Bonsecamin: A New Cyclic Pentapeptide Discovered through Heterologous Expression of a Cryptic Gene Cluster
Abstract
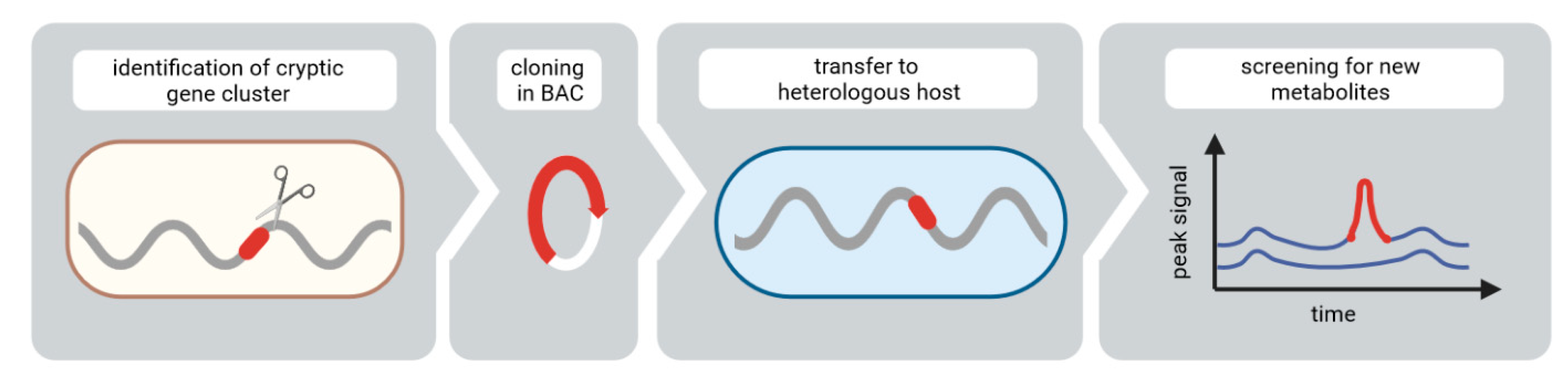
:1. Introduction
2. Materials and Methods
2.1. General Procedures
2.2. DNA Isolation and Manipulation
2.3. Metabolite Extraction
2.4. Mass Spectrometry (MS) Analysis of Metabolites
2.5. Extract Purification
2.6. Structure Elucidation by NMR Spectroscopy, Optical Rotation and Marfey’s Method
2.7. Genome Mining and Bioinformatic Analysis
3. Results and Discussion
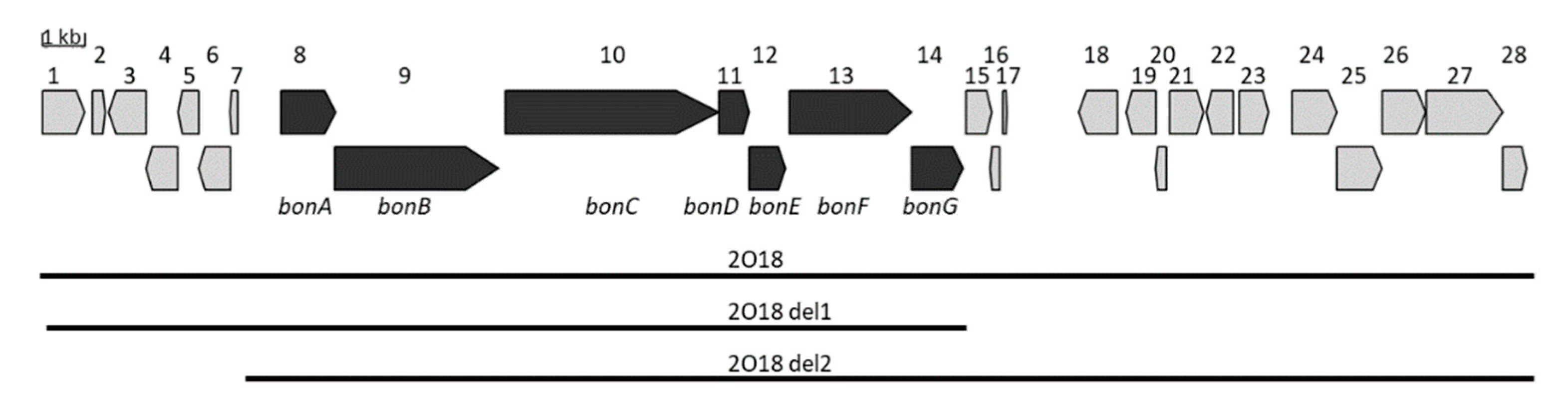
3.1. Identification and Expression of the NRPS Gene Cluster
3.2. Purification and Structure Elucidation
3.3. Determination of the Minimal Gene Cluster
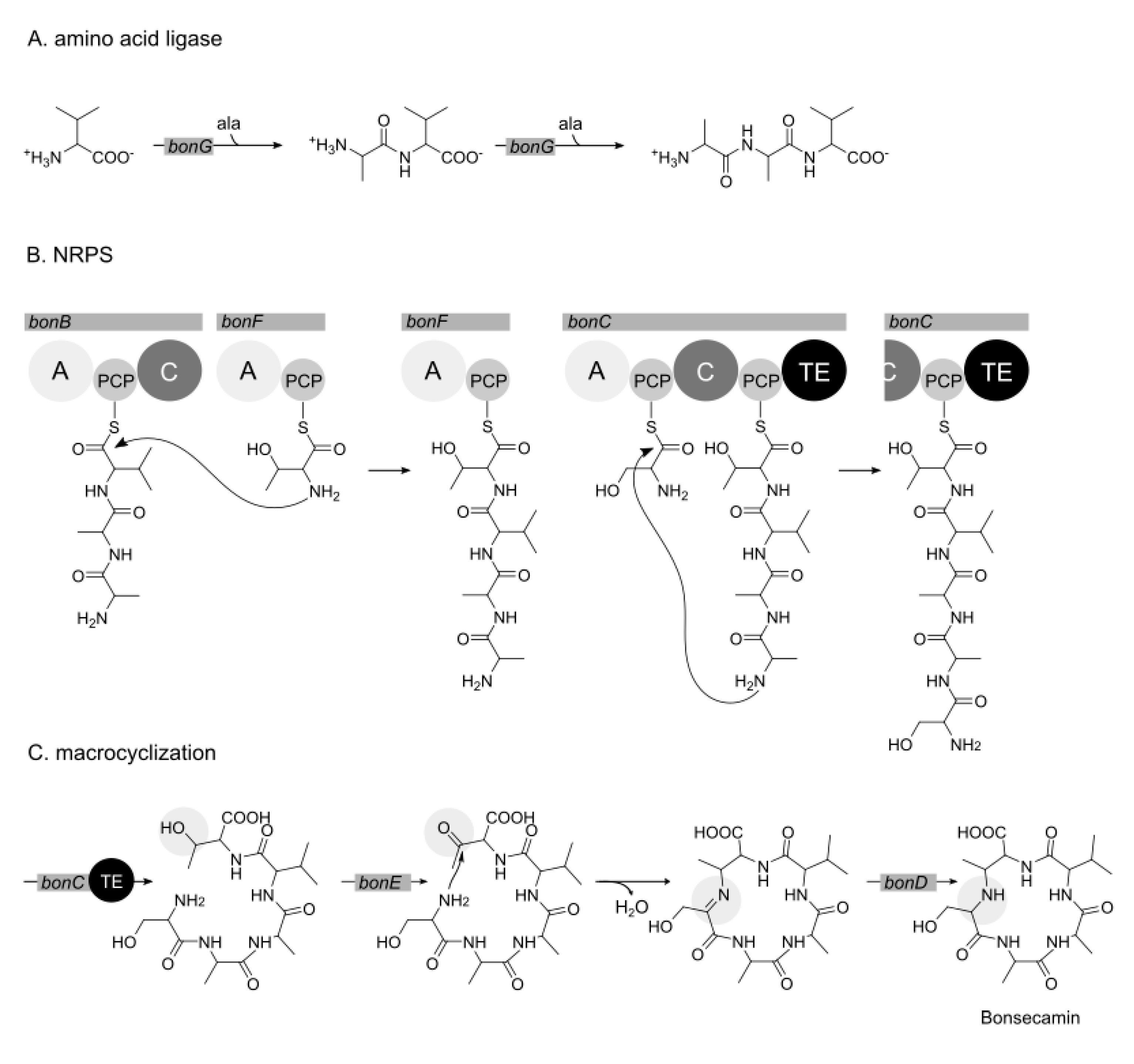
3.4. Biosynthesis of Bonsecamin
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohammadipanah, F.; Matasyoh, J.; Hamedi, J.; Klenk, H.-P.; Laatsch, H. Persipeptides A and B, two cyclic peptides from Streptomyces sp. UTMC 1154. Bioorg. Med. Chem. 2012, 20, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shaaban, K.A.; Elshahawi, S.I.; Ponomareva, L.V.; Sunkara, M.; Copley, G.C.; Hower, J.C.; Morris, A.J.; Kharel, M.K.; Thorson, J.S. Mullinamides A and B, new cyclopeptides produced by the Ruth Mullins coal mine fire isolate Streptomyces sp. RM-27-46. J. Antibiot. (Tokyo) 2014, 67, 571–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, W.-S.; Wang, J.-D.; Wang, X.-J.; Zhang, J. Bingchamides A and B, two novel cyclic pentapeptides from the Streptomyces bingchenggensis: Fermentation, isolation, structure elucidation and biological properties. J. Antibiot. (Tokyo) 2009, 62, 501–505. [Google Scholar] [CrossRef] [Green Version]
- Ji, Z.; Wei, S.; Fan, L.; Wu, W. Three novel cyclic hexapeptides from Streptomyces alboflavus 313 and their antibacterial activity. Eur. J. Med. Chem. 2012, 50, 296–303. [Google Scholar] [CrossRef]
- Umezawa, K.; Ikeda, Y.; Uchihata, Y.; Naganawa, H.; Kondo, S. Chloptosin, an apoptosis-inducing dimeric cyclohexapeptide produced by Streptomyces. J. Org. Chem. 2000, 65, 459–463. [Google Scholar] [CrossRef]
- Song, Y.; Li, Q.; Liu, X.; Chen, Y.; Zhang, Y.; Sun, A.; Zhang, W.; Zhang, J.; Ju, J. Cyclic Hexapeptides from the Deep South China Sea-Derived Streptomyces scopuliridis SCSIO ZJ46 Active Against Pathogenic Gram-Positive Bacteria. J. Nat. Prod. 2014, 77, 1937–1941. [Google Scholar] [CrossRef]
- Cai, G.; Napolitano, J.G.; McAlpine, J.B.; Wang, Y.; Jaki, B.U.; Suh, J.-W.; Yang, S.H.; Lee, I.-A.; Franzblau, S.G.; Pauli, G.F.; et al. Hytramycins V and I, Anti-Mycobacterium tuberculosis Hexapeptides from a Streptomyces hygroscopicus Strain. J. Nat. Prod. 2013, 76, 2009–2018. [Google Scholar] [CrossRef]
- He, H.; Williamson, R.T.; Shen, B.; Graziani, E.I.; Yang, H.Y.; Sakya, S.M.; Petersen, P.J.; Carter, G.T. Mannopeptimycins, Novel Antibacterial Glycopeptides from Streptomyces h ygroscopicus, LL-AC98. J. Am. Chem. Soc. 2002, 124, 9729–9736. [Google Scholar] [CrossRef]
- Dawson, R. The toxicology of microcystins. Toxicon 1998, 36, 953–962. [Google Scholar] [CrossRef]
- Raymond, K.N.; Dertz, E.A.; Kim, S.S. Enterobactin: An archetype for microbial iron transport. Proc. Natl. Acad. Sci. USA 2003, 100, 3584–3588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, M.P.; Petersen, P.J.; Weiss, W.J.; Janso, J.E.; Luckman, S.W.; Lenoy, E.B.; Bradford, P.A.; Testa, R.T.; Greenstein, M. Mannopeptimycins, new cyclic glycopeptide antibiotics produced by Streptomyces hygroscopicus LL-AC98: Antibacterial and mechanistic activities, Antimicrob. Agents Chemother. 2003, 47, 62–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turan, T.; Karacay, O.; Tulunay, G.; Boran, N.; Koc, S.; Bozok, S.; Kose, M.F. Results with EMA/CO (etoposide, methotrexate, actinomycin D, cyclophosphamide, vincristine) chemotherapy in gestational trophoblastic neoplasia. Int. J. Gynecol. Cancer 2006, 16, 1432–1438. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Manda, T.; Matsumoto, S.; Mukumoto, S.; Nishigaki, F.; Kawamura, I.; Shimomura, K. FR901228, a novel antitumor bicyclic depsipeptide produced by chromobacterium violaceum no. 968. III. Antitumor activities on experimental tumors in mice. J. Antibiot. (Tokyo) 1994, 47, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Borel, J.F.; Feurer, C.; Gubler, H.U.; Stähelin, H. Biological effects of cyclosporin A: A new antilymphocytic agent. Agents Actions 1976, 6, 468–475. [Google Scholar] [CrossRef]
- Kolata, G. FDA Speeds Approval of Cyclosporin. Science 1983, 221, 1273. [Google Scholar] [CrossRef]
- Sieber, S.A.; Marahiel, M.A. Learning from Nature’s Drug Factories: Nonribosomal Synthesisof MacrocyclicPeptides. J. Bacteriol. 2003, 185, 7036–7043. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez Estévez, M.; Gummerlich, N.; Myronovskyi, M.; Zapp, J.; Luzhetskyy, A. Benzanthric Acid, a Novel Metabolite From Streptomyces albus Del14 Expressing the Nybomycin Gene Cluster. Front. Chem. 2020, 7, 896. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M.R.; Myronovskyi, M.; Rosenkränzer, B.; Paululat, T.; Petzke, L.; Ristau, J.; Luzhetskyy, A. Novel fredericamycin variant overproduced by a streptomycin-resistant streptomyces albus subsp. Chlorinus Strain. Mar. Drugs 2020, 18, 284. [Google Scholar] [CrossRef]
- Lasch, C.; Stierhof, M.; Estévez, M.R.; Myronovskyi, M.; Zapp, J.; Luzhetskyy, A. Dudomycins: New Secondary Metabolites Produced after Heterologous Expression of an Nrps Cluster from Streptomyces albus ssp. Chlorinus Nrrl B-24108. Microorganisms 2020, 8, 1800. [Google Scholar] [CrossRef]
- Myronovskyi, M.; Rosenkränzer, B.; Stierhof, M.; Petzke, L.; Seiser, T.; Luzhetskyy, A. Identification and Heterologous Expression of the Albucidin Gene Cluster from the Marine Strain Streptomyces Albus Subsp. Chlorinus NRRL B-24108. Microorganisms 2020, 8, 237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez Estévez, M.; Myronovskyi, M.; Gummerlich, N.; Nadmid, S.; Luzhetskyy, A. Heterologous Expression of the Nybomycin Gene Cluster from the Marine Strain Streptomyces albus subsp. chlorinus NRRL B-24108. Mar. Drugs 2018, 16, 435. [Google Scholar] [CrossRef] [Green Version]
- Green, M.R.; Sambrook, J. Molecular Cloning: A Laboratory Manual, 4th ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2012. [Google Scholar]
- Kieser, T.; Bibb, M.J.; Buttner, M.J.; Chater, K.F.; Hopwood, D.A. Practical Streptomyces Genetics. A Laboratory Manual.; John Innes Foundation: Norwich, UK, 2000. [Google Scholar]
- Rebets, Y.; Kormanec, J.; Lutzhetskyy, A.; Bernaerts, K.; Anné, J. Cloning and expression of metagenomic dna in streptomyces lividans and subsequent fermentation for optimized production. Methods Mol. Biol. 2017, 1539, 99–144. [Google Scholar]
- Court, D.L.; Sawitzke, J.A.; Thomason, L.C. Genetic Engineering Using Homologous Recombination. Annu. Rev. Genet. 2002, 36, 361–388. [Google Scholar] [CrossRef]
- Medema, M.H.; Blin, K.; Cimermancic, P.; De Jager, V.; Zakrzewski, P.; Fischbach, M.A.; Weber, T.; Takano, E.; Breitling, R. antiSMASH: Rapid identification, annotation and analysis of secondary metabolite biosynthesis gene clusters in bacterial and fungal genome sequences. Nucleic Acids Res. 2011, 39, W339–W346. [Google Scholar] [CrossRef] [PubMed]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Myronovskyi, M.; Rosenkränzer, B.; Nadmid, S.; Pujic, P.; Normand, P.; Luzhetskyy, A. Generation of a cluster-free Streptomyces albus chassis strains for improved heterologous expression of secondary metabolite clusters. Metab. Eng. 2018, 49, 316–324. [Google Scholar] [CrossRef]
- Harrison, K.J.; Crécy-Lagard, V. de; Zallot, R. Gene Graphics: A genomic neighborhood data visualization web application. Bioinformatics 2018, 34, 1406–1408. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.B.; Walsh, C.T. Action and Timing of BacC and BacD in the Late Stages of Biosynthesis of the Dipeptide Antibiotic Bacilysin. Biochemistry 2013, 52, 889–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antle, V.D.; Liu, D.; McKellar, B.R.; Caperelli, C.A.; Hua, M.; Vince, R. Substrate Specificity of Glycinamide Ribonucleotide Synthetase from Chicken Liver. J. Biol. Chem. 1996, 271, 8192–8195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopp, F.; Mahlert, C.; Grünewald, J.; Marahiel, M.A. Peptide Macrocyclization: The Reductase of the Nostocyclopeptide Synthetase Triggers the Self-Assembly of a Macrocyclic Imine. J. Am. Chem. Soc. 2006, 128, 16478–16479. [Google Scholar] [CrossRef]
- Evans, B.S.; Ntai, I.; Chen, Y.; Robinson, S.J.; Kelleher, N.L. Proteomics-Based Discovery of Koranimine, a Cyclic Imine Natural Product. J. Am. Chem. Soc. 2011, 133, 7316–7319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zipperer, A.; Konnerth, M.C.; Laux, C.; Berscheid, A.; Janek, D.; Weidenmaier, C.; Burian, M.; Schilling, N.A.; Slavetinsky, C.; Marschal, M.; et al. Human commensals producing a novel antibiotic impair pathogen colonization. Nature 2016, 535, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Schracke, N.; Linne, U.; Mahlert, C.; Marahiel, M.A. Synthesis of Linear Gramicidin Requires the Cooperation of Two Independent Reductases†. Biochemistry 2005, 44, 8507–8513. [Google Scholar] [CrossRef] [PubMed]

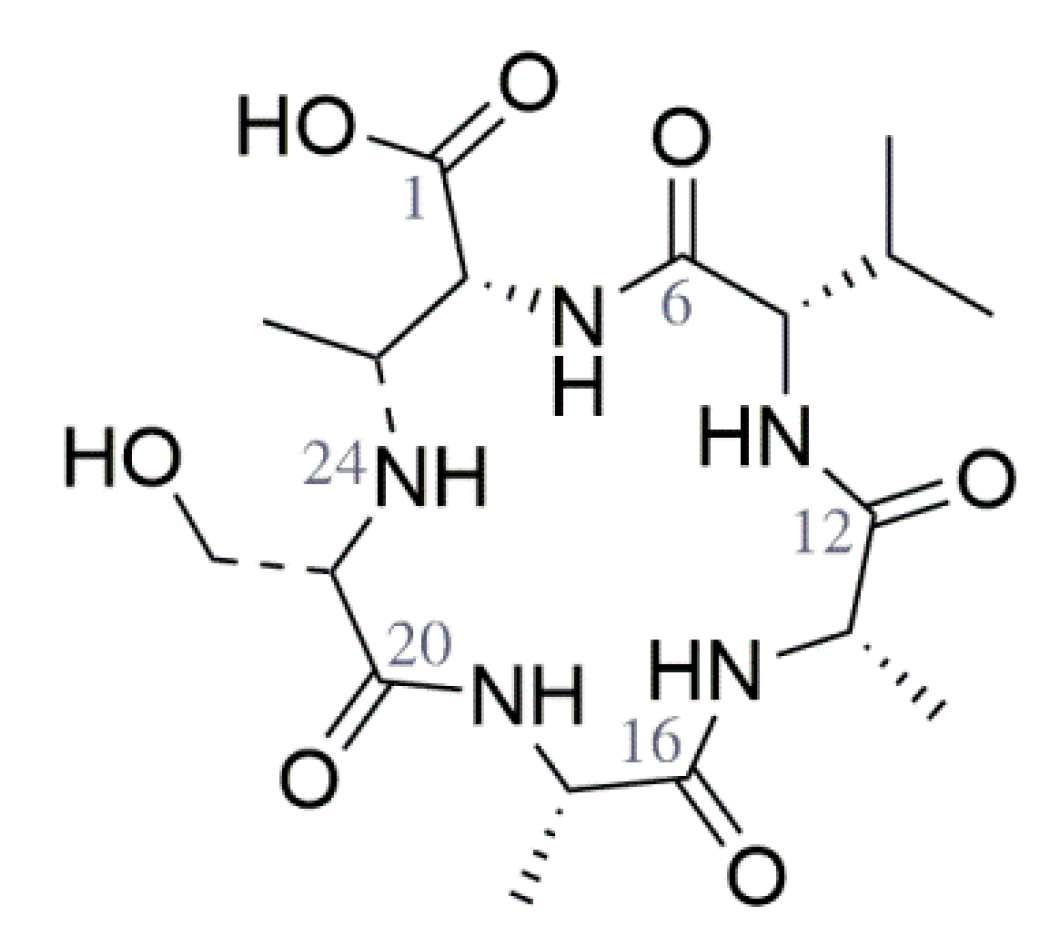
| Pos. | δC/δN Type | δH, mult. (J in Hz) | COSY | Key HMBC (H-) | |
|---|---|---|---|---|---|
| 2,3-DABA | 1 | 170.5*, C | |||
| 2 | 58.53, CH | 3.96, dd (7.5, 2.5) | 3, 5 | 1 | |
| 3 | 56.23, CH | 3.23, m | 2, 4 | 23 | |
| 4 | 13.41, CH3 | 0.52, d (6.3) | 3 | 2 | |
| 5 | 122.4, NH | 7.06, d (7.5) | 2 | 1, 6 | |
| Val | 6 | 169.6*, C | |||
| 7 | 58.40, CH | 4.01, t (9.1) | 8, 11 | 6, 12 | |
| 8 | 30.24, CH | 1.96, m | 7, 9, 10 | ||
| 9 | 18.39, CH3 | 0.83, d (7.0) | 8 | 7 | |
| 10 | 19.44, CH3 | 0.84, d (7.0) | 8 | 7 | |
| 11 | 114.5, NH | 7.84, d (9.1) | 7 | 12 | |
| Ala | 12 | 172.2*, C | |||
| 13 | 50.67, CH | 4.21, dq (8.6, 7.5) | 14, 15 | 12, 16 | |
| 14 | 17.46, CH3 | 1.32, d (7.5) | 13 | 12 | |
| 15 | 120.5, NH | 8.14, d (8.6) | 13 | 16 | |
| Ala | 16 | 169.8*, C | |||
| 17 | 50.56, CH | 4.08, quin (7.2) | 18, 19 | 16, 20 | |
| 18 | 17.23, CH3 | 1.24, d (7.2) | 17 | 16 | |
| 19 | 121.7, NH | 7.87, d (7.2) | 17 | 16 | |
| Ser | 20 | 172.9*, C | |||
| 21 | 62.38, CH | 2.80, m | 22, 24 | 3, 20 | |
| 22 | 61.04, CH2 | 3.44, m | 21, 23 | ||
| 3.57, m | |||||
| 23 | ----, OH | 5.01, m | 22 | ||
| 24 | 48.3, NH | 2.31, d (5.5) | 21 | 2, 4, 20, 21, 22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lasch, C.; Stierhof, M.; Estévez, M.R.; Myronovskyi, M.; Zapp, J.; Luzhetskyy, A. Bonsecamin: A New Cyclic Pentapeptide Discovered through Heterologous Expression of a Cryptic Gene Cluster. Microorganisms 2021, 9, 1640. https://doi.org/10.3390/microorganisms9081640
Lasch C, Stierhof M, Estévez MR, Myronovskyi M, Zapp J, Luzhetskyy A. Bonsecamin: A New Cyclic Pentapeptide Discovered through Heterologous Expression of a Cryptic Gene Cluster. Microorganisms. 2021; 9(8):1640. https://doi.org/10.3390/microorganisms9081640
Chicago/Turabian StyleLasch, Constanze, Marc Stierhof, Marta Rodríguez Estévez, Maksym Myronovskyi, Josef Zapp, and Andriy Luzhetskyy. 2021. "Bonsecamin: A New Cyclic Pentapeptide Discovered through Heterologous Expression of a Cryptic Gene Cluster" Microorganisms 9, no. 8: 1640. https://doi.org/10.3390/microorganisms9081640
APA StyleLasch, C., Stierhof, M., Estévez, M. R., Myronovskyi, M., Zapp, J., & Luzhetskyy, A. (2021). Bonsecamin: A New Cyclic Pentapeptide Discovered through Heterologous Expression of a Cryptic Gene Cluster. Microorganisms, 9(8), 1640. https://doi.org/10.3390/microorganisms9081640





