Nationwide Incidence of Chigger Mite Populations and Molecular Detection of Orientia tsutsugamushi in the Republic of Korea, 2020
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Weekly Incidence of Chigger Mite Populations
2.3. Identification of Chigger Mites
2.4. Surveillance of O. tsutsugamushi in Chigger Mites from Wild Rodents
2.5. Molecular Detection of O. tsutsugamushi from Chigger Mites
2.6. DNA Sequencing and Phylogenetic Analysis
2.7. Geographical and Climate Analyses
3. Results
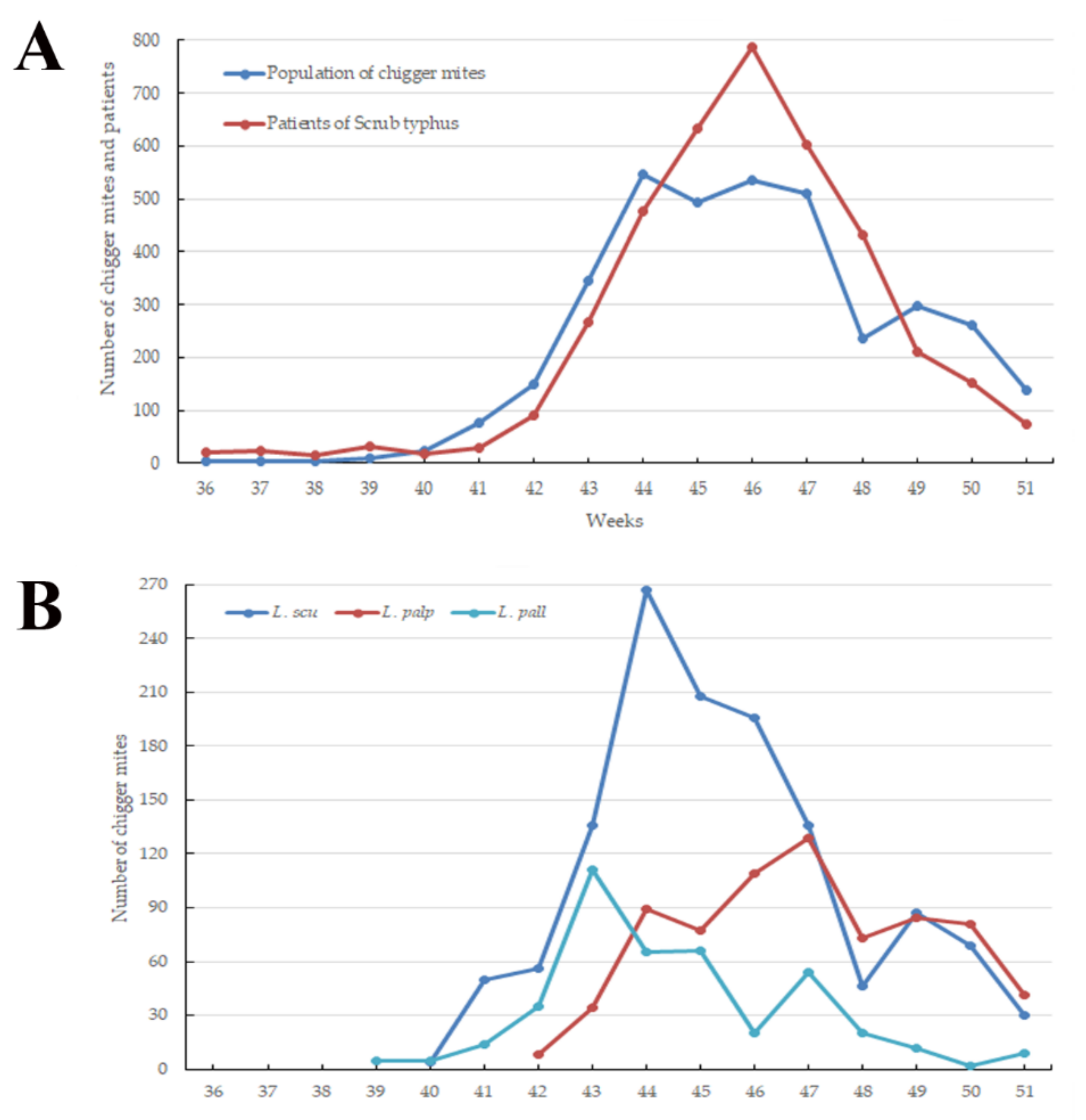
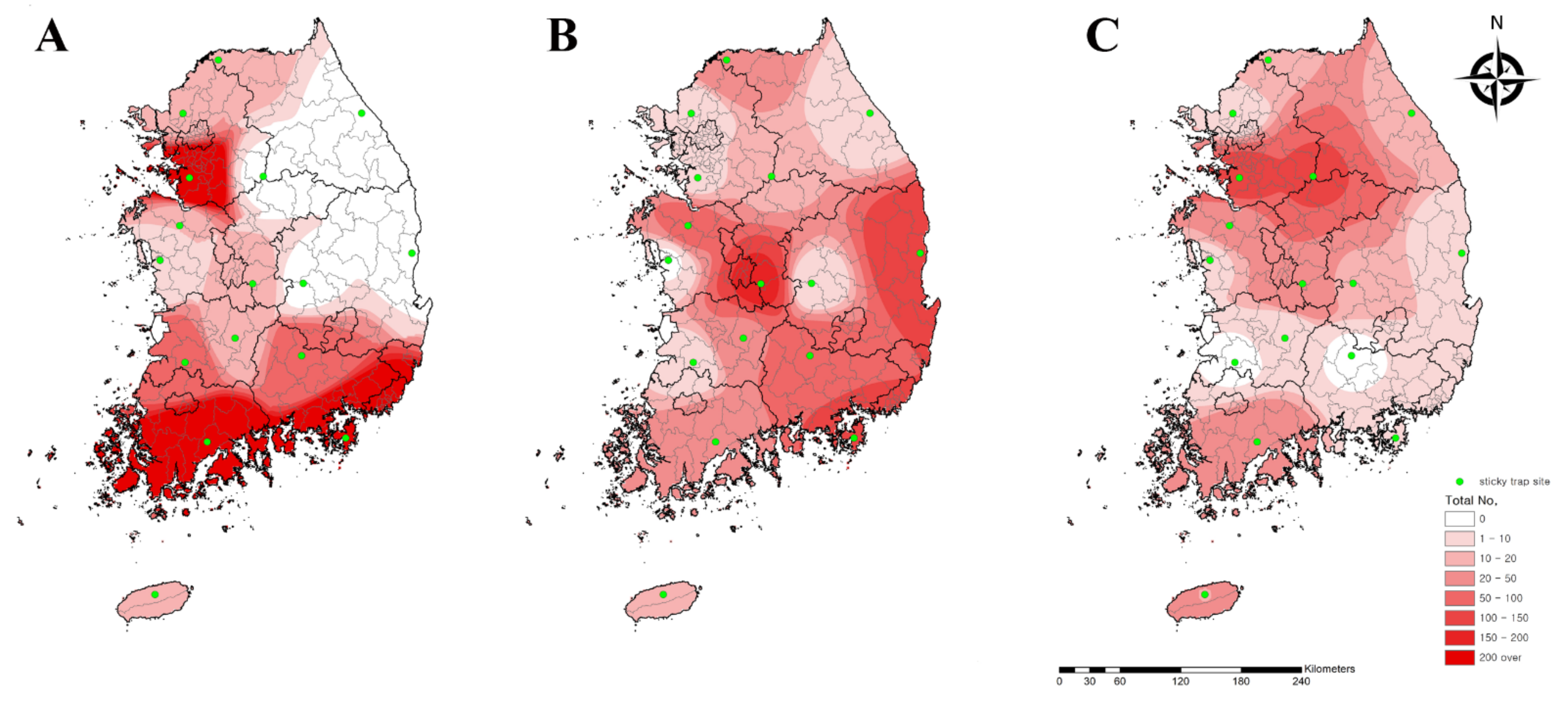
3.1. Weekly Prevalence of Chigger Mite Populations
3.2. Prevalence of O. tsutsugamushi in Chigger Mites from Wild Rodents
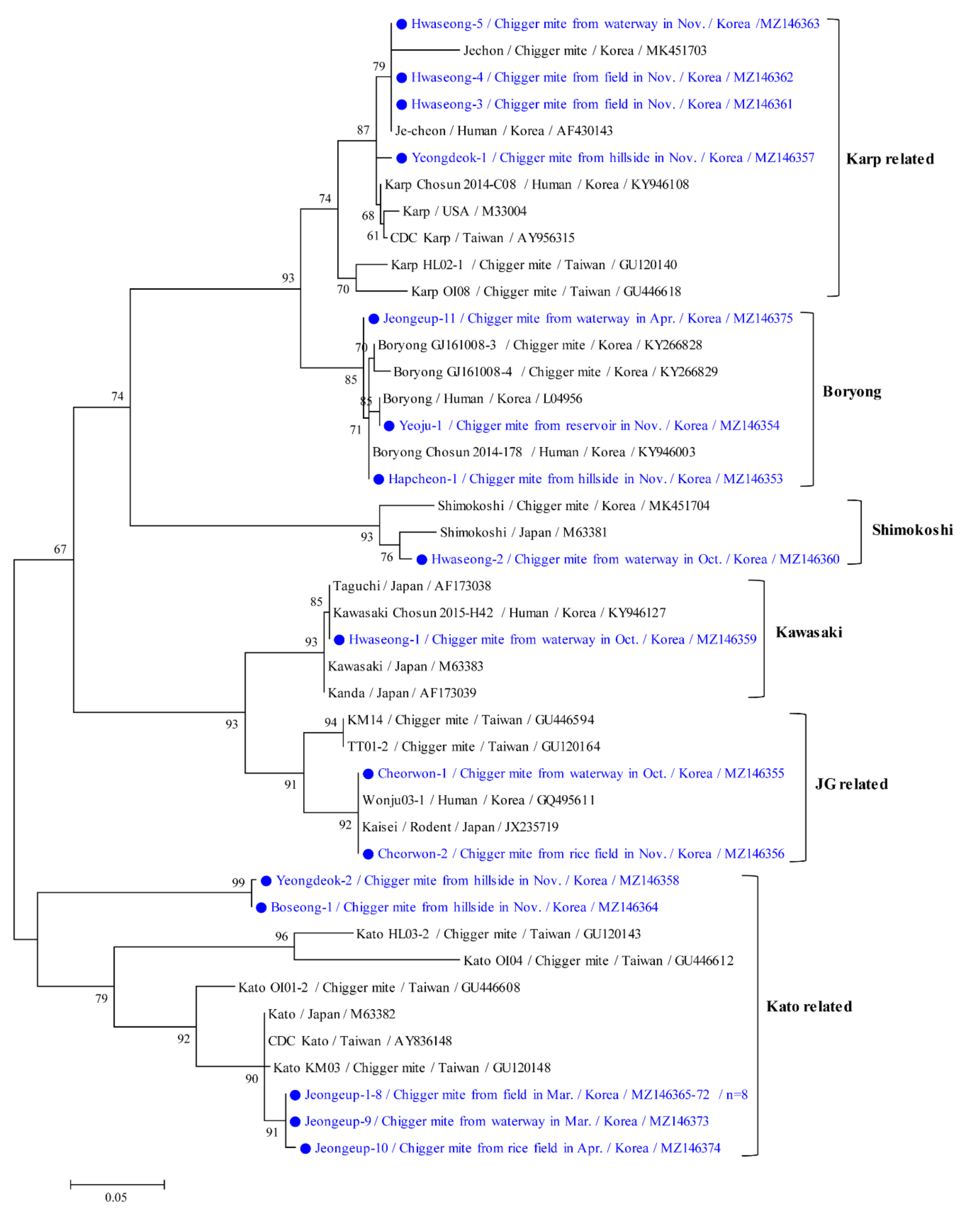
3.3. Molecular and Phylogenetic Analyses
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luce-Fedrow, A.; Lehman, M.L.; Kelly, D.J.; Mullins, K.; Maina, A.N.; Stewart, R.L.; Ge, H.; John, H.S.; Jiang, J.; Richards, A.L. A review of scrub typhus (Orientia tsutsugamushi and Related Organisms): Then, now, and tomorrow. Trop. Med. Infect. Dis. 2018, 3, 8. [Google Scholar] [CrossRef] [Green Version]
- Kelly, D.J.; Fuerst, P.A.; Ching, W.M.; Richards, A.L. Scrub typhus: The geographic distribution of phenotypic and genotypic variants of Orientia tsutsugamushi. Clin. Infect. Dis. 2009, 48 (Suppl. 3), S203–S230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korea Diseases Control and Prevention Agency. Infectious Disease Portal. The Results of the National Infectious Disease Surveillance. 2020. Available online: http://kdca.go.kr/npt/biz/npp/ist/bass/bassDissStatsMain.do (accessed on 1 March 2021).
- Bahk, Y.Y.; Jun, H.; Park, S.H.; Jung, H.; Jegal, S.; Kim-Jeon, M.D.; Roh, J.Y.; Lee, W.G.; Ahn, S.K.; Lee, J.; et al. Surveillance of chigger mite vectors for tsutsugamushi disease in the Hwaseong area, Gyeonggi-do, Republic of Korea, 2015. Korean J. Parasitol. 2020, 58, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Song, B.G.; Lee, W.G.; Lee, H.I.; Cho, S.H. Geographical distribution of chigger mites as scrub typhus vectors in the republic of Korea, 2019. Korea KDCA Public Health Wkly. Rep. 2020, 13, 817–831. [Google Scholar]
- Chung, M.H.; Kang, J.S. History of tsutsugamushi disease in Korea. Infect. Chemother. 2019, 51, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.Y.; Kim, H.C.; Lee, Y.S.; Seo, J.H.; Lim, J.W.; Yong, T.S.; Klein, T.A.; Lee, W.J. Geographical distribution and relative abundance of vectors of scrub typhus in the Republic of Korea. Korean J. Parasitol. 2009, 47, 381–386. [Google Scholar] [CrossRef]
- Kim, H.C.; Lee, I.Y.; Chong, S.T.; Richards, A.L.; Gu, S.H.; Song, J.W.; Lee, J.S.; Klein, T.A. Serosurveillance of scrub typhus in small mammals collected from military training sites near the DMZ, Northern Gyeonggi-do, Korea, and analysis of the relative abundance of chiggers from mammals examined. Korean J. Parasitol. 2010, 48, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.Y.; Song, B.G.; Park, W.I.; Shin, E.H.; Park, C.; Park, M.Y.; Chang, K.S.; Lee, W.G.; Lee, H.I.; Shin, E.H. Coincidence between geographical distribution of Leptotrombidium scutellare and scrub typhus incidence in South Korea. PLoS ONE 2014, 9, e113193. [Google Scholar]
- Kim, S.Y.; Gill, B.; Song, B.G.; Chu, H.; Park, W.I.; Lee, H.I.; Shin, E.H.; Cho, S.H.; Roh, J.Y. Annual fluctuation in chigger mite populations and Orientia Tsutsugamushi Infections in scrub typhus endemic regions of South Korea. Osong Public Health Res. Perspect. 2019, 10, 351–358. [Google Scholar] [CrossRef] [Green Version]
- Pham, X.D.; Otsuka, Y.; Suzuki, H.; Takaoka, H. Detection of Orientia tsutsugamushi (Rickettsiales: Rickettsiaceae) in unengorged chiggers (Acari: Trombiculidae) from Oita Prefecture, Japan, by nested polymerase chain reaction. J. Med. Entomol. 2001, 38, 308–311. [Google Scholar] [CrossRef]
- McIntyre, K.M.; Setzkorn, C.; Hepworth, P.J.; Morand, S.; Morse, A.P.; Baylis, M. Systematic assessment of the climate sensitivity of important human and domestic animals pathogens in Europe. Sci. Rep. 2017, 7, 7134. [Google Scholar] [CrossRef] [Green Version]
- Park, S.W.; Ha, N.Y.; Ryu, B.; Bang, J.H.; Song, H.; Kim, Y.; Kim, G.; Oh, M.D.; Cho, N.H.; Lee, J.K. Urbanization of scrub typhus disease in South Korea. PLoS Negl. Trop. Dis. 2015, 9, e0003814. [Google Scholar] [CrossRef]
- Lee, W.G.; Yang, S.C. Introduction of regional center for vector surveillance against climate change. Korea KDCA Public Health Wkly. Rep. 2014, 7, 936–938. [Google Scholar]
- Ree, H.I. Fauna and key to the chigger mites of Korea (Acarina: Trombiculidae and Leeuwenhoekiidae). Kor. J. System. Zool. 1990, 6, 57–70. [Google Scholar]
- Takahashi, M.; Murata, M.; Misumi, H.; Hori, E.; Kawamura, A., Jr.; Tanaka, H. Failed vertical transmission of Rickettsia tsutsugamushi (Rickettsiales: Rickettsiaceae) acquired from rickettsemic mice by Leptotrombidium pallidum (Acari: Trombiculidae). J. Med. Entomol. 1994, 31, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Elliott, I.; Pearson, I.; Dahal, P.; Thomas, N.V.; Roberts, T.; Newton, P.N. Scrub typhus ecology: A systematic review of Orientia in vectors and hosts. Parasit. Vectors 2019, 12, 513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frances, S.P.; Watcharapichat, P.; Phulsuksombati, D.; Tanskul, P. Transmission of Orientia tsutsugamushi, the aetiological agent for scrub typhus, to co-feeding mites. Parasitology 2000, 120, 601–607. [Google Scholar] [CrossRef]
- Takahashi, M.; Murata, M.; Nogami, S.; Hori, E.; Kawamura, A., Jr.; Tanaka, H. Transovarial transmission of Rickettsia tsutsugamushi in Leptotrombidium pallidum successively reared in the laboratory. Jpn. J. Exp. Med. 1988, 58, 213–218. [Google Scholar] [PubMed]
- Takahashi, M.; Urakami, H.; Misumi, H.; Noda, S.; Yamamoto, S.; Suzuki, H.; Matsumoto, I. Detection and serotyping of Orientia tsutsugamushi from the unfed larval trombiculid mite Leptotrombidium scutellare. Med. Entomol. Zool. 2002, 53, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Misumi, H.; Takahashi, M.; Urakami, H.; Matsumoto, I. Distributions of infective spots composed of unfed larvae infected with Orientia tsutsugamushi in Leptotrombidium mites and their annual fluctuations on the soil surface in an endemic area of tsutsugamushi disease (Acari: Trombiculidae). Med. Entomol. Zool. 2002, 53, 227–247. [Google Scholar] [CrossRef] [Green Version]
- Kawamura, A., Jr.; Nogami, S. Transovarian transmission. In Tsutsugamushi Disease; Kawamura, A., Jr., Tanaka, H., Tamura, A., Eds.; University of Tokyo Press: Tokyo, Japan, 1995; pp. 247–266. [Google Scholar]
- Lee, H.I.; Shim, S.K.; Song, B.G.; Choi, E.N.; Hwang, K.J.; Park, M.Y.; Park, C.; Shin, E.H. Detection of Orientia tsutsugamushi, the causative agent of scrub typhus, in a novel mite species, Eushoengastia koreaensis, in Korea. Vector Borne Zoonotic Dis. 2011, 11, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Yu, D.S.; Lee, G.S.; Seo, J.J.; Chung, J.K.; Lee, J.I. Epidemiological characteristics of rodents and chiggers with Orientia Tsutsugamushi in the Republic of Korea. Korean J. Parasitol. 2020, 58, 559–564. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, S.H.; Park, D.W.; Jung, S.H.; Park, H.J.; Seo, M.H.; Song, H.J.; Lee, J.Y.; Kim, D.M.; Kim, C.M.; et al. Molecular epidemiology of an Orientia tsutsugamushi gene encoding a 56-kDa type-specific antigen in chiggers, small mammals, and patients from the southwest region of Korea. Am. J. Trop. Med. Hyg. 2018, 98, 616–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.J.; Lee, I.Y.; Song, H.J.; Kim, J.; Park, H.J.; Song, D.; Jang, W.J. Geographical distribution of Orientia tsutsugamushi strains in chiggers from three provinces in Korea. Microbiol. Immunol. 2018, 62, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Chung, J.K.; Kim, S.H.; Cho, S.J.; Ha, Y.D.; Jung, S.H.; Park, H.J.; Song, H.J.; Lee, J.Y.; Kim, D.M.; et al. Seroepidemiological survey of zoonotic diseases in small mammals with PCR detection of Orientia tsutsugamushi in chiggers, Gwangju, Korea. Korean J. Parasitol. 2016, 54, 307–313. [Google Scholar] [CrossRef] [Green Version]
- Kawamura, A., Jr.; Nogami, S. Seasonal prevalence of the disease in Japan. In Tsutsugamushi Disease; Kawamura, A., Jr., Tanaka, H., Tamura, A., Eds.; University of Tokyo Press: Tokyo, Japan, 1995; pp. 10–20. [Google Scholar]
- Ree, H.I.; Lee, I.Y.; Jeon, S.H.; Yoshida, Y. Geographical distribution of vectors and sero-strains of tsutsugamushi disease at mid-south inland of Korea. Korean J. Parasitol. 1997, 35, 171–179. [Google Scholar] [CrossRef] [PubMed]

| Regions | No. of Collected Chigger Mites per Species | Total | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L. scu | L. palp | N. kwa | N. tam | L. pall | L. tec | H. miy | L. ori | N. asa | N. jap | N. mit | N. nag | N. gar | E. kor | L. zet | Un | ||
| Yeoju | 0 | 15 | 0 | 0 | 102 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 122 |
| Paju | 15 | 4 | 7 | 5 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 36 |
| Hwaseong | 380 | 8 | 0 | 53 | 115 | 8 | 3 | 8 | 0 | 0 | 18 | 1 | 0 | 0 | 0 | 0 | 594 |
| Gangneung | 0 | 1 | 156 | 1 | 16 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 175 |
| Cheorwon | 16 | 35 | 157 | 193 | 18 | 119 | 0 | 7 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 550 |
| Okcheon | 14 | 159 | 0 | 19 | 50 | 0 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 253 |
| Boryeong | 3 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3 | 0 | 0 | 16 |
| Yesan | 10 | 65 | 0 | 28 | 30 | 0 | 24 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 160 |
| Jeongeup | 66 | 6 | 1 | 108 | 0 | 0 | 0 | 0 | 0 | 12 | 0 | 12 | 6 | 0 | 0 | 0 | 211 |
| Jinan | 11 | 46 | 73 | 41 | 1 | 0 | 8 | 0 | 27 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 207 |
| Boseong | 301 | 37 | 53 | 0 | 34 | 0 | 5 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 431 |
| Gimcheon | 0 | 2 | 0 | 0 | 18 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 22 |
| Yeongdeok | 0 | 146 | 5 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 3 | 158 |
| Geoje | 393 | 112 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 507 |
| Hapcheon | 61 | 78 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 139 |
| Seogwipo | 15 | 11 | 0 | 0 | 20 | 0 | 0 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 56 |
| Total | 1285 | 725 | 452 | 448 | 418 | 127 | 53 | 29 | 27 | 23 | 18 | 13 | 8 | 7 | 1 | 3 | 3637 |
| Species | No. of Collected Rodents (%) | No. of Collected Chigger Mites | Chigger Index |
|---|---|---|---|
| Apodemus agrarius | 381 (76.4) | 45,222 | 118.7 |
| Crocidura spp. | 94 (18.8) | 992 | 10.6 |
| Craseomys regulus | 12 (2.4) | 2314 | 192.8 |
| Micromys minutus | 9 (1.8) | 1118 | 124.2 |
| Apodemus peninsulae | 2 (0.4) | 283 | 141.5 |
| Microtus fortis | 1 (0.2) | 224 | 224.0 |
| Total | 499 | 50,153 | 100.5 |
| Regions | No. of Collected Rodents/Installed Traps | No. of Rodents Infested with Chigger Mites (%) | No. of Collected Chigger Mites | Chigger Index | No. of Tested Chigger Mites (No. of Pools) | No. of Ot-Positive Chigger Mite Pools | MIR (%) of Chigger Mites |
|---|---|---|---|---|---|---|---|
| Yeoju | 34/400 | 31 (91.2) | 6623 | 194.8 | 3309 (129) | 1 | 0.03 |
| Paju | 26/400 | 14 (53.8) | 2099 | 80.7 | 1073 (38) | 0 | 0 |
| Hwaseong | 29/400 | 26 (89.7) | 4175 | 144.0 | 2086 (80) | 5 | 0.2 |
| Gangneung | 74/400 | 51 (68.9) | 3903 | 52.7 | 1949 (94) | 0 | 0 |
| Cheorwon | 40/400 | 33 (82.5) | 3357 | 83.9 | 1672 (76) | 2 | 0.1 |
| Cheongju | 29/400 | 22 (75.9) | 2973 | 102.5 | 1482 (63) | 0 | 0 |
| Boryeong | 24/400 | 19 (79.2) | 1170 | 48.8 | 582 (21) | 0 | 0 |
| Yesan | 26/400 | 23 (88.5) | 3458 | 133.0 | 1728 (72) | 0 | 0 |
| Jeongeup | 29/400 | 24 (82.8) | 3011 | 103.8 | 1506 (61) | 11 | 0.7 |
| Jinan | 44/400 | 39 (88.6) | 6628 | 150.6 | 3307 (134) | 0 | 0 |
| Boseong | 26/400 | 22 (84.6) | 2930 | 112.7 | 1463 (61) | 1 | 0.07 |
| Gimcheon | 19/400 | 11 (57.9) | 613 | 32.3 | 245 (14) | 0 | 0 |
| Yeongdeok | 21/400 | 20 (95.2) | 3126 | 148.9 | 1562 (63) | 2 | 0.1 |
| Geoje | 24/400 | 18 (75.0) | 1782 | 74.3 | 894 (27) | 0 | 0 |
| Hapcheon | 24/400 | 18 (75.0) | 3797 | 158.2 | 1897 (49) | 1 | 0.1 |
| Seogwipo | 30/400 | 11 (36.7) | 508 | 16.9 | 182 (16) | 0 | 0 |
| Total | 499/6400 | 382 (76.6) | 50,153 | 100.5 | 24,937 (998) | 23 | 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, M.-G.; Song, B.-G.; Kim, T.-K.; Noh, B.-E.; Lee, H.S.; Lee, W.-G.; Lee, H.I. Nationwide Incidence of Chigger Mite Populations and Molecular Detection of Orientia tsutsugamushi in the Republic of Korea, 2020. Microorganisms 2021, 9, 1563. https://doi.org/10.3390/microorganisms9081563
Seo M-G, Song B-G, Kim T-K, Noh B-E, Lee HS, Lee W-G, Lee HI. Nationwide Incidence of Chigger Mite Populations and Molecular Detection of Orientia tsutsugamushi in the Republic of Korea, 2020. Microorganisms. 2021; 9(8):1563. https://doi.org/10.3390/microorganisms9081563
Chicago/Turabian StyleSeo, Min-Goo, Bong-Goo Song, Tae-Kyu Kim, Byung-Eon Noh, Hak Seon Lee, Wook-Gyo Lee, and Hee Il Lee. 2021. "Nationwide Incidence of Chigger Mite Populations and Molecular Detection of Orientia tsutsugamushi in the Republic of Korea, 2020" Microorganisms 9, no. 8: 1563. https://doi.org/10.3390/microorganisms9081563






