Real-Time PCR for Molecular Detection of Zoonotic and Non-Zoonotic Giardia spp. in Wild Rodents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Material and Reference Sequences
2.2. DNA Extraction and Real-Time PCR
3. Results
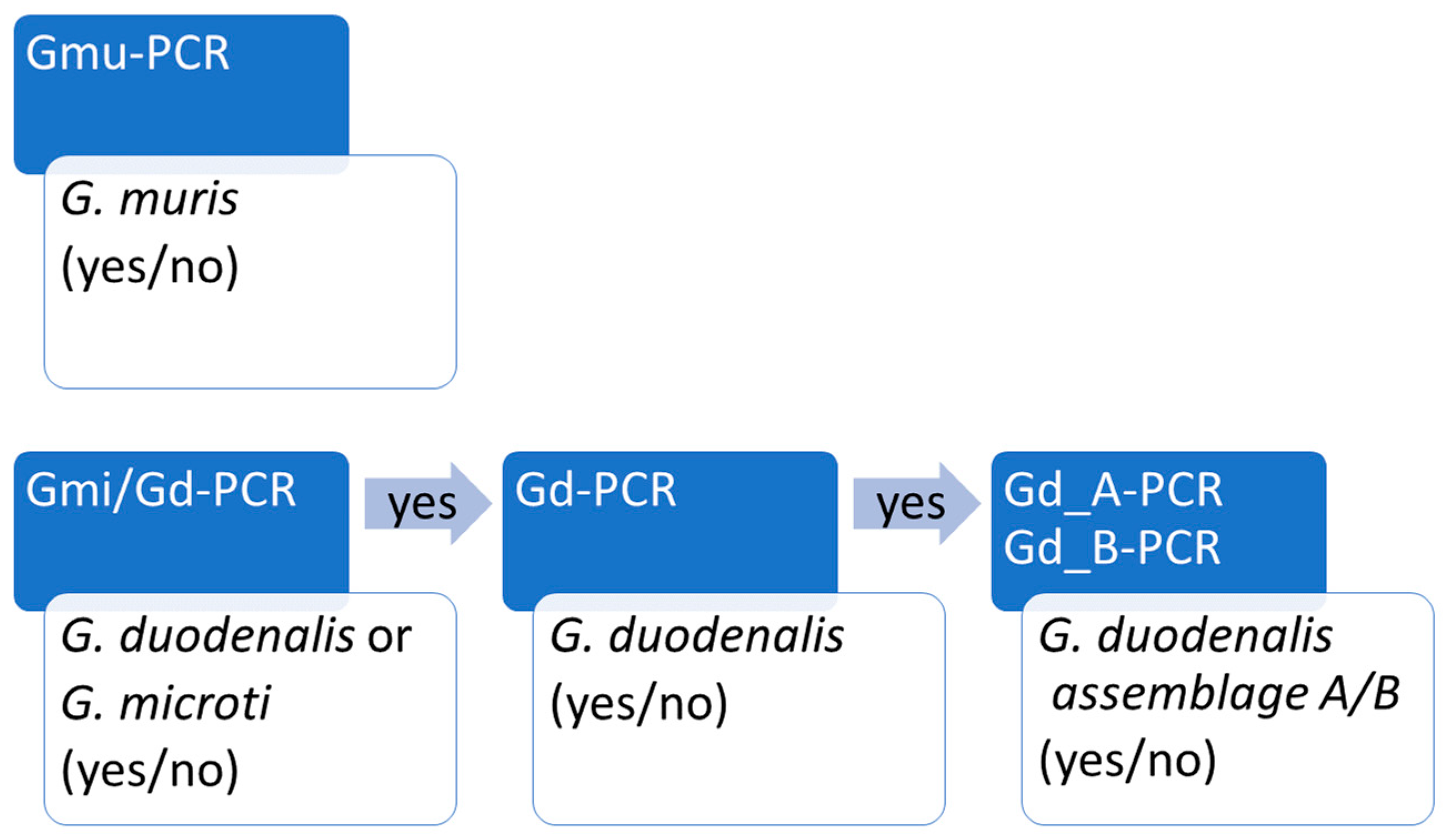
3.1. Development of Real Time PCR Workflow to Distinguish G. muris, G. microti and G. duodenalis
3.2. Specificity of Real-Time PCR Assays
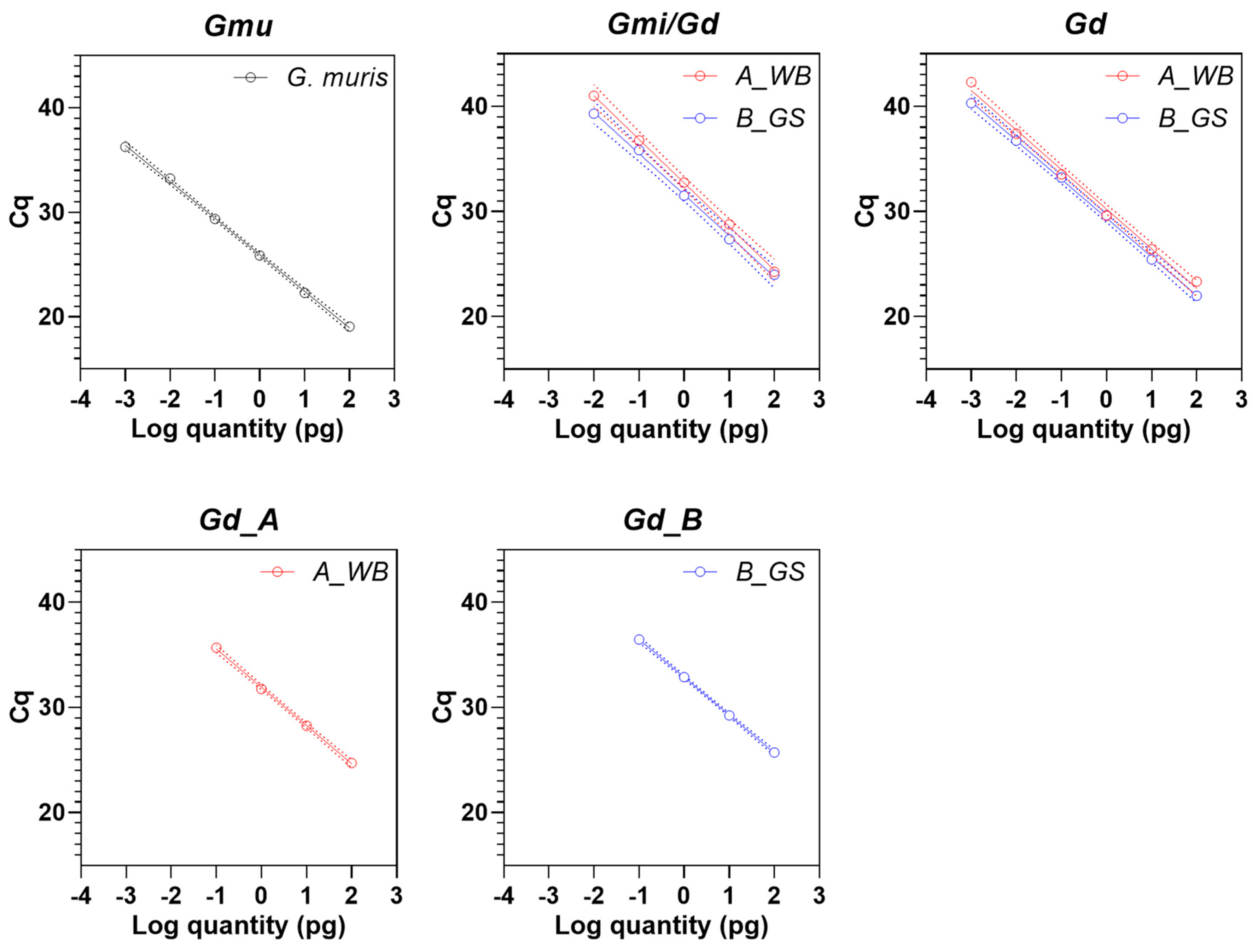
3.3. Analytical Sensitivity of Real-Time PCR Assays
3.4. Reanalysis of Known Giardia Positive Wild Rodent Samples Confirms Applicability of PCR Workflow for Detection of G. muris, G. microti and Zoonotic G. duodenalis Assemblages
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cacciò, S.M.; Lalle, M.; Svärd, S. Host specificity in the Giardia duodenalis species complex. Infect. Genet. Evol. 2018, 66, 335–345. [Google Scholar] [CrossRef]
- Ryan, U.; Cacciò, S.M. Zoonotic potential of Giardia. Int. J. Parasitol. 2013, 43, 943–956. [Google Scholar] [CrossRef]
- Monis, P.; Caccio, S.M.; Thompson, R.A. Variation in Giardia: Towards a taxonomic revision of the genus. Trends Parasitol. 2009, 25, 93–100. [Google Scholar] [CrossRef]
- Helmy, Y.A.; Spierling, N.G.; Schmidt, S.; Rosenfeld, U.M.; Reil, D.; Imholt, C.; Jacob, J.; Ulrich, R.G.; Aebischer, T.; Klotz, C. Occurrence and distribution of Giardia species in wild rodents in Germany. Parasites Vectors 2018, 11, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, A.R.C.; Monis, P.T. Taxonomy of giardia species. In Giardia: A Model Organism; Luján, H.D., Svärd, S., Eds.; Springer: Wien, Austria; New York, NY, USA, 2011; pp. 3–15. [Google Scholar]
- Feng, Y.; Xiao, L. Zoonotic potential and molecular epidemiology of giardia species and giardiasis. Clin. Microbiol. Rev. 2011, 24, 110–140. [Google Scholar] [CrossRef] [Green Version]
- Woschke, A.; Faber, M.; Stark, K.; Holtfreter, M.; Mockenhaupt, F.; Richter, J.; Regnath, T.; Sobottka, I.; Reiter-Owona, I.; Diefenbach, A.; et al. Suitability of current typing procedures to identify epidemiologically linked human Giardia duodenalis isolates. PLoS Negl. Trop. Dis. 2021, 15, e0009277. [Google Scholar] [CrossRef] [PubMed]
- Fayer, R.; Santín, M.; Trout, J.M.; DeStefano, S.; Koenen, K.; Kaur, T. Prevalence of Microsporidia, Cryptosporidium spp., and Giardia spp. in beavers (Castor canadensis) in Massachusetts. J. Zoo Wildl. Med. 2006, 37, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Galán-Puchades, M.; Trelis, M.; Sáez-Durán, S.; Cifre, S.; Gosálvez, C.; Sanxis-Furió, J.; Pascual, J.; Bueno-Marí, R.; Franco, S.; Peracho, V.; et al. One health approach to zoonotic parasites: Molecular detection of intestinal protozoans in an urban population of Norway rats, Rattus norvegicus, in Barcelona, Spain. Pathogens 2021, 10, 311. [Google Scholar] [CrossRef]
- Lecova, L.; Hammerbauerova, I.; Tumova, P.; Nohynkova, E. Companion animals as a potential source of Giardia intestinalis infection in humans in the Czech Republic—A pilot study. Vet Parasitol. Reg. Stud. Rep. 2020, 21, 100431. [Google Scholar] [CrossRef] [PubMed]
- Meerburg, B.G.; Singleton, G.; Kijlstra, A. Rodent-borne diseases and their risks for public health. Crit. Rev. Microbiol. 2009, 35, 221–270. [Google Scholar] [CrossRef]
- Padovan, D. Infectious Diseases of Wild Rodents; Corvus Publishing Company: Anacortes, WA, USA, 2006; 348p. [Google Scholar]
- Perec-Matysiak, A.; Buńkowska-Gawlik, K.; Zaleśny, G.; Hildebrand, J. Small rodents as reservoirs of Cryptosporidium spp. and Giardia spp. in south-western Poland. Ann. Agric. Environ. Med. 2015, 22, 1–5. [Google Scholar] [CrossRef]
- Erlandsen, S.L.; Sherlock, L.A.; Bemrick, W.J.; Ghobrial, H.; Jakubowski, W. Prevalence of Giardia spp. in beaver and muskrat populations in northeastern states and Minnesota: Detection of intestinal trophozoites at necropsy provides greater sensitivity than detection of cysts in fecal samples. Appl. Environ. Microbiol. 1990, 56, 31–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heitman, T.L.; Frederick, L.M.; Viste, J.R.; Guselle, N.J.; Morgan, U.M.; Thompson, R.C.; Olson, M.E. Prevalence of Giardia and Cryptosporidium and characterization of Cryptosporidium spp. isolated from wildlife, human, and agricultural sources in the North Saskatchewan River Basin in Alberta, Canada. Can. J. Microbiol. 2002, 48, 530–541. [Google Scholar] [CrossRef]
- Tsui, C.K.; Miller, R.; Uyaguari-Diaz, M.; Tang, P.; Chauve, C.; Hsiao, W.; Isaac-Renton, J.; Prystajecky, N. Beaver fever: Whole-genome characterization of waterborne outbreak and sporadic isolates to study the zoonotic transmission of giardiasis. mSphere 2018, 3, e00090-18. [Google Scholar] [CrossRef] [Green Version]
- Gherman, C.M.; Kalmár, Z.; Györke, A.; Mircean, V. Occurrence of Giardia duodenalis assemblages in farmed long-tailed chinchillas Chinchilla lanigera (Rodentia) from Romania. Parasites Vectors 2018, 11, 86. [Google Scholar] [CrossRef] [Green Version]
- Levecke, B.; Meulemans, L.; Dalemans, T.; Casaert, S.; Claerebout, E.; Geurden, T. Mixed Giardia duodenalis assemblage A, B, C and E infections in pet chinchillas (Chinchilla lanigera) in Flanders (Belgium). Vet. Parasitol. 2011, 177, 166–170. [Google Scholar] [CrossRef]
- Backhans, A.; Jacobson, M.; Hansson, I.; Lebbad, M.; Lambertz, S.T.; Gammelgård, E.; Saager, M.; Akande, O.; Fellström, C. Occurrence of pathogens in wild rodents caught on Swedish pig and chicken farms. Epidemiol. Infect. 2012, 141, 1885–1891. [Google Scholar] [CrossRef]
- Fernandez-Alvarez, A.; Martin-Alonso, A.; Abreu-Acosta, N.; Feliu, C.; Hugot, J.-P.; Valladares, B.; Foronda, P. Identification of a novel assemblage G subgenotype and a zoonotic assemblage B in rodent isolates of Giardia duodenalis in the Canary Islands, Spain. Parasitology 2013, 141, 206–215. [Google Scholar]
- Sulaiman, I.M.; Fayer, R.; Bern, C.; Gilman, R.H.; Trout, J.M.; Schantz, P.M.; Das, P.; Lal, A.A.; Xiao, L. Triosephosphate isomerase gene characterization and potential zoonotic transmission of Giardia duodenalis. Emerg. Infect. Dis. 2003, 9, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Van Keulen, H.; Feely, D.E.; Macechko, P.T.; Jarroll, E.L.; Erlandsen, S.L. The sequence of Giardia small subunit rRNA shows that voles and muskrats are parasitized by a unique species Giardia microti. J. Parasitol. 1998, 84, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, R.; Zhao, W.; Qi, M.; Zhao, J.; Zhang, L.; Li, J.; Liu, A. Genotyping and subtyping of Giardia and Cryptosporidium isolates from commensal rodents in China. Parasitology 2015, 142, 800–806. [Google Scholar] [CrossRef]
- Araujo, N.S.; Mundim, M.J.; Gomes, M.A.; Amorim, R.M.; Viana, J.C.; Queiroz, R.P.; Rossi, M.A.; Cury, M.C. Giardia duodenalis: Pathological alterations in gerbils, Meriones unguiculatus, infected with different dosages of trophozoites. Exp. Parasitol. 2008, 118, 449–457. [Google Scholar] [CrossRef]
- Bartelt, L.A.; Roche, J.; Kolling, G.; Bolick, D.; Noronha, F.; Naylor, C.; Hoffman, P.; Warren, C.; Singer, S.; Guerrant, R. Persistent G. lamblia impairs growth in a murine malnutrition model. J. Clin. Investig. 2013, 123, 2672–2684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fink, M.Y.; Shapiro, D.; Singer, S.M. Giardia lamblia: Laboratory maintenance, lifecycle induction, and infection of murine models. Curr. Protoc. Microbiol. 2020, 57, e102. [Google Scholar] [CrossRef]
- Pham, J.K.; Nosala, C.; Scott, E.Y.; Nguyen, K.F.; Hagen, K.D.; Starcevich, H.N.; Dawson, S.C. Transcriptomic profiling of high-density Giardia foci encysting in the murine proximal intestine. Front. Cell Infect. Microbiol. 2017, 7, 227. [Google Scholar] [CrossRef] [Green Version]
- Bajer, A. Between-year variation and spatial dynamics of Cryptosporidium spp. and Giardia spp. infections in naturally infected rodent populations. Parasitology 2008, 135, 1629–1649. [Google Scholar] [CrossRef] [PubMed]
- Caccio, S.M.; Beck, R.; Almeida, A.; Bajer, A.; Pozio, E. Identification of Giardia species and Giardia duodenalis assemblages by sequence analysis of the 5.8S rDNA gene and internal transcribed spacers. Parasitology 2010, 137, 919–925. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, R.M.; Meloni, B.P.; Groth, D.M.; Wetherall, J.D.; Reynoldson, J.A.; Thompson, R.C. Ribosomal RNA sequencing reveals differences between the genotypes of Giardia isolates recovered from humans and dogs living in the same locality. J. Parasitol. 1997, 83, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Lalle, M.; Pozio, E.; Capelli, G.; Bruschi, F.; Crotti, D.; Caccio, S.M. Genetic heterogeneity at the beta-giardin locus among human and animal isolates of Giardiaduodenalis and identification of potentially zoonotic subgenotypes. Int. J. Parasitol. 2005, 35, 207–213. [Google Scholar] [CrossRef]
- Read, C.; Walters, J.; Robertson, I.D.; Thompson, R.C. Correlation between genotype of Giardia duodenalis and diarrhoea. Int. J. Parasitol. 2002, 32, 229–231. [Google Scholar] [CrossRef]
- Sprong, H.; Caccio, S.M.; van der Giessen, J.W.; Zoopnet Network and Partners. Identification of zoonotic genotypes of Giardia duodenalis. PLoS Negl. Trop. Dis. 2009, 3, e558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, R.C.; Ash, A. Molecular epidemiology of Giardia and Cryptosporidium infections. Infect. Genet. Evol. 2016, 315–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keister, D.B. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans. R Soc. Trop. Med. Hyg. 1983, 77, 487–488. [Google Scholar] [CrossRef]
- Appelbee, A.J.; Frederick, L.M.; Heitman, T.L.; Olson, M.E. Prevalence and genotyping of Giardia duodenalis from beef calves in Alberta, Canada. Vet. Parasitol. 2003, 112, 289–294. [Google Scholar] [CrossRef]
- Minetti, C.; Lamden, K.; Durband, C.; Cheesbrough, J.; Fox, A.; Wastling, J.M. Determination of Giardia duodenalis assemblages and multi-locus genotypes in patients with sporadic giardiasis from England. Parasites Vectors 2015, 8, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pijnacker, R.; Mughini-Gras, L.; Heusinkveld, M.; Roelfsema, J.; van Pelt, W.; Kortbeek, T. Different risk factors for infection with Giardia lamblia assemblages A and B in children attending day-care centres. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 2005–2013. [Google Scholar] [CrossRef] [PubMed]
- Verweij, J.J.; Blange, R.A.; Templeton, K.; Schinkel, J.; Brienen, E.A.; van Rooyen, M.A.; van Lieshout, L.; Polderman, A.M. Simultaneous detection of Entamoeba histolytica, Giardia lamblia, and Cryptosporidium parvum in fecal samples by using multiplex real-time PCR. J. Clin. Microbiol. 2004, 42, 1220–1223. [Google Scholar] [CrossRef] [Green Version]
- Vanni, I.; Caccio, S.M.; van Lith, L.; Lebbad, M.; Svard, S.G.; Pozio, E.; Tosini, F. Detection of Giardia duodenalis assemblages A and B in human feces by simple, assemblage-specific PCR assays. PLoS Negl. Trop. Dis. 2012, 6, e1776. [Google Scholar] [CrossRef]
- Xu, F.; Jimenez-Gonzalez, A.; Einarsson, E.; Astvaldsson, A.; Peirasmaki, D.; Eckmann, L.; Andersson, J.O.; Svard, S.G.; Jerlstrom-Hultqvist, J. The compact genome of Giardia muris reveals important steps in the evolution of intestinal protozoan parasites. Microb. Genom. 2020, 6, e000402. [Google Scholar]
- Xu, F.; Jex, A.; Svard, S.G. A chromosome-scale reference genome for Giardia intestinalis WB. Sci. Data 2020, 7, 38. [Google Scholar] [CrossRef] [Green Version]
- Tumova, P.; Mazanek, L.; Lecova, L.; Dluhosova, J.; Typovska, H.; Kotrasova, V.; Tichackova, V.; Nohynkova, E. A natural zoonotic giardiasis: Infection of a child via Giardia cysts in pet chinchilla droppings. Parasitol. Int. 2018, 67, 759–762. [Google Scholar] [CrossRef]
- Pantchev, N.; Broglia, A.; Paoletti, B.; Vrhovec, M.G.; Bertram, A.; Nockler, K.; Caccio, S.M. Occurrence and molecular typing of Giardia isolates in pet rabbits, chinchillas, guinea pigs and ferrets collected in Europe during 2006–2012. Vet. Rec. 2014, 175, 18. [Google Scholar] [CrossRef] [Green Version]
- Soares, R.M.; de Souza, S.L.; Silveira, L.H.; Funada, M.R.; Richtzenhain, L.J.; Gennari, S.M. Genotyping of potentially zoonotic Giardia duodenalis from exotic and wild animals kept in captivity in Brazil. Vet. Parasitol. 2011, 180, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Kilonzo, C.; Li, X.; Vivas, E.J.; Jay-Russell, M.T.; Fernandez, K.L.; Atwill, E.R. Fecal shedding of zoonotic food-borne pathogens by wild rodents in a major agricultural region of the central California coast. Appl. Environ. Microbiol. 2013, 79, 6337–6344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uiterwijk, M.; Nijsse, R.; Kooyman, F.N.J.; Wagenaar, J.A.; Mughini-Gras, L.; Koop, G.; Ploeger, H.W. Comparing four diagnostic tests for Giardia duodenalis in dogs using latent class analysis. Parasites Vectors 2018, 11, 439. [Google Scholar] [CrossRef] [PubMed]
- Van Lith, L.; Soba, B.; Vizcaino, V.V.; Svard, S.; Sprong, H.; Tosini, F.; Pozio, E.; Caccio, S.M. A real-time assemblage-specific PCR assay for the detection of Giardia duodenalis assemblages A, B and E in fecal samples. Vet. Parasitol. 2015, 211, 28–34. [Google Scholar] [CrossRef] [PubMed]

| Target Species Specificity (Abbreviation of qPCR Assay) and Oligonucleotides | Oligonucleotide Sequence | Reference |
|---|---|---|
| G. muris (Gmu) | ||
| Gm80F | 5‘-GACGGCTCGGTACAACG-3‘ | This study |
| Gm169R | 5‘-CTCTTGAGCACTCGTCTTGG-3‘ | This study |
| Gm104P | FAM-5‘-ACCGGGGGTGAAGGCTAGACGG-3‘-BHQ1 | This study |
| G. microti/G. duodenalis (Gmi/Gd) | ||
| Giardia-80F | 5‘-GACGGCTCAGGACAACGGTT-3‘ | [39] |
| GiaR | 5‘-CTGCGTCACGCTGCTCG-3‘ | [32] |
| Giardia-127T | TexasRed-5’-CGGACACCGCTGGCAACCCGG-3’-BHQ2 | This study |
| G. duodenalis (Gd) | ||
| Giardia-127F | 5‘-CGGACACCGCTGGCAA-3‘ | This study |
| GiaR | 5‘-CTGCGTCACGCTGCTCG-3‘ | [32] |
| Giardia-152T | HEX-5‘-GCCCGCCCTTGCGCGCACG-3‘-BHQ2 | This study |
| G. duodenalis Assemblage A (Gd_A) | ||
| 4E1-HP-Af | 5’-AAAGAGATAGTTCGCGATGTC-3’ | [38,40] |
| 4E1-HP-Ar | 5’-ATTAACAAACAGGGAGACGTATG-3’ | [38,40] |
| 4E1-HP-Atp | VIC-5’-aggcacacggtttacaccg-3’-BHQ1 | [38] |
| G. duodenalis Assemblage B (Gd_B) | ||
| 4E1-HP-Bf | 5’-GAAGTCATCTCTGGGGCAAG-3’ | [38,40] |
| 4E1-HP-Br | 5’-GAAGTCTAGATAAACGTGTCGG-3’ | [38,40] |
| 4E1-HP-Btp | TexasRed-5’-TACACTGTTCGTATGACCACTGTCGATA-3’-BHQ2 | [38] |
| Sample Species | Material for DNA Extraction | PCR-Assay | ||||
|---|---|---|---|---|---|---|
| Gmu | Gmi/Gd | Gd | Gd_A | Gd_B | ||
| G. muris | Feces of lab mouse 1 | + | − | − | − | − |
| G. muris | Purified cysts | + | − | − | − | − |
| G. duodenalis A 2 (WB6) | In vitro culture | − | + | + | + | − |
| G. duodenalis B (GS) 2 | In vitro culture | − | + | + | − | + |
| G. duodenalis B (GS) 2 | Feces of lab mouse 1 | − | + | + | − | + |
| G. microti | Feces of wild bank vole 1 | − | + | − | − | − |
| Balamutia mandrilaris | In vitro culture | − | − | − | − | − |
| Entamoeba histolytica | In vitro culture | − | − | − | − | − |
| Toxoplasma gondii | In vitro culture | − | − | − | − | − |
| Leishmania donovani | In vitro culture | − | − | − | − | − |
| Rodent Species | Isolate # [4] | Previous Result [4] | qPCR-Assay (Cq-Value) | ||||
|---|---|---|---|---|---|---|---|
| Gmu | Gmi/Gd | Gd | Gd_A | Gd_B | |||
| Microtus agrestis | 118 | G. muris | 27.6 | ||||
| Apodemus agrarius | 220 | G. muris | 35.0 | ||||
| Apodemus agrarius | 243 | G. muris | 36.0 | ||||
| Apodemus agrarius | 301 | G. muris | 36.1 | 41.3 | 37.1 | ||
| Apodemus agrarius | 311 | G. muris | 32.5 | ||||
| Myodes glareolus | 328 | G. muris | 36.8 | ||||
| Myodes glareolus | 334 | G. muris | 27.9 | ||||
| Apodemus flavicollis | 385 | G. muris | 35.5 | ||||
| Apodemus flavicollis | 511 | G. muris | 30.1 | ||||
| Microtus arvalis | 443 | G. microti | 36.9 | ||||
| Myodes glareolus | 451 | G. microti | 36.6 | ||||
| Myodes glareolus | 516 | G. microti | 34.1 | ||||
| Microtus arvalis | 603 | G. microti | 34.8 | ||||
| Microtus arvalis | 495 | G. microti | 34.3 | ||||
| Microtus arvalis | 496 | G. microti | 30.9 | ||||
| Myodes glareolus | 502 | G. microti | 36.5 | ||||
| Myodes glareolus | 508 | G. microti | 29.1 | ||||
| Apodemus flavicollis | 518 | G. microti | 31.4 | ||||
| Apodemus flavicollis | 524 | G. microti | 31.1 | ||||
| Myodes glareolus | 559 | G. microti | 32.9 | ||||
| Myodes glareolus | 561 | G. microti | 29.7 | ||||
| Microtus arvalis | 566 | G. microti | 31.9 | ||||
| Microtus arvalis | 568 | G. microti | 31.9 | ||||
| Myodes glareolus | 041 | G. duodenalis Ass. A | |||||
| Myodes glareolus | 056 | G. duodenalis Ass. A | 40.2 | 39.6 | |||
| Apodemus sp. | 207 | G. duodenalis Ass. A | |||||
| Myodes glareolus | 340 | G. duodenalis Ass. B | 33.0 | 30.2 | 34.5 | ||
| Apodemus sylvaticus | 305 | Unknown 1 | |||||
| Apodemus flavicollis | 348 | Unknown 1 | 41.5 | ||||
| Apodemus flavicollis | 376 | Unknown 1 | |||||
| Apodemus sp. | 400 | Unknown 1 | |||||
| Apodemus flavicollis | 520 | Unknown 1 | |||||
| Apodemus agrarius | 576 | Unknown 1 | 32.2 | ||||
| Myodes glareolus | 554 | Unknown 1 | |||||
| Myodes glareolus | 555 | Unknown 1 | 31.1 | ||||
| Microtus arvalis | 567 | Unknown 1 | 35.8 | ||||
| Microtus arvalis | 569 | Unknown 1 | |||||
| Myodes glareolus | 591 | Unknown 1 | 36.2 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klotz, C.; Radam, E.; Rausch, S.; Gosten-Heinrich, P.; Aebischer, T. Real-Time PCR for Molecular Detection of Zoonotic and Non-Zoonotic Giardia spp. in Wild Rodents. Microorganisms 2021, 9, 1610. https://doi.org/10.3390/microorganisms9081610
Klotz C, Radam E, Rausch S, Gosten-Heinrich P, Aebischer T. Real-Time PCR for Molecular Detection of Zoonotic and Non-Zoonotic Giardia spp. in Wild Rodents. Microorganisms. 2021; 9(8):1610. https://doi.org/10.3390/microorganisms9081610
Chicago/Turabian StyleKlotz, Christian, Elke Radam, Sebastian Rausch, Petra Gosten-Heinrich, and Toni Aebischer. 2021. "Real-Time PCR for Molecular Detection of Zoonotic and Non-Zoonotic Giardia spp. in Wild Rodents" Microorganisms 9, no. 8: 1610. https://doi.org/10.3390/microorganisms9081610
APA StyleKlotz, C., Radam, E., Rausch, S., Gosten-Heinrich, P., & Aebischer, T. (2021). Real-Time PCR for Molecular Detection of Zoonotic and Non-Zoonotic Giardia spp. in Wild Rodents. Microorganisms, 9(8), 1610. https://doi.org/10.3390/microorganisms9081610





