Norw. J. Agric. Sci. 1994, 17, 173–184. [Google Scholar]
- Frederick, Z.A.; Villani, S.M.; Cox, K.D. The effect of delayed-dormant chemical treatments on demethylation inhibitor (DMI) sensitivity in a DMI-resistant population of Venturia inaequalis. Plant Dis. 2015, 99, 1751–1756. [Google Scholar] [CrossRef] [Green Version]
- Pereira, W.V.; Morales, R.G.F.; Bauer, A.I.G.; Kudlawiec, K.; May-De-Mio, L.L. Discontinuance of tebuconazole in the field restores sensitivity of Monilinia fructicola in stone fruit orchards. Plant Pathol. 2020, 69, 68–76. [Google Scholar] [CrossRef]
- Misonou, T.; Fukatsu, R. Studies on the infection and control of pear scab. II. Dispersion of conidia and their role as the origin of infection. Bull. Chiba Agric. Exp. Stn. 1970, 10, 91–98, (In Japanese with English summary). [Google Scholar]
- Gao, L.; Berrie, A.; Yang, J.; Xu, X. Within- and between-orchard variability in the sensitivity of Venturia inaequalis to myclobutanil, a DMI fungicide, in the UK. Pest Manag. Sci. 2009, 65, 1241–1249. [Google Scholar] [CrossRef]
- Kwak, Y.; Min, J.; Song, J.; Kim, M.; Lee, H.; Kim, H.T. Relationship of resistance to benzimidazole fungicides with mutation of β-tubulin gene in Venturia nashicola. Res. Plant Dis. 2017, 23, 150–158, (In Korean with English abstract). [Google Scholar] [CrossRef]
- Gerberich, K.M.; Beckerman, J. Rapid detection of fungicide resistance in Venturia inaequalis. Phytopathology 2011, 101, S2.3. [Google Scholar]
- Yaegashi, H.; Hirayama, K.; Akahira, T.; Ito, T. Point mutation in CYP51A1 of Venturia inaequalis is associated with low sensitivity to sterol demethylation inhibitors. J. Gen. Plant Pathol. 2020, 86, 245–249. [Google Scholar] [CrossRef]
- Schnabel, G.; Jones, A.L. The 14α-demethylase (CYP51A1) gene is overexpressed in Venturia inaequalis strains resistant to myclobutanil. Phytopathology 2001, 91, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Cools, H.J.; Fraaije, B.A. Update on mechanism of azole resistance in Mycosphaerella graminicola and implications for future control. Pest Manag. Sci. 2013, 64, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Cools, H.J.; Bayon, C.; Atkins, S.; Lucas, J.A.; Fraaije, B.A. Overexpression of the sterol 14α-demethylase gene (MgCYP51) in Mycosphaerella graminicola isolates confers a novel azole fungicide sensitivity phenotype. Pest Manag. Sci. 2012, 68, 1034–1040. [Google Scholar] [CrossRef]
- Becher, R.; Wirsel, S.G.R. Fungal cytochrome P450 sterol 14α-demethylase (CYP51) and azole resistance in plant and human pathogens. Appl. Microbiol. Biotechnol. 2012, 95, 825–840. [Google Scholar] [CrossRef]
- Leroux, P.; Albertini, C.; Gautier, A.; Gredt, M.; Walker, A.S. Mutations in the CYP51 gene correlated with changes in sensitivity to sterol 14α-demethylation inhibitors in field isolates of Mycosphaerella graminicola. Pest Manag. Sci. 2007, 63, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Mair, W.; Lopez-Ruiz, F.; Stammler, G.; Clark, W.; Burnett, F.; Hollomon, D.; Ishii, H.; Thind, T.S.; Brown, J.K.M.; Fraaije, B.; et al. Proposal for a unified nomenclature for target-site mutations associated with resistance to fungicides. Pest Manag. Sci. 2016, 72, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Huf, A.; Rehfus, A.; Lorenz, K.H.; Bryson, R.; Voegele, R.T.; Stammler, G. Proposal for a new nomenclature for CYP51 haplotypes in Zymoseptoria tritici and analysis of their distribution in Europe. Plant Pathol. 2018, 67, 1706–1712. [Google Scholar] [CrossRef]
- Price, C.L.; Parker, J.E.; Warrilow, A.G.S.; Kelly, D.E.; Kelly, S.L. Azole fungicides—understanding resistance mechanisms in agricultural fungal pathogens. Pest Manag. Sci. 2015, 71, 1054–1058. [Google Scholar] [CrossRef] [PubMed]
- Leroux, P.; Walker, A.S. Multiple mechanisms account for resistance to sterol 14α-demethylation inhibitors in field isolates of Mycosphaerella graminicola. Pest Manag. Sci. 2011, 67, 44–59. [Google Scholar] [CrossRef]
- De Waard, M.; Andrade, A.C.; Hayashi, K.; Schoonbeek, H.J.; Stergiopoulos, I.; Zwiers, L.H. Impact of fugal drug transporters on fungicide sensitivity, multidrug resistance and virulence. Pest Manag. Sci. 2006, 62, 195–207. [Google Scholar] [CrossRef] [Green Version]
- Kretschmer, M.; Leroch, M.; Mosbach, A.; Walker, A.S.; Fillinger, S.; Mernke, D.; Schoonbeek, H.J.; Pradier, J.M.; Leroux, P.; de Waard, M.A.; et al. Fungicide-driven evolution and molecular basis of multidrug resistance in the field populations of the grey mould fungus Botrytis cinerea. PLoS Pathog. 2009, 5, e1000696. [Google Scholar] [CrossRef]
- Leroux, P.; Walker, A.S. Activity of fungicides and modulators of membrane drug transporters in field strains of Botrytis cinerea displaying multidrug resistance. Eur. J. Plant Pathol. 2013, 135, 683–693. [Google Scholar] [CrossRef]
- Hahn, M.; Leroch, M. Multidrug efflux transporters. In Fungicide Resistance in Plant Pathogens—Principles and a Guide to Practical Management; Ishii, H., Hollomon, D.W., Eds.; Springer: Tokyo, Japan, 2015; pp. 233–248. [Google Scholar]
- Palani, P.V.; Lalithakumari, D. Resistance of Venturia inaequalis to the sterol biosynthesis-inhibiting fungicide, penconazole [1-(2-(2,4-dichlorophenyl)pentyl)-1H-1,2,4-triazole]. Mycol. Res. 1999, 103, 157–1164. [Google Scholar]
- Ishii, H.; Bryson, P.K.; Kayamori, M.; Miyamoto, T.; Yamaoka, Y.; Schnabel, G. Cross-resistance to the new fungicide mefentrifluconazole in DMI-resistant fungal pathogens. Pestic. Biochem. Physiol. 2021, 171, 104737. [Google Scholar] [CrossRef]
- Tesh, S.A.; Tesh, J.M.; Fegert, I.; Buesen, R.; Schneider, S.; Mentzel, T.; van Ravenzwaay, B.; Stinchcombe, S. Innovative selection approach for a new antifungal agent mefentrifluconazole (Revysol®) and the impact upon its toxicity profile. Regul. Toxicol. Phamacol. 2019, 106, 152–168. [Google Scholar] [CrossRef]
- Nakao, S.; Watanabe, H.; Yano, T.; Yamaoka, Y.; Ishii, H. Control efficacy of the systemic acquired resistance (SAR) inducer acibenzolar S methyl against Venturia nashicola in Japanese pear orchards. J. Gen. Plant Pathol. 2021. [Google Scholar] [CrossRef]
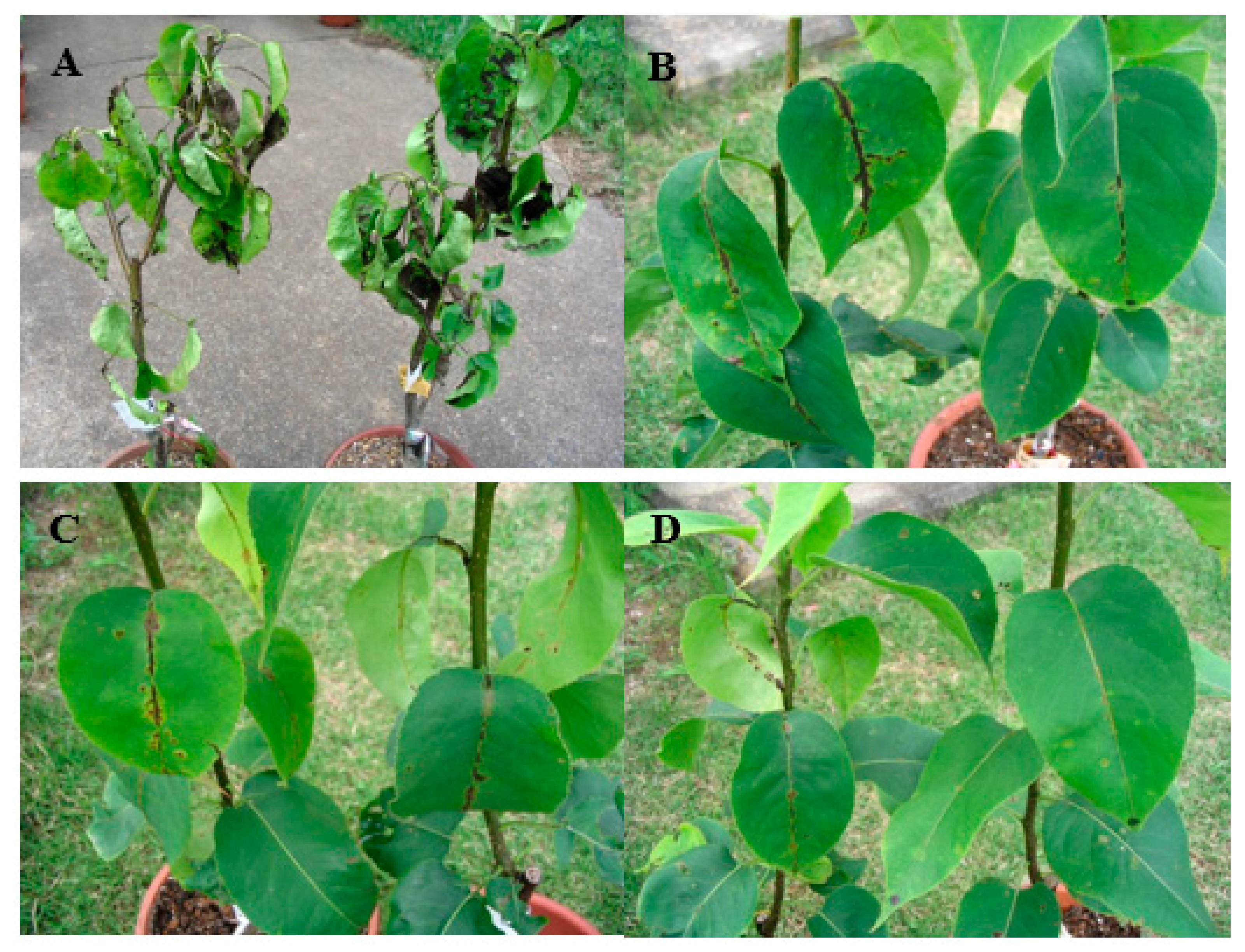


| Year of Isolation | Location | Sensitivity to Fenarimol in Planta (Control, %) 1 | Sensitivity to Fenarimol in Culture (Average EC50, mg/L) 2 |
|---|---|---|---|
| 2005 | Ukiha 2, Fukuoka | 2.1 * | 0.227 |
| Kurokawa, Fukuoka | 3.7 * | 1.012 | |
| Ukiha 1, Fukuoka | 21.6 * | 0.328 | |
| Chikuzen, Fukuoka | 37.8 * | 0.147 | |
| Kurume, Fukuoka | 55.3 | 0.370 | |
| Ninaibaru, Fukuoka | 77.7 * | 0.292 | |
| Tagawa, Fukuoka | 100 | 0.253 | |
| NIAES, Ibaraki 3 | 94.6 | 0.142 | |
| 2006 | Usui, Fukuoka | 25.0 | 0.233 |
| Kaho, Fukuoka | 31.7 | 0.186 | |
| Akizuki, Fukuoka | 32.4 | 0.196 | |
| Akaike, Fukuoka | 37.4 | 0.278 | |
| Kurokawa, Fukuoka | 70.1 | 3.945 | |
| NIAES, Ibaraki 3 | 100 | 0.142 |
| Isolate | Year of Isolation | Location | Response to Fenarimol or Difenoconazole in Culture 1 | Sensitivity to Fenarimol or Difenoconazole in Culture (EC50, mg/L) 2 | Deduced Amino-Acid Change of CYP51 |
|---|---|---|---|---|---|
| Kurokawa 4 | 2005 | Asakura, Fukuoka | Sensitive | 0.151 | Q359 (-), G428R |
| Kurokawa 39 | 2005 | Asakura, Fukuoka | Sensitive | 0.166 | Q359 (-), G428R |
| Kurokawa 18 | 2005 | Asakura, Fukuoka | Less-sensitive | 1.948 | Y102N, D291G, Q359 (-), G445D |
| Kurokawa 20 | 2005 | Asakura, Fukuoka | Less-sensitive | 0.504 | Y102N, A340T, Q359 (-), R366P |
| Kurokawa 26 | 2005 | Asakura, Fukuoka | Less-sensitive | 1.970 | Y102N, Q110H, V131F |
| Kurokawa 9 | 2005 | Asakura, Fukuoka | Resistant 3 | 1.067 | Q359 (-), G428R |
| Kurokawa 21 | 2005 | Asakura, Fukuoka | Resistant | 21.299 | G60S, Y102N, Q359 (-), G428R |
| Kurokawa 22 | 2005 | Asakura, Fukuoka | Resistant | 5.906 | Y102N, S310P, P324S, Q359 (-), G428R |
| 2 Housui 1 | 2017 | Imari, Saga | Sensitive | 0.003 | Q359 (-) |
| 1 Housui 9 | 2017 | Imari, Saga | Less-sensitive | 0.884 | Q359 (-) |
| 1 Housui 18 | 2017 | Imari, Saga | Less-sensitive | 2.675 | Q359 (-) |
| 1 Housui 21 | 2017 | Imari, Saga | Less-sensitive | 1.125 | Q359 (-) |
| 2 Housui 10 | 2017 | Imari, Saga | Less-sensitive | 0.551 | Not tested |
| 2 Housui 14 | 2017 | Imari, Saga | Less-sensitive | 0.526 | Q359 (-) |
| 2 Housui 24 | 2017 | Imari, Saga | Less-sensitive | 0.512 | Q359 (-) |
| 1 Housui 13 | 2017 | Imari, Saga | Resistant | 0.619 | Q359 (-) |
| 2 Housui 11 | 2017 | Imari, Saga | Resistant | 0.033 | Not tested |
| H Mizu 3 | 2018 | Yame, Fukuoka | (Sensitive) | (0.000) | Q359 (-) |
| H Mizu 5 | 2018 | Yame, Fukuoka | (Sensitive) | (0.044) | Q359 (-) |
| S Mizu 2 | 2018 | Yame, Fukuoka | (Less-sensitive) | (1.104) | Q359 (-), Y446H |
| S Mizu 3 | 2018 | Yame, Fukuoka | (Less-sensitive) | (0.196) | Q359 (-) |
| S Mizu 4 | 2018 | Yame, Fukuoka | (Less-sensitive) | (0.257) | Q359 (-), Y446H |
| S Mizu 5 | 2018 | Yame, Fukuoka | (Less-sensitive) | (0.593) | Q359 (-) |
| Isolate | Time After Inoculation | Control (%) | Scab Incidence (%) on DW-Sprayed Reference Trees |
|---|---|---|---|
| Kurokawa 9 | 3 weeks | 85.7 | 93.3 |
| 1 month | 20.0 | 100.0 | |
| Kurokawa 21 | 3 weeks | 49.9 | 93.3 |
| 1 month | 6.7 | 100.0 | |
| Kurokawa 22 | 3 weeks | 0.0 | 86.7 |
| 1 month | 0.0 | 100.0 | |
| NIAES 1 | 3 weeks | 100.0 | 33.3 |
| 1 month | 100.0 | 73.3 |
| Timing of Disease Assessment | Origin of Conidia Inoculated | Treatment | Scab Incidence (%) | |||
|---|---|---|---|---|---|---|
| 3 weeks after inoculation | Kurokawa | Fenarimol 1 | 33.2 | 19.5 | ||
| Difenoconazole 2 | 100 | 0.0 | ||||
| Hexaconazole 3 | 100 | 0.0 | ||||
| Distilled water | 29.2 | |||||
| NIAES | Fenarimol | 100 | 0.0 | |||
| Difenoconazole | 100 | 0.0 | ||||
| Hexaconazole | 100 | 0.0 | ||||
| Distilled water | 17.9 | |||||
| 1 month after inoculation | Kurokawa | Fenarimol | −14.7 | 88.4 | ||
| Difenoconazole | 100 | 0.0 | ||||
| Hexaconazole | 21.7 | 60.4 | ||||
| Distilled water | 77.1 | |||||
| NIAES | Fenarimol | 65.9 | 20.9 | |||
| Difenoconazole | 100 | 0 | ||||
| Hexaconazole | 90.9 | 5.6 | ||||
| Distilled water | 61.3 | |||||
| Isolate | EC50 (mg/L) of Fungicide | ||
|---|---|---|---|
| Fenarimol | Difenoconazole | Hexaconazole | |
| Kurokawa 4 | 0.151 | <0.010 | 0.020 |
| Kurokawa 9 | 1.067 | <0.010 | <0.010 |
| Kurokawa 18 | 1.948 | <0.010 | 0.072 |
| Kurokawa 20 | 0.504 | <0.010 | 0.023 |
| Kurokawa 21 | 21.299 | 0.032 | 0.113 |
| Kurokawa 22 | 5.906 | 3.199 | 0.091 |
| Kurokawa 26 | 1.970 | <0.010 | 0.028 |
| Kurokawa 39 | 0.166 | <0.010 | 0.022 |
| Baseline | 0.142 ± 0.215 | 0.023 ± 0.142 | 0.007 ± 0.016 |
| Isolate | Sensitivity to Fenarimol | EC50 (mg/L) of Fenarimol | |
|---|---|---|---|
| 1st trial | 2nd trial | ||
| Kurokawa 4 | Sensitive | 0.151 | 0.028 |
| Kurokawa 39 | Sensitive | 0.166 | 0.131 |
| Kurokawa 18 | Less-Sensitive | 1.948 | 0.973 |
| Kurokawa 20 | Less-Sensitive | 0.504 | 0.347 |
| Kurokawa 26 | Less-Sensitive | 1.970 | 0.680 |
| Kurokawa 9 | Resistant 1 | 1.067 | 0.263 |
| Kurokawa 21 | Resistant 1 | 21.299 | 1.721 |
| Kurokawa 22 | Resistant 1 | 5.906 | 7.879 |
| AA Position | Mutation | Orthology |
|---|---|---|
| 60 | G60S | |
| 102 | Y102N | |
| 110 | Q110H | |
| 131 | V131F | V131F is placed between D130 (SEPTTR_D134 1) and Y133 (ERYSGT 2/BRYSGH 2/UNCINE 3/MONIFG 4/_Y136 or LEPTNO_Y144 5) |
| 291 | D291G | |
| 310 | S310P | |
| 324 | P324S | |
| 340 | A340T | |
| 359 | Q359- | |
| 366 | R366P | |
| 428 | G428R | |
| 445 | G445D | SEPTTR_G460 6 |
| 446 | Y446H | CERBE_Y464 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishii, H.; Cools, H.J.; Nishimura, K.; Borghi, L.; Kikuhara, K.; Yamaoka, Y. DMI-Fungicide Resistance in Venturia nashicola, the Causal Agent of Asian Pear Scab—How Reliable Are Mycelial Growth Tests in Culture? Microorganisms 2021, 9, 1377. https://doi.org/10.3390/microorganisms9071377
Ishii H, Cools HJ, Nishimura K, Borghi L, Kikuhara K, Yamaoka Y. DMI-Fungicide Resistance in Venturia nashicola, the Causal Agent of Asian Pear Scab—How Reliable Are Mycelial Growth Tests in Culture? Microorganisms. 2021; 9(7):1377. https://doi.org/10.3390/microorganisms9071377
Chicago/Turabian StyleIshii, Hideo, Hans Jorgen Cools, Kumiko Nishimura, Lorenzo Borghi, Kenji Kikuhara, and Yuichi Yamaoka. 2021. "DMI-Fungicide Resistance in Venturia nashicola, the Causal Agent of Asian Pear Scab—How Reliable Are Mycelial Growth Tests in Culture?" Microorganisms 9, no. 7: 1377. https://doi.org/10.3390/microorganisms9071377
APA StyleIshii, H., Cools, H. J., Nishimura, K., Borghi, L., Kikuhara, K., & Yamaoka, Y. (2021). DMI-Fungicide Resistance in Venturia nashicola, the Causal Agent of Asian Pear Scab—How Reliable Are Mycelial Growth Tests in Culture? Microorganisms, 9(7), 1377. https://doi.org/10.3390/microorganisms9071377






