Multi-Trait Wheat Rhizobacteria from Calcareous Soil with Biocontrol Activity Promote Plant Growth and Mitigate Salinity Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
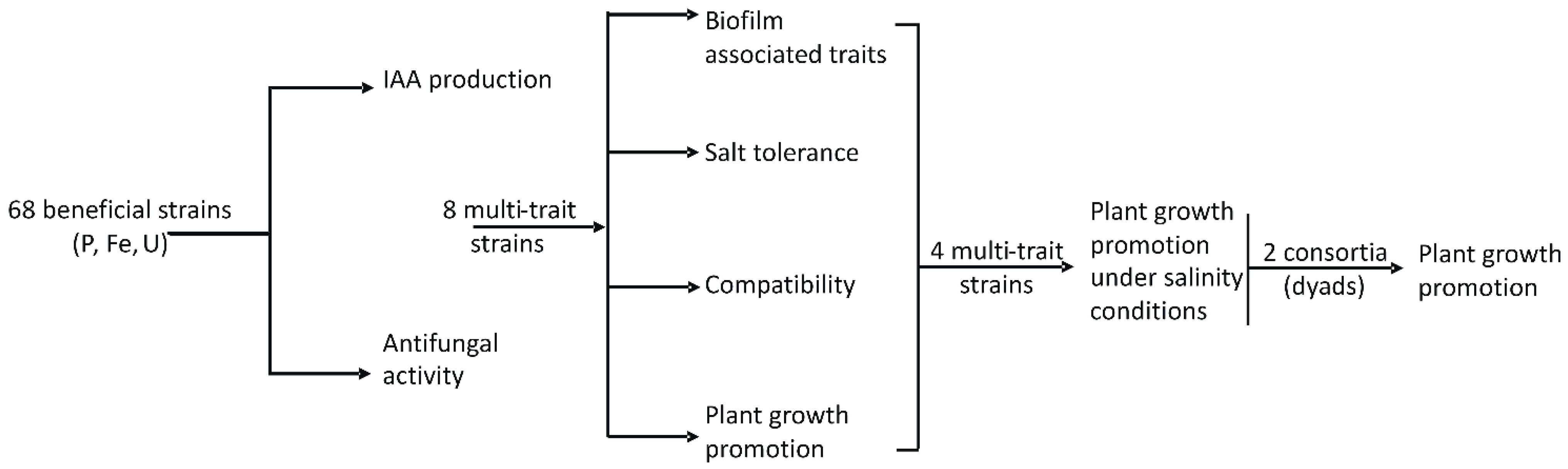
2.2. Experimental Strategy
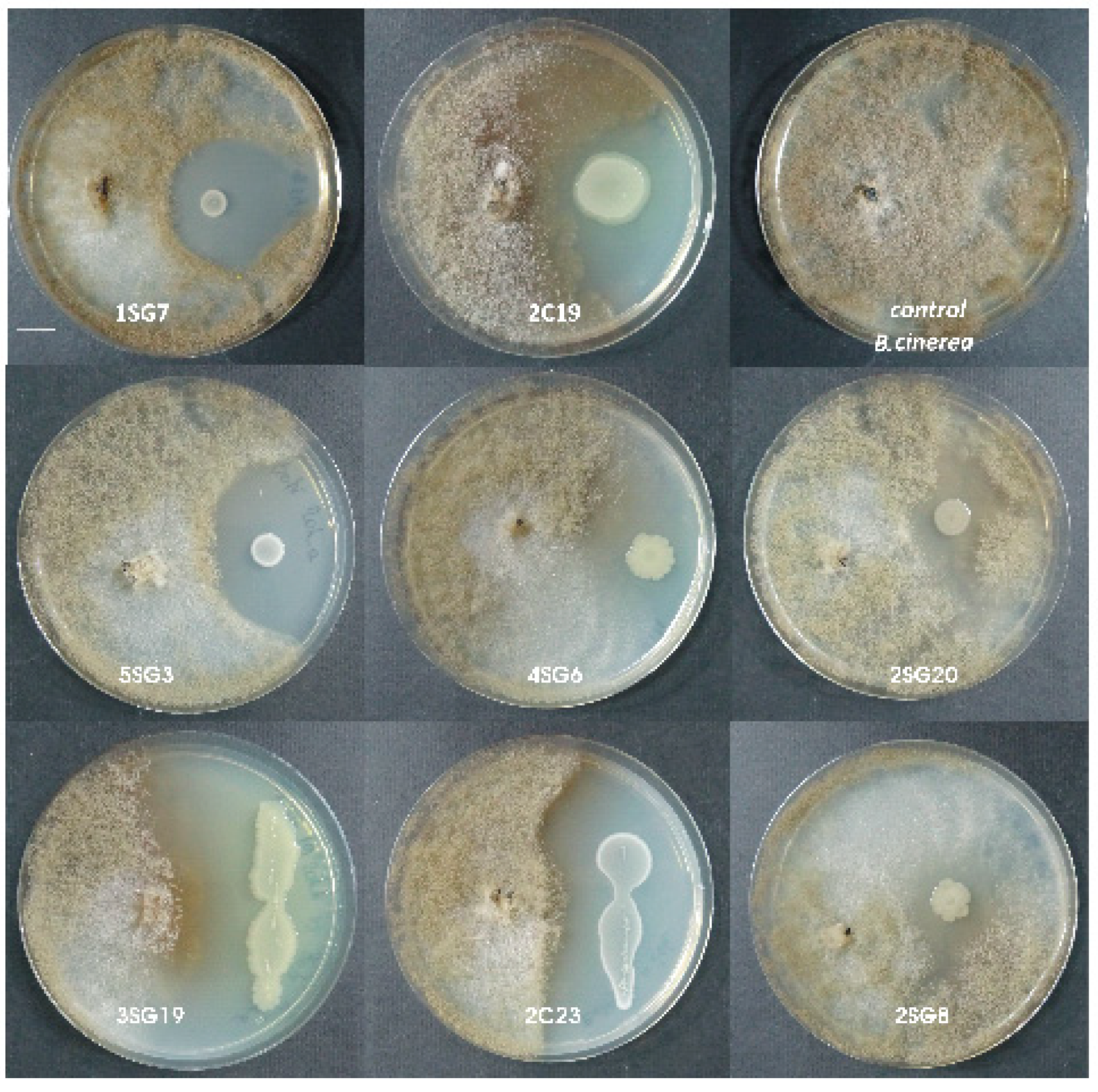
2.3. Antifungal Activity Against Phytopathogenic Fungi
2.4. IAA Production
2.5. Selection of 8 Bacterial Strains Based on Their Best Trait Analysis
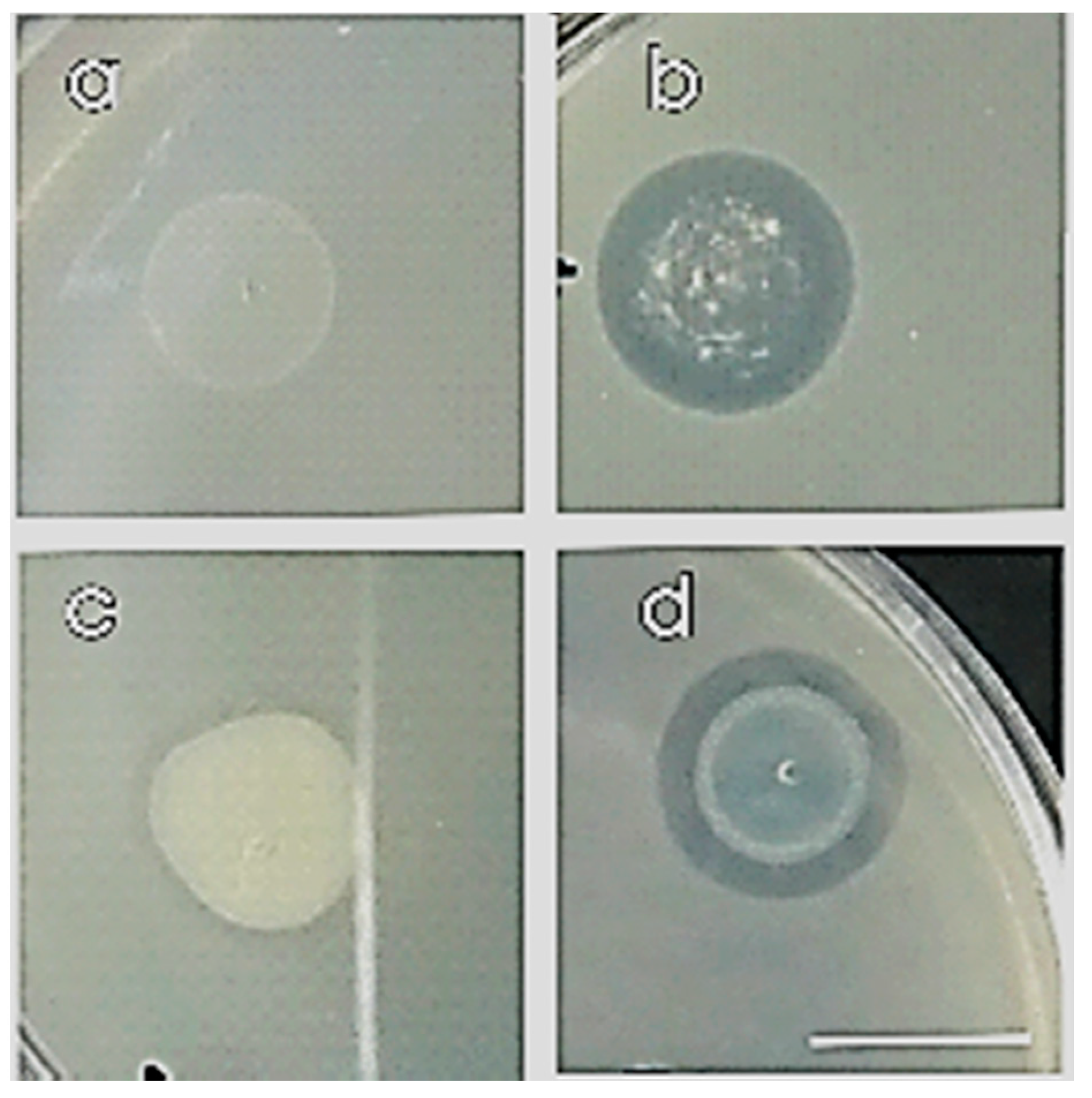
2.6. Biofilm Associated Traits
2.6.1. Swarming Motility and Temperature Tolerance
2.6.2. Biofilm Formation
2.7. Salinity Tolerance of Bacterial Strains
2.8. Strain Compatibility
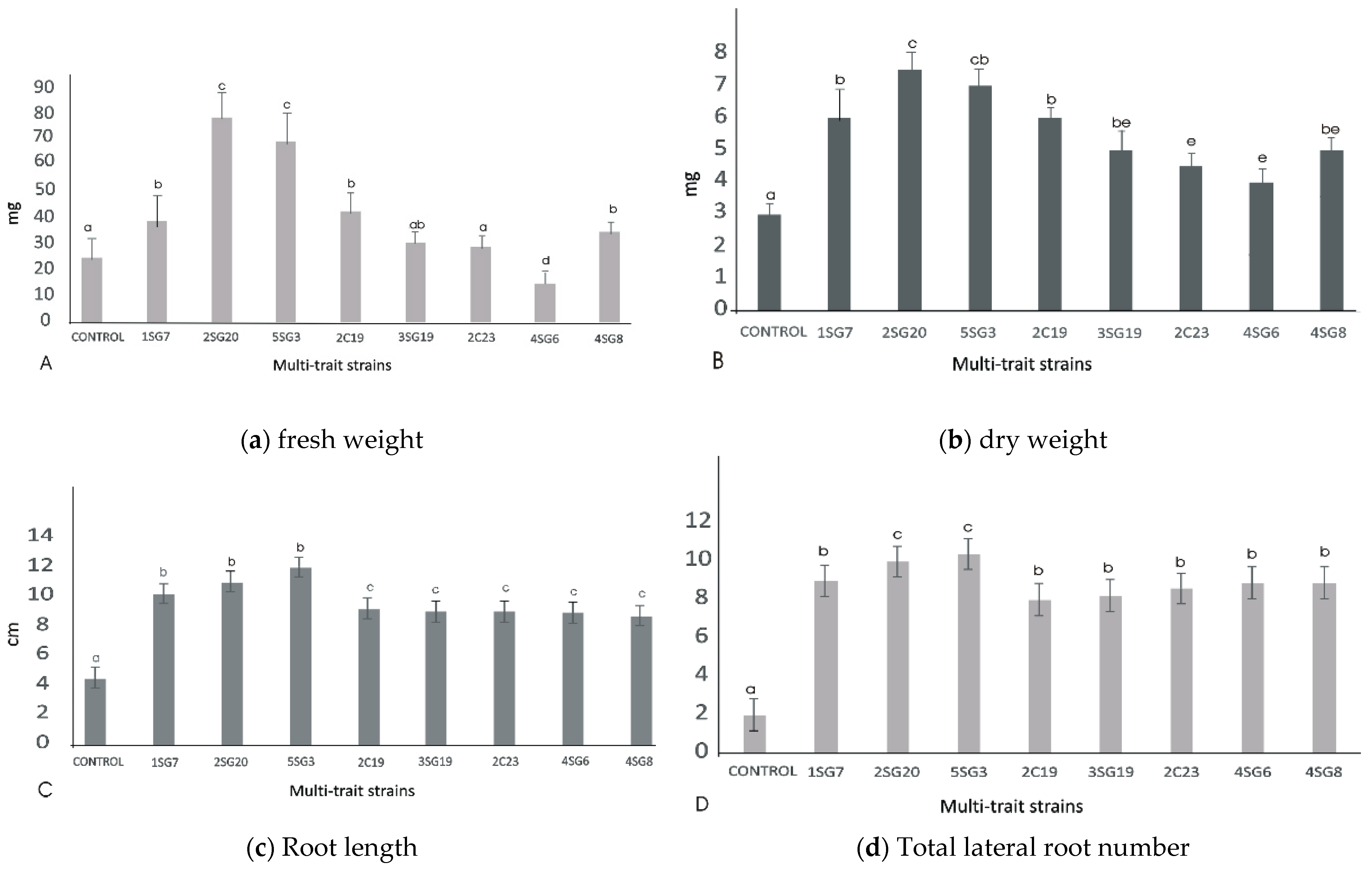
2.9. Screening of the 8 Selected Bacterial Strains for Plant Growth Promotion on Arabidopsis thaliana (Col-0)
2.10. Screening of the 4 Selected Bacterial Strains for Plant Growth Promotion on A. thaliana (Col-0) under Salinity Conditions
2.11. Screening of 2 Selected Compatible Bacterial Strain Dyads for Plant Growth Promotion on A. thaliana (Col-0)
2.12. Statistical Analysis
3. Results
3.1. Antifungal Activity Against Phytopathogenic Fungi
3.2. IAA Production
3.3. First Bacterial Strain Selection
3.4. Further Beneficial Traits
3.4.1. Biofilm Associated Traits
Swarming Motility and Temperature Tolerance
Biofilm Formation
3.4.2. Salinity Tolerance
Salt Tolerance of Bacterial Strains
3.4.3. Strain Compatibility
3.4.4. Plant Growth Promotion on A. thaliana (Col-0)
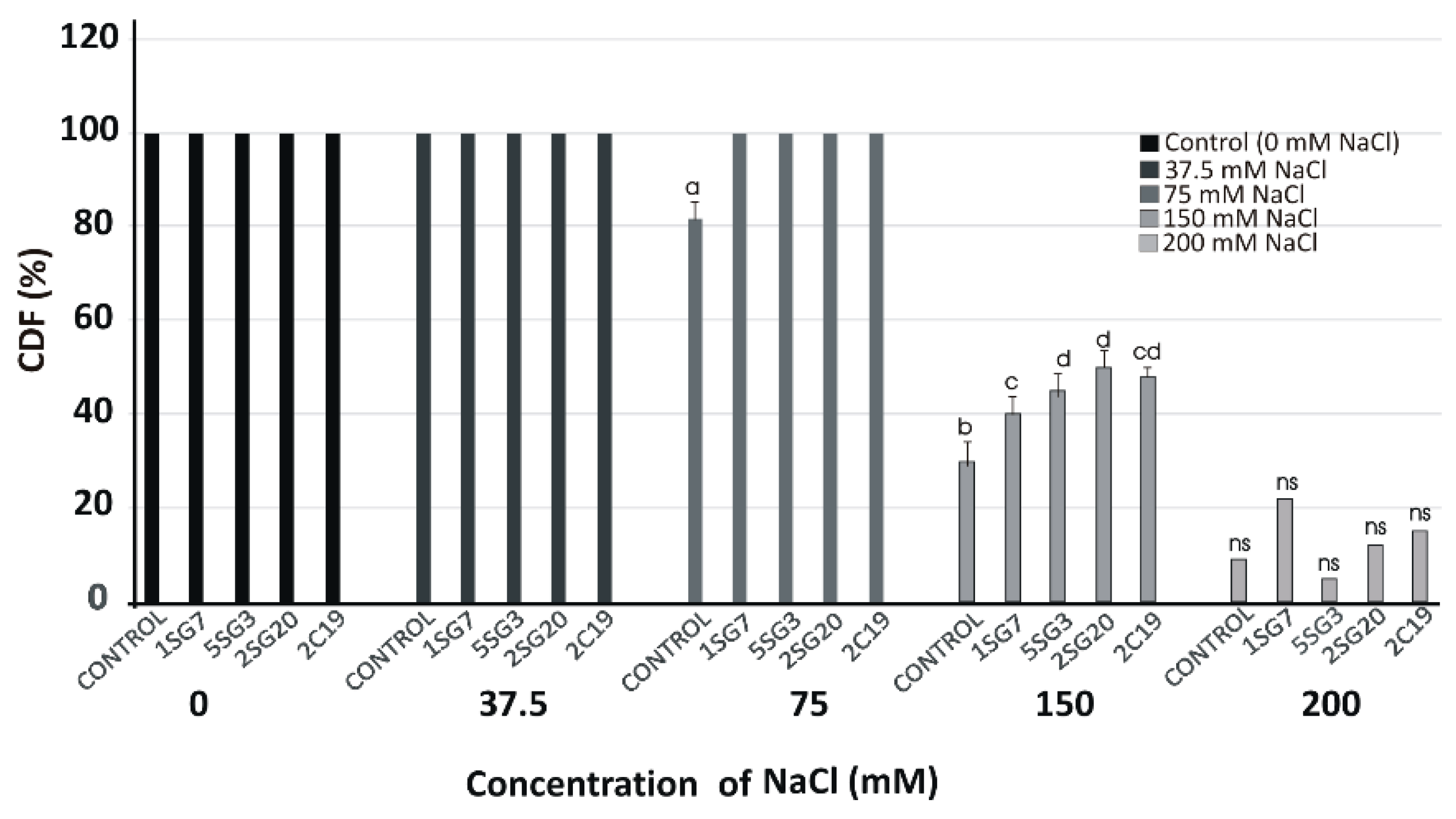
3.5. Plant Growth Promotion on A. thaliana (Col-0) under Salinity Conditions
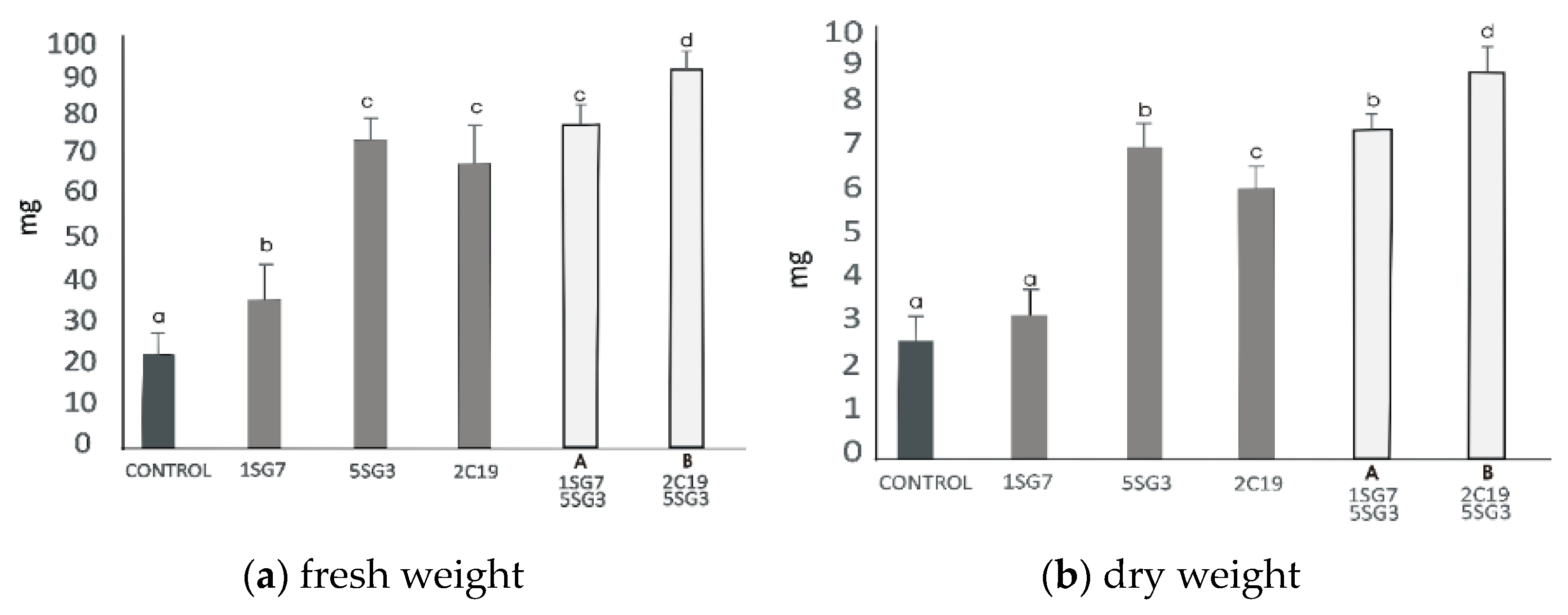
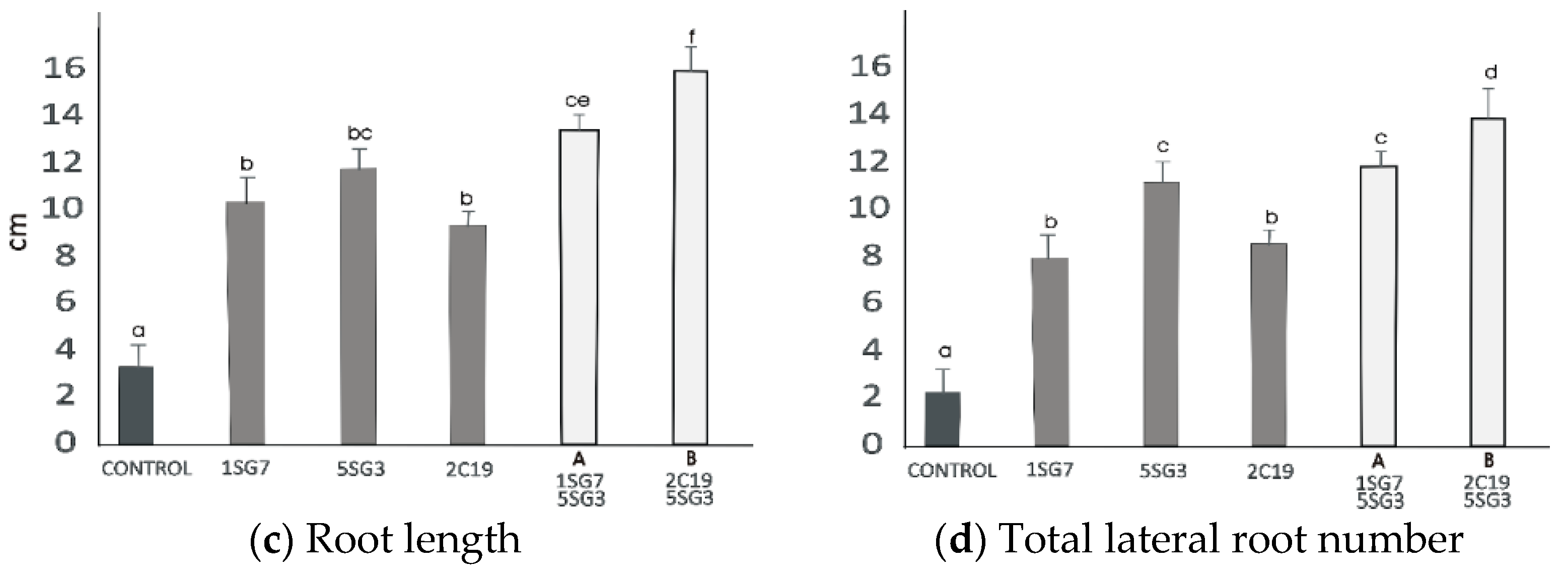
3.6. Screening of 2 Selected Compatible Bacterial Strain Dyads as Mixtures for Plant Growth Promotion on A. thaliana (Col-0)
3.7. Overall Additional Beneficial Traits of the Selected Strains
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Panke-Buisse, K.; Poole, A.; Goodrich, J.; Ley, R.E.; Kao-Kniffin, J. Selection on soil microbiomes reveals reproducible impacts on plant function. ISME J. 2015, 9, 980–989. [Google Scholar] [CrossRef]
- Bandyopadhyay, P.; Bhuyan, S.K.; Yadava, P.K.; Varma, A.; Tuteja, N. Emergence of plant and rhizospheric microbiota as stable interactomes. Protoplasma 2017, 254, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, K.; Kumar, N.; Shandilya, C.; Mohapatra, S.; Bhayana, S.; Varma, A. Revisiting Plant-Microbe Interactions and Microbial Consortia Application for Enhancing Sustainable Agriculture: A Review. Front. Microbiol. 2020, 21, 560406. [Google Scholar] [CrossRef] [PubMed]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Leong, J.; Teintze, M.; Schroth, M.N. Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 1980, 286, 885–886. [Google Scholar] [CrossRef]
- Suslow, T.V.; Kloepper, J.W.; Schroth, M.N.; Burr, T.J. Beneficial bacteria enhance plant growth. Calif. Agric. Exp. Stn. 1979, 33, 15–17. [Google Scholar]
- Bhardwaj, D.; Ansari, M.W.; Sahoo, R.K.; Tuteja, N. Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb. Cell Fact. 2014, 13, 66. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, R.K.; Ansari, M.W.; Pradhan, M.; Dangar, T.K.; Mohanty, S.; Tuteja, N. Phenotypic and molecular characterization of efficient native Azospirillum strains from rice fields for crop improvement. Protoplasma 2014, 251, 943–953. [Google Scholar] [CrossRef]
- Glick, B.R. The enhancement of plant growth by free-living bacteria. Can. J. Microbiol. 1995, 41, 109–117. [Google Scholar] [CrossRef]
- Rodríguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef]
- Vessey, J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant. Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Thomloudi, E.-E.; Tsalgatidou, P.C.; Diuka, D.; Spantidos, T.-N.; Dimou, M.; Venieraki, A.; Katinakis, P. Multistrain versus single-strain plant growth promoting microbial inoculants—The compatibility issue. Hell. Plant Prot. J. 2019, 12, 61–77. [Google Scholar] [CrossRef] [Green Version]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant Growth Promoting Rhizobacteria (PGPR) as Green Bioinoculants: Recent Developments, Constraints, and Prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Ryu, C.M.; Zhang, S. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 2004, 94, 1259–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarma, B.K.; Yadav, S.K.; Singh, S.; Singh, H.B. Microbial consortium-mediated plant defense against phytopathogens: Readdressing for enhancing efficacy. Soil Biol. Biochem. 2015, 87, 25–33. [Google Scholar] [CrossRef]
- Friedman, J.; Higgins, L.M.; Gore, J. Community structure follows simple assembly rules in microbial microcosms. Nat. Ecol. Evol. 2017, 1, 0109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molina-Romero, D.; Baez, A.; Quintero-Hernández, V.; Castañeda-Lucio, M.; Fuentes-Ramírez, L.E.; Bustillos-Cristales, M.d.R.; Rodríguez-Andrade, O.; Morales-García, Y.E.; Munive, A.; Muñoz-Rojas, J. Compatible bacterial mixture, tolerant to desiccation, improves maize plant growth. PLoS ONE 2017, 12, e0187913. [Google Scholar] [CrossRef] [PubMed]
- Crégut, M.; Piutti, S.; Slezack-Deschaumes, S.; Benizri, E. Compartmentalization and regulation of arylsulfatase activities in Streptomyces sp., Microbacterium sp. and Rhodococcus sp. soil isolates in response to iNorganic sulfate limitation. Microbiol. Res. 2013, 168, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Stressler, T.; Seitl, I.; Kuhn, A.; Fischer, L. Detection, production, and application of microbial arylsulfatases. Appl. Microbiol. Biotechnol. 2016, 100, 9053–9067. [Google Scholar] [CrossRef]
- Zhu, Y.; Qiao, C.; Li, H.; Li, L.; Xiao, A.; Ni, H.; Jiang, Z. Improvement thermostability of Pseudoalteromonas carrageenovora arylsulfatase by rational design. Int. J. Biol. Macromol. 2018, 108, 953–959. [Google Scholar] [CrossRef]
- Zhu, Y.; Liang, M.; Li, H.; Ni, H.; Li, L.; Li, Q.; Jiang, Z. A mutant of Pseudoalteromonas carrageenovora arylsulfatase with enhanced enzyme activity and its potential application in improvement of the agar quality. Food Chem. 2020, 320, 126652. [Google Scholar] [CrossRef]
- Miech, C.; Dierks, T.; Selmer, T.; von Figura, K.; Schmidt, B. Arylsulfatase from Klebsiella pneumoniae carries a formylglycine generated from a serine. J. Biol. Chem. 1998, 273, 4835–4837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stressler, T.; Leisibach, D.; Lutz-Wahl, S.; Kuhn, A.; Fischer, L. Homologous expression and biochemical characterization of the arylsulfatase from Kluyveromyces lactis and its relevance in milk processing. Appl. Microbiol. Biotechnol. 2016, 100, 5401–5414. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.E.; Kim, K.H.; Bae, Y.J.; Lee, J.H.; Jang, Y.H.; Nam, S.W. Purification and characterization of the recombinant arylsulfatase cloned from Pseudoalteromonas carrageenovora. Protein Expr. Purif. 2005, 39, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Marino, T.; Russo, N.; Toscano, M. Catalytic mechanism of the arylsulfatase promiscuous enzyme from Pseudomonas aeruginosa. Chemistry 2013, 19, 2185–2192. [Google Scholar] [CrossRef]
- Henderson, M.J.; Milazzo, F.H. Arylsulfatase in Salmonella typhimurium: Detection and influence of carbon source and tyramine on its synthesis. J. Bacteriol. 1979, 139, 80–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murooka, Y.; Yim, M.H.; Harada, T. Formation and purification of Serratia marcescens arylsulfatase. Appl. Environ. Microbiol. 1980, 39, 812–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.G.; Shin, J.G.; Jeon, M.J.; Lee, S.H. Heterologous expression and characterization of a recombinant thermophilic arylsulfatase from Thermotoga maritima. Biotechnol. Bioproc. E. 2013, 8, 897–902. [Google Scholar] [CrossRef]
- Niu, R.; Jing, H.; Chen, Z.; Xu, J.; Dai, J.; Yan, Z. Differentiating malignant colorectal tumor patients from benign colorectal tumor patients by assaying morning urinary arylsulfatase activity. Asia Pac. J. Clin. Oncol. 2012, 8, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Blum, S.C.; Lehmann, J.; Solomon, D.; Caires, E.F.; Alleoni, L.R.F. Sulfur forms in organic substrates affecting S mineralization in soil. Geoderma 2013, 200–201, 156–164. [Google Scholar] [CrossRef]
- Schloter, M.; Dilly, O.; Munch, J.C. Indicators for evaluating soil quality. Agric. Ecosyst. Environ. 2003, 98, 255–262. [Google Scholar] [CrossRef]
- Pulleman, M.; Creamer, R.; Hamer, U.; Helder, J.; Pelosi, C.; Pérès, G.; Rutgers, M. Soil biodiversity, biological indicators and soil ecosystem services-an overview of European approaches. Curr. Opin. Environ. Sustain. 2012, 4, 529–538. [Google Scholar] [CrossRef]
- Kucharski, J.; Tomkiel, M.; Baćmaga, M.; Borowik, A.; Wyszkowska, J. Enzyme activity and microorganisms diversity in soil contaminated with the boreal 58 WG herbicide. J. Environ. Sci. Health B 2016, 51, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Klose, S.; Moore, J.M.; Tabatabai, M.A. Arylsulfatase activity of microbial biomass in soils as affected by cropping systems. Biol. Fertil. Soils 1999, 29, 46–54. [Google Scholar] [CrossRef]
- García-Sánchez, M.; Klouza, M.; Holečková, Z.; Tlustoš, P.; Száková, J. Organic and inorganic amendment application on mercury-polluted soils: Effects on soil chemical and biochemical properties. Environ. Sci. Pollut. Res. Int. 2016, 23, 14254–14268. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Dutta, D.; Karmakar, R.; Ray, D.P. Structure-toxicity relationship of chloroacetanilide herbicides: Relative impact on soil microorganisms. Environ. Toxicol. Pharmacol. 2012, 34, 307–314. [Google Scholar] [CrossRef]
- Schreinemachers, P.; Tipraqsa, P. Agricultural pesticides and land use intensification in high, middle and low income countries. Food Policy 2012, 37, 616–626. [Google Scholar] [CrossRef]
- Garau, G.; Silvetti, M.; Castaldi, P.; Mele, E.; Deiana, P.; Deiana, S. Stabilising metal(loid)s in soil with iron and aluminium-based products: Microbial, biochemical and plant growth impact. J. Environ. Manag. 2014, 139, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.; Pampulha, M.E. Effects of long-term heavy metal contamination on soil microbial characteristics. J. Biosci. Bioeng. 2006, 102, 157–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Lei, P.; Wang, Q.; Ma, J.; Zhan, Y.; Jiang, K.; Xu, Z.; Xu, H. The Endophyte Pantoea alhagi NX-11 Alleviates Salt Stress Damage to Rice Seedlings by Secreting Exopolysaccharides. Front. Microbiol. 2020, 10, 3112. [Google Scholar] [CrossRef] [PubMed]
- Barnawal, D.; Bharti, N.; Pandey, S.S.; Pandey, A.; Chanotiya, C.S.; Kalra, A. Plant growth promoting rhizobacteria enhances wheat salt and drought stress tolerance by altering endogenous phytohormone levels and TaCTR1/TaDREB2 expression. Physiol. Plant. 2017, 161, 502–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Kulkarni, J.; Jha, B. Halotolerant rhizobacteria promote growth and enhance salinity tolerance in peanut. Front. Microbiol. 2016, 7, 1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heydarian, Z.; Gruber, M.; Glick, B.R.; Hegedus, D.D. Gene Expression Patterns in Roots of Camelina sativa with Enhanced Salinity Tolerance Arising from Inoculation of Soil with Plant Growth Promoting Bacteria Producing 1-Aminocyclopropane-1-Carboxylate Deaminase or Expression the Corresponding acdS Gene. Front. Microbiol. 2018, 9, 1297. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Zhao, S.; Tian, C.Y. Effect of halotolerant rhizobacteria isolated from halophytes on the growth of sugar beet (Beta vulgaris L.) under salt stress. FEMS Microbiol. Lett. 2017, 364, a001438. [Google Scholar] [CrossRef]
- Yasmeen, T.; Ahmad, A.; Arif, M.S.; Mubin, M.; Rehman, K.; Shahzad, S.M.; Iqbal, S.; Rizwan, M.; Ali, S.; Alyemeni, M.N.; et al. Biofilm forming rhizobacteria enhance growth and salt tolerance in sunflower plants by stimulating antioxidant enzymes activity. Plant Physiol. Biochem. 2020, 156, 242–256. [Google Scholar] [CrossRef]
- Khati, P.; Parul; Bhatt, P.; Nisha; Kumar, R.; Sharma, A. Effect of nanozeolite and plant growth promoting rhizobacteria on maize. 3 Biotech. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Bouranis, D.L.; Venieraki, A.; Chorianopoulou, S.N.; Katinakis, P. Impact of Elemental Sulfur on the Rhizospheric Bacteria of Durum Wheat Crop Cultivated on a Calcareous Soil. Plants 2019, 8, 379. [Google Scholar] [CrossRef] [Green Version]
- Whalen, J.K.; Warman, P.R. Arylsulfatase activity in soil and soil extracts using natural and artificial substrates. Biol. Fertil. Soils 1996, 22, 373–378. [Google Scholar] [CrossRef]
- Pikovskaya, R. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya 1948, 17, 362–370. [Google Scholar]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Gordon, S.A.; Weber, R.P. Colorimetric estimation of indoleacetic acid. Plant Physiol. 1951, 26, 192–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bric, J.M.; Bostock, R.M.; Silverstone, S.E. Rapid in situ assay for indoleacetic Acid production by bacteria immobilized on a nitrocellulose membrane. Appl. Environ. Microbiol. 1991, 57, 535–538. [Google Scholar] [CrossRef] [Green Version]
- O’Toole, G.A.; Kolter, R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 1998, 30, 295–304. [Google Scholar] [CrossRef]
- Geis, A.; Singh, J.; Teuber, M. Potential of lactic streptococci to produce bacteriocin. Appl. Environ. Microbiol. 1983, 45, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Wintermans, P.C.; Bakker, P.A.; Pieterse, C.M. Natural genetic variation in Arabidopsis for responsiveness to plant growth-promoting rhizobacteria. Plant Mol. Biol. 2016, 90, 623–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Jha, C.K.; Saraf, M. Plant growth promoting rhizobacteria (PGPR): A review. E3 J. Agric. Res. Dev. 2015, 5, 108–119. [Google Scholar]
- Chowdhury, S.; Hartmann, A.; Gao, X.; Borriss, R. Biocontrol mechanism by root-associated Bacillus amyloliquefaciens FZB42—A review. Front. Microbiol. 2015, 6, 780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, J.; Weyens, N.; Croes, S.; Beckers, B.; Meiresonne, L.; Van Peteghem, P.; Carleer, R.; Vangronsveld, J. Phytoremediation of Metal Contaminated Soil Using Willow: Exploiting Plant-Associated Bacteria to Improve Biomass Production and Metal Uptake. Int. J. Phytoremediation 2015, 17, 1123–1136. [Google Scholar] [CrossRef]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Nasrulhaq Boyce, A. Role of plant growth promoting rhizobacteria in agricultural sustainability—A review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef] [PubMed]
- Villarreal Sanchez, J.A.; Diaz Jimenez, L.; Escobedo Bocardo, J.C.; Cardenas Palomo, J.O.; Guerra Escamilla, N.E.; Luna Alvarez, J.S. Effect of Marine Microorganisms on Limestone as an Approach for Calcareous Soil. Sustainability 2018, 10, 2078. [Google Scholar] [CrossRef] [Green Version]
- Paschalidis, C.; Sotiropoulos, S.; Papakonstantinou, L.; Petropoulos, D.; Kavvadias, V.; Paschalidis, D.; Christodoulou, C. Soil Resources and the Role in Agriculture Sector of Greek Economy. Environ. Ecol. Res. 2020, 8, 70–75. [Google Scholar]
- Grover, M.; Ali, S.Z.; Sandhya, V.; Rasul, A.; Venkateswarlu, B. Role of microorganisms in adaptation of agriculture crops to abiotic stresses. World J. Microbiol. Biotechnol. 2011, 27, 1231–1240. [Google Scholar] [CrossRef]
- Aeron, A.; Khare, E.; Jha, C.K.; Meena, V.S.; Aziz, S.M.A.; Islam, M.T.; Kim, K.; Meena, S.K.; Pattanayak, A.; Rajashekara, H.; et al. Revisiting the plant growth-promoting rhizobacteria: Lessons from the past and objectives for the future. Arch. Microbiol. 2020, 202, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Yazawa, S.; Ushijima, Y.A. Promising strain of endophytic Streptomyces sp. for biological control of cucumber anthracnose. J. Gen. Plant Pathol. 2009, 75, 27–36. [Google Scholar] [CrossRef]
- Coombs, J.T.; Michelsen, P.P.; Franco, C.M.M. Evaluation of endophytic actinobacteria as antagonists of Gaeumannomyces graminis var. tritici in wheat. Biol. Control. 2004, 29, 359–366. [Google Scholar] [CrossRef]
- John, R.P.; Tyagi, R.D.; Brar, S.K.; Surampalli, R.Y.; Prévost, D. Bio-encapsulation of microbial cells for targeted agricultural delivery. Crit. Rev. Biotechnol. 2011, 31, 211–226. [Google Scholar] [CrossRef]
- Dong, L.; Li, Y.; Xu, J.; Yang, J.; Wei, G.; Shen, L.; Ding, W.; Chen, S. Biofertilizers regulate the soil microbial community and enhance Panax ginseng yields. Chin. Med. 2019, 23, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Pirttilä, A.M.; Mohammad Parast Tabas, H.; Baruah, N.; Koskimäki, J.J. Biofertilizers and Biocontrol Agents for Agriculture: How to Identify and Develop New Potent Microbial Strains and Traits. Microorganisms 2021, 9, 817. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Askari, K.; Kumari, M. Optimization of indole acetic acid production by isolated bacteria from Stevia rebaudiana rhizosphere and its effects on plant growth. J. Genet. Eng. Biotechnol. 2018, 16, 581–586. [Google Scholar] [CrossRef]
- Jaiswal, A.; Das, K.; Koli, D.K.; Pabbi, S. Characterization of cyanobacteria for IAA and siderophore production and their effect on rice seed germination. Int. J. Curr. Microbiol. App. Sci. 2018, 5, 212–222. [Google Scholar]
- Vandana, U.K.; Rajkumari, J.; Singha, L.P.; Satish, L.; Alavilli, H.; Sudheer, P.D.V.N.; Chauhan, S.; Ratnala, R.; Satturu, V.; Mazumder, P.B.; et al. The Endophytic Microbiome as a Hotspot of Synergistic Interactions, with Prospects of Plant Growth Promotion. Biology 2021, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.L.; Waqas, M.; Kang, S.M.; Al-Harrasi, A.; Hussain, J.; Al-Rawahi, A.; Al-Khiziri, S.; Ullah, I.; Ali, L.; Jung, H.Y.; et al. Bacterial endophyte Sphingomonas sp. LK11 produces gibberellins and IAA and promotes tomato plant growth. J. Microbiol. 2014, 52, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Contesto, C.; Milesi, S.; Mantelin, S.; Zancarini, A.; Desbrosses, G.; Varoquaux, F.; Bellini, C.; Kowalczyk, M.; Touraine, B. The auxin-signaling pathway is required for the lateral root response of Arabidopsis to the rhizobacterium Phyllobacterium brassicacearum. Planta 2010, 232, 1455–1470. [Google Scholar] [CrossRef]
- Spaepen, S.; Vanderleyden, J. Auxin and plant-microbe interactions. Cold Spring Harb. Perspect. Biol. 2011, 3, a001438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venieraki, A.; Tsalgatidou, P.C.; Georgakopoulos, D.G.; Dimou, M.; Katinakis, P. Swarming motility in plant-associated bacteria. Hell. Plant. Protect. J. 2016, 9, 16–27. [Google Scholar] [CrossRef] [Green Version]
- Kasim, W.A.; Gaafar, R.M.; Abou-Ali, R.M.; Omar, M.N.; Hewait, H.M. Effect of biofilm forming plant growth promoting rhizobacteria on salinity tolerance in barley. Ann. Agric. Sci. 2016, 61, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Ansari, F.A.; Ahmad, I. Biofilm development, plant growth promoting traits and rhizosphere colonization by Pseudomonas entomophila FAP1: A Promising PGPR. Adv. Microbiol. 2018, 8, 235. [Google Scholar] [CrossRef] [Green Version]
- Haque, M.; Mosharaf, K.; Khatun, M.; Haque, A.; Biswas, S.; Islam, S.; Islam, M.; Shozib, H.B.; Miah, M.U.; Molla, A.H.; et al. Biofilm Producing Rhizobacteria With Multiple Plant Growth-Promoting Traits Promote Growth of Tomato Under Water-Deficit Stress. Front. Microbiol. 2020, 11, 542053. [Google Scholar] [CrossRef]
- De Souza, R.; Ambrosini, A.; Passaglia, L.M.P. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef]
- Saikia, J.; Sarma, R.K.; Dhandia, R.; Yadav, A.; Bharali, R.; Gupta, V.K.; Saikia, R. Alleviation of drought stress in pulse crops with ACC deaminase producing rhizobacteria isolated from acidic soil of Northeast India. Sci. Rep. 2018, 8, 3560. [Google Scholar] [CrossRef]
- Shilev, S. Plant-Growth-Promoting Bacteria Mitigating Soil Salinity Stress in Plants. Appl. Sci. 2020, 10, 7326. [Google Scholar] [CrossRef]
- Etesami, H.; Beattie, G.A. Mining Halophytes for Plant Growth-Promoting Halotolerant Bacteria to Enhance the Salinity Tolerance of Non-halophytic Crops. Front. Microbiol. 2018, 9, 148. [Google Scholar] [CrossRef] [Green Version]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt-Tolerant Plant Growth Promoting Rhizobacteria for Enhancing Crop Productivity of Saline Soils. Front. Microbiol. 2019, 10, 2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dodd, I.C.; Zinovkina, N.Y.; Safronova, V.I.; Belimov, A.A. Rhizobacterial mediation of plant hormone status. Ann. Appl. Biol. 2010, 157, 361–379. [Google Scholar] [CrossRef]
- Glick, B.R.; Todorovic, B.; Czarny, J.; Cheng, Z.; Duan, J.; McConkey, B. Promotion of plant growth by bacterial ACC deaminase. Crit. Rev. Plant. Sci. 2007, 26, 227–242. [Google Scholar] [CrossRef]
- Timmusk, S.; El-Daim, I.A.A.; Copolovici, L.; Tanilas, T.; Kännaste, A.; Behers, L.; Nevo, E.; Seisenbaeva, G.; Stenström, E.; Niinemets, Ü. Drought-tolerance of wheat improved by rhizosphere bacteria from harsh environments: Enhanced biomass production and reduced emissions of stress volatiles. PLoS ONE 2014, 9, e96086. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, S.K.; Singh, D.P. Effect of salt-tolerant plant growth promoting rhizobacteria on wheat plants and soil health in a saline environment. Plant Biol. 2015, 17, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Tabassum, B.; Abd_Allahd, E.F. Bacillus subtilis: A plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J. Biol. Sci. 2019, 26, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D.; Jabborova, D.; Berg, G. Synergistic interactions between Bradyrhizobium japonicum and the endophyte Stenotrophomonas rhizophila and their effects on growth, and nodulation of soybean under salt stress. Plant Soil 2016, 405, 35–45. [Google Scholar] [CrossRef]
- Ofek, M.; Ruppel, S.; Waisel, Y. Effects of salinity on rhizosphere bacterial communities associated with different root types of Vicia faba L. In Biosaline Agriculture and Salinity Tolerance in Plants; Ozturk, M., Waisel, Y., Khan, A., Gork, G., Eds.; Birkhauser Verlag: Basel, Switzerland, 2006; pp. 1–21. [Google Scholar]
- Ajar, N.Y.; Priyanka, V.; Bhanumati, S.; Vinay, S.C.; Archna, S.; Anil, K.S. Plant Growth Promoting Bacteria: Biodiversity and Multifunctional Attributes for Sustainable Agriculture. Adv. Biotech. Micro. 2017, 5, 555671. [Google Scholar]
- Chu, T.N.; Tran, B.T.H.; Van Bui, L.; Hoang, M.T.T. Plant growth-promoting rhizobacterium Pseudomonas PS01 induces salt tolerance in Arabidopsis thaliana. BMC Res. Notes 2019, 12, 11. [Google Scholar] [CrossRef] [Green Version]
- Bano, A.; Fatima, M. Salt tolerance in Zea mays (L.) following inoculation with Rhizobium and Pseudomonas. Biol. Fertil. Soils. 2009, 45, 405–413. [Google Scholar] [CrossRef]
- Cho, S.M.; Park, J.Y.; Han, S.H.; Anderson, A.J.; Yang, K.Y.; Gardener, B.M.S.; Kim, Y.C. Identification and transcriptional analysis of priming genes in Arabidopsis thaliana induced by root colonization with Pseudomonas chlororaphis O6. Plant Pathol. J. 2011, 27, 272–279. [Google Scholar] [CrossRef] [Green Version]
- Baek, D.; Rokibuzzaman, M.; Khan, A.; Kim, M.C.; Park, H.J.; Yun, D.J.; Chung, Y.R. Plant-growth promoting Bacillus oryzicola YC7007 modulates stress-response gene expression and provides protection from salt stress. Front. Plant Sci. 2020, 9, 1646. [Google Scholar] [CrossRef] [Green Version]
- Akram, W.; Aslam, H.; Rashid Ahmad, S.; Anjum, T.; Ahmad Yasin, N.; Ullah Khan, W.; Ahmad, A.; Guo, J.; Wu, T.; Luo, W.; et al. Bacillus megaterium strain A12 ameliorates salinity stress in tomato plants through multiple mechanisms. J. Plant Interact. 2019, 14, 506–518. [Google Scholar] [CrossRef] [Green Version]
- Mahdi, I.; Fahsi, N.; Hafidi, M.; Allaoui, A.; Biskri, L. Plant Growth Enhancement using Rhizospheric Halotolerant Phosphate Solubilizing Bacterium Bacillus licheniformis QA1 and Enterobacter asburiae QF11 Isolated from Chenopodium quinoa Willd. Microorganisms 2020, 8, 948. [Google Scholar] [CrossRef]
- Møller, I.S.; Tester, M. Salinity tolerance of Arabidopsis: A good model for cereals? Trends Plant Sci. 2007, 12, 534–540. [Google Scholar] [CrossRef]
- Xu, W.-F.; Shi, W.-M.; Ueda, A.; Takabe, T. Mechanisms of Salt Tolerance in Transgenic Arabidopsis thaliana Carrying a Peroxisomal Ascorbate Peroxidase Gene from Barley. Pedosphere 2008, 18, 486–495. [Google Scholar] [CrossRef]
- Guetsky, R.; Elad, Y.; Shtienberg, D.; Dinoor, A. Improved biocontrol of Botrytis cinerea on detached strawberry leaves by adding nutritional supplements to a mixture of Pichia guilermondii and Bacillus mycoides. Biocontrol Sci. Technol. 2002, 12, 625–630. [Google Scholar] [CrossRef]
- Garcia, R.A.M.; Ten Hoopen, G.M.; Kass, D.C.; Garita, V.A.S.; Krauss, U. Evaluation of mycoparasites as biocontrol agents of Rosellinia root rot in cocoa. Biol. Control. 2003, 27, 210–227. [Google Scholar] [CrossRef]
- Kelsic, E.D.; Zhao, J.; Vetsigian, K.; Kishony, R. Counteraction of antibiotic production and degradation stabilizes microbial communities. Nature 2015, 521, 516–519. [Google Scholar] [CrossRef] [Green Version]
- Kamou, N.N.; Dubey, M.; Tzelepis, G.; Menexes, G.; Papadakis, E.N.; Karlsson, M.; Lagopodi, A.L.; Jensen, D.F. Investigating the compatibility of the biocontrol agent Clonostachys rosea IK726 with prodigiosin-producing Serratia rubidaea S55 and phenazine-producing Pseudomonas chlororaphis ToZa7. Arch. Microbiol. 2016, 198, 369–377. [Google Scholar] [CrossRef]
- Castanheira, N.L.; Dourado, A.C.; Pais, I.; Semedo, J.; Scotti-Campos, P.; Borges, N.; Carvalho, G.; Barreto Crespo, M.T.; Fareleira, P. Colonization and beneficial effects on annual ryegrass by mixed inoculation with plant growth promoting bacteria. Microbiol. Res. 2017, 198, 47–55. [Google Scholar] [CrossRef]
- Pangesti, N.; Vandenbrande, S.; Pineda, A.; Dicke, M.; Raaijmakers, J.M.; Van Loon, J.J. Antagonism between two root-associated beneficial Pseudomonas strains does not affect plant growth promotion and induced resistance against a leaf-chewing herbivore. FEMS Microbiol. Ecol. 2017, 93, fix038. [Google Scholar] [CrossRef] [Green Version]
- Santiago, C.D.; Yagi, S.; Ijima, M.; Nashimoto, T.; Sawada, M.; Ikeda, S.; Asano, K.; Orikasa, Y.; Ohwada, T. Bacterial compatibility in combined inoculations enhances the growth of potato seedlings. Microbes Environ. 2017, 32, 14–23. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; McInroy, J.A.; Hu, C.H.; Kloepper, J.W. Mixtures of plant-growth-promoting rhizobacteria enhance biological control of multiple plant diseases and plant-growth promotion in the presence of pathogens. Plant Dis. 2018, 102, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Jousset, A.; Becker, J.; Chatterjee, S.; Karlovsky, P.; Scheu, S.; Eisenhauer, N. Biodiversity and species identity shape the antifungal activity of bacterial communities. Ecology 2014, 95, 1184–1190. [Google Scholar] [CrossRef]
- Mehrabi, Z.; McMillan, V.E.; Clark, I.M.; Canning, G.; Hammond-Kosack, K.E.; Preston, G.; Hirsch, P.R.; Mauchline, T.H. Pseudomonas spp. diversity is negatively associated with suppression of the wheat take-all pathogen. Sci. Rep. 2016, 6, 29905. [Google Scholar] [CrossRef]
- Jetiyanon, K.; Fowler, W.D.; Kloepper, J.W. Broad-spectrum protection against several pathogens by PGPR mixtures under field conditions in Thailand. Plant Dis. 2003, 87, 1390–1394. [Google Scholar] [CrossRef] [Green Version]
- Gallardo-Navarro, Ó.A.; Santillán, M. Three-Way Interactions in an Artificial Community of Bacterial Strains Directly Isolated from the Environment and Their Effect on the System Population Dynamics. Front. Microbiol. 2019, 13, 2555. [Google Scholar] [CrossRef]
- Ali, S.; Hameed, S.; Shahid, M.; Iqbal, M.; Lazarovits, G.; Imran, A. Functional characterization of potential PGPR exhibiting broad-spectrum antifungal activity. Microbiol. Res. 2020, 232, 126389. [Google Scholar] [CrossRef]
- Aguirre-von-Wobeser, E.; Eguiarte, L.E.; Souza, V.; Soberón-Chávez, G. Theoretical analysis of the cost of antagonistic activity for aquatic bacteria in oligotrophic environments. Front. Microbiol. 2015, 6, 490. [Google Scholar] [CrossRef] [Green Version]
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shin, H.S.; Patra, J.K. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 2018, 206, 131–140. [Google Scholar] [CrossRef]
- Pagie, L.; Hogeweg, P. Colicin diversity: A result of eco-evolutionary dynamics. J. Theor. Biol. 1999, 196, 251–261. [Google Scholar] [CrossRef] [Green Version]
- Frean, M.; Abraham, E.R. Rock–scissors–paper and the survival of the weakest. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2001, 268, 1323–1327. [Google Scholar] [CrossRef] [Green Version]



| Isolate | P Solubilization | Siderophore | Urease Activity | Identification Based on 16S rRNA | Accession Number | Antifungal Activity Inhibition Rate (IR) | IAA Production μg/mL |
|---|---|---|---|---|---|---|---|
| 1.SG.7 | - | + | + | Bacillus sp. | LR027398 | 56.5 | 2.35 ± 0.6 |
| 2.SG.20 | + | + | + | P. koreensis | LR027423 | 50.2 | 18.25 ± 0.9 |
| 5.SG.3 | + | + | + | B. amyloliquefaciens | LR027456 | 62.9 | 28.35 ± 0.8 |
| 2.C.19 | + | + | + | P. moraviensis | LR027411 | 56.5 | 25.15 ± 0.5 |
| 3.SG.19 | + | + | + | P. fluorescens | LR027436 | 53.9 | 15.41 ± 0.2 |
| 2.C.23 | + | + | + | P. fluorescens | LR027412 | 55.6 | 22.61 ± 0.8 |
| 4.SG.6 | + | + | + | P. koreensis | LR027448 | 54.5 | 20.16 ± 0.9 |
| 2.SG.8 | + | + | + | P. koreensis | LR027414 | 52.8 | 11.2 ± 0.9 |
| [48] | present study | ||||||
| Isolate | 1.SG.7 | 2.SG.20 | 5.SG.3 | 2.C.19 | 3.SG.19 | 2.C.23 | 4.SG.6 | 2.SG.8 |
|---|---|---|---|---|---|---|---|---|
| 1.SG.7 | + | + | + | + | + | - | + | + |
| 2.SG.20 | + | + | + | + | + | - | + | + |
| 5.SG.3 | + | + | + | + | + | - | + | + |
| 2.C.19 | + | + | + | + | + | + | + | + |
| 3.SG.19 | + | + | + | + | + | + | + | - |
| 2.C.23 | - | - | - | + | + | + | + | + |
| 4.SG.6 | + | + | + | + | + | + | + | + |
| 2.SG.8 | + | + | + | - | + | + | + | + |
| Isolate | Biocontrol Activity | IAA Production | Plant Growth Promotion | Plant Growth Promotion in Salinity | Lateral Roots | Strain Salinity Tolerance | Temperature Tolerance | Biofilm Formation | Compatibility in Dyads |
|---|---|---|---|---|---|---|---|---|---|
| 1.SG.7 | |||||||||
| 2.SG.20 | |||||||||
| 5.SG.3 | |||||||||
| 2.C.19 | |||||||||
| 3.SG.19 | |||||||||
| 2.C.23 | |||||||||
| 4.SG.6 | |||||||||
| 2.SG.8 | |||||||||
| strong | |||||||||
| medium | |||||||||
| low | |||||||||
| not applicable | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venieraki, A.; Chorianopoulou, S.N.; Katinakis, P.; Bouranis, D.L. Multi-Trait Wheat Rhizobacteria from Calcareous Soil with Biocontrol Activity Promote Plant Growth and Mitigate Salinity Stress. Microorganisms 2021, 9, 1588. https://doi.org/10.3390/microorganisms9081588
Venieraki A, Chorianopoulou SN, Katinakis P, Bouranis DL. Multi-Trait Wheat Rhizobacteria from Calcareous Soil with Biocontrol Activity Promote Plant Growth and Mitigate Salinity Stress. Microorganisms. 2021; 9(8):1588. https://doi.org/10.3390/microorganisms9081588
Chicago/Turabian StyleVenieraki, Anastasia, Styliani N. Chorianopoulou, Panagiotis Katinakis, and Dimitris L. Bouranis. 2021. "Multi-Trait Wheat Rhizobacteria from Calcareous Soil with Biocontrol Activity Promote Plant Growth and Mitigate Salinity Stress" Microorganisms 9, no. 8: 1588. https://doi.org/10.3390/microorganisms9081588






