Effect of Lactococcus lactis Strain Plasma on HHV-6 and HHV-7 Shedding in Saliva: A Prospective Observational Study
Abstract
1. Introduction
2. Materials and Methods
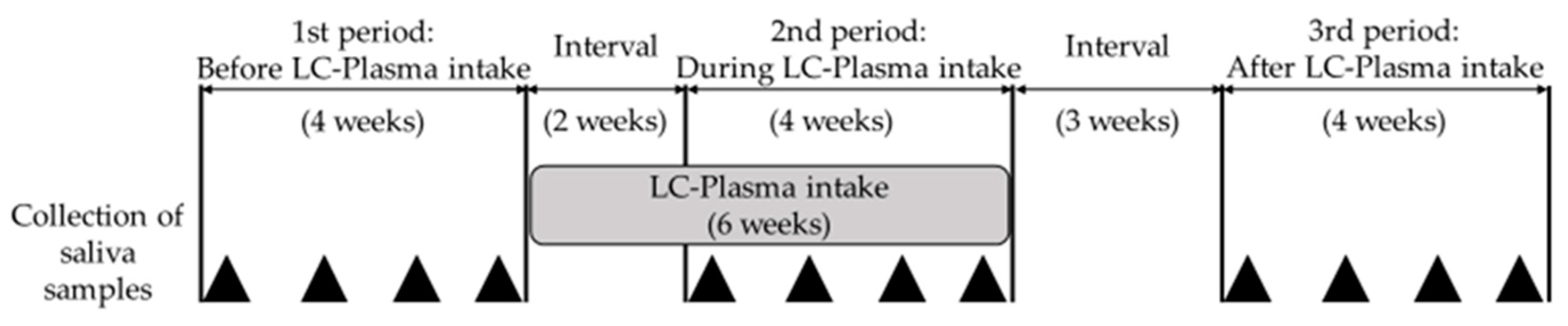
2.1. Study Design
2.2. DNA Extraction and Real-Time PCR
2.3. Statistical Analysis
2.4. Ethics Approval
3. Results
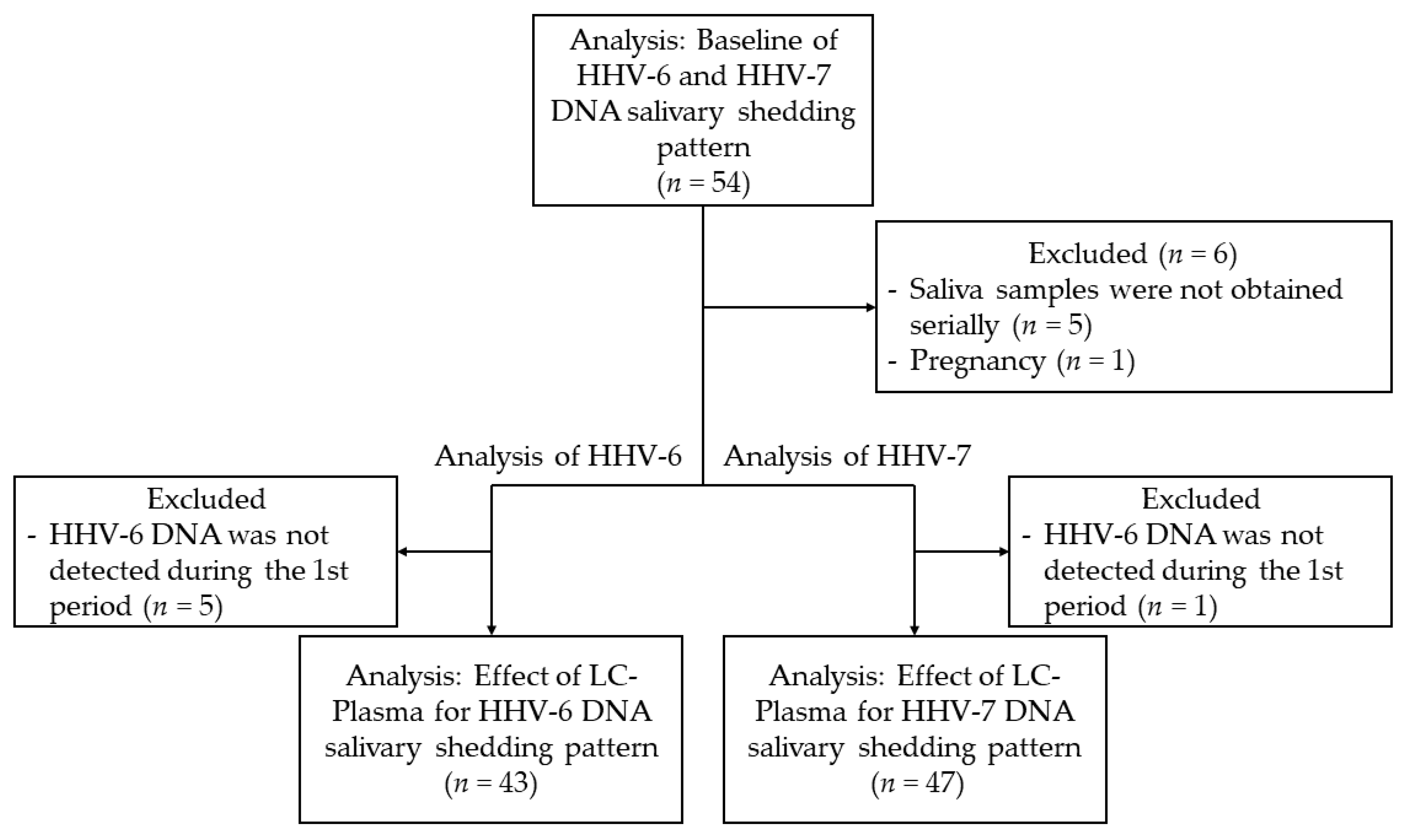
3.1. Study Subjects and Collected Samples
3.2. Baseline of HHV-6 and HHV-7 Shedding Patterns
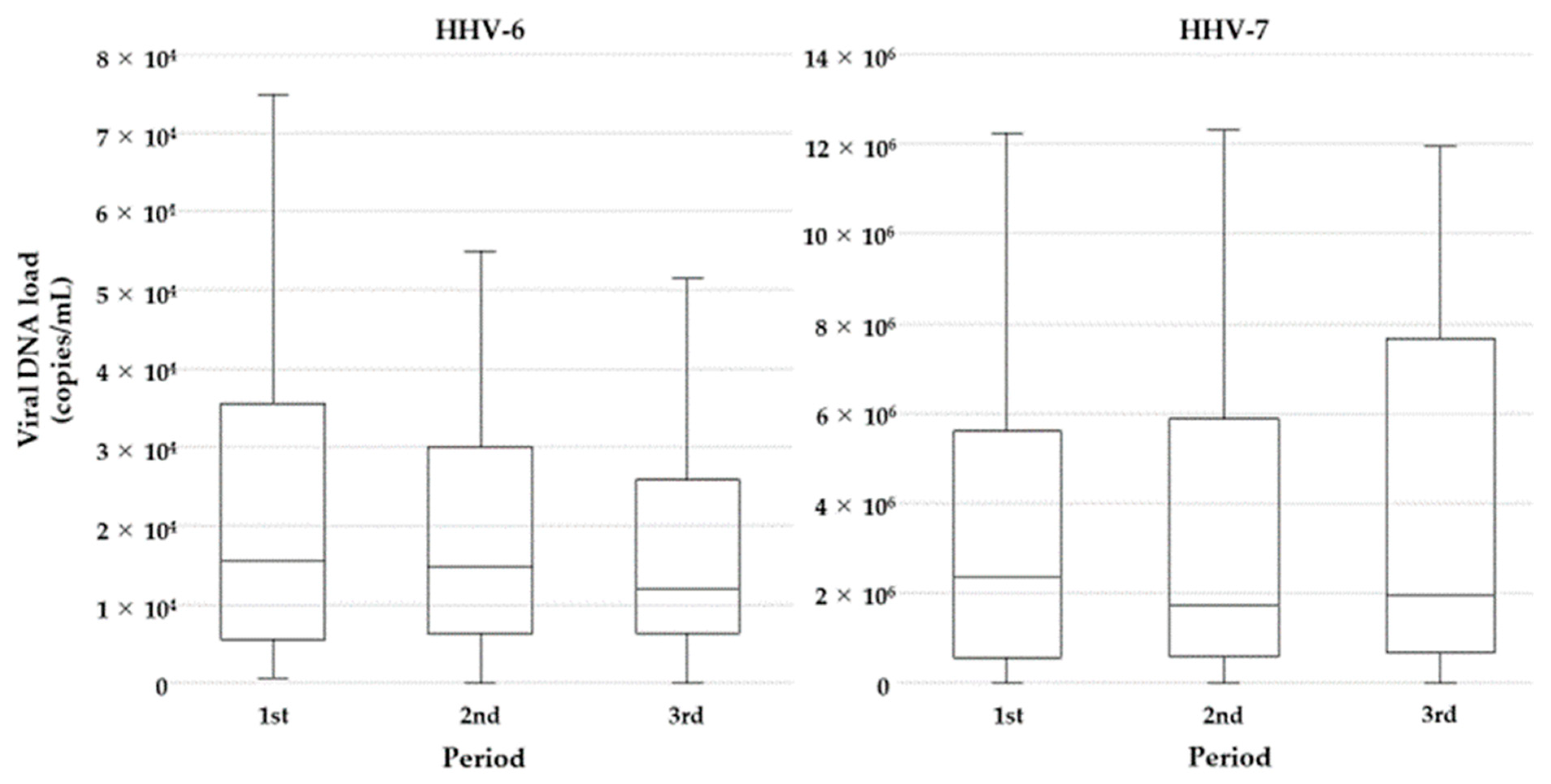
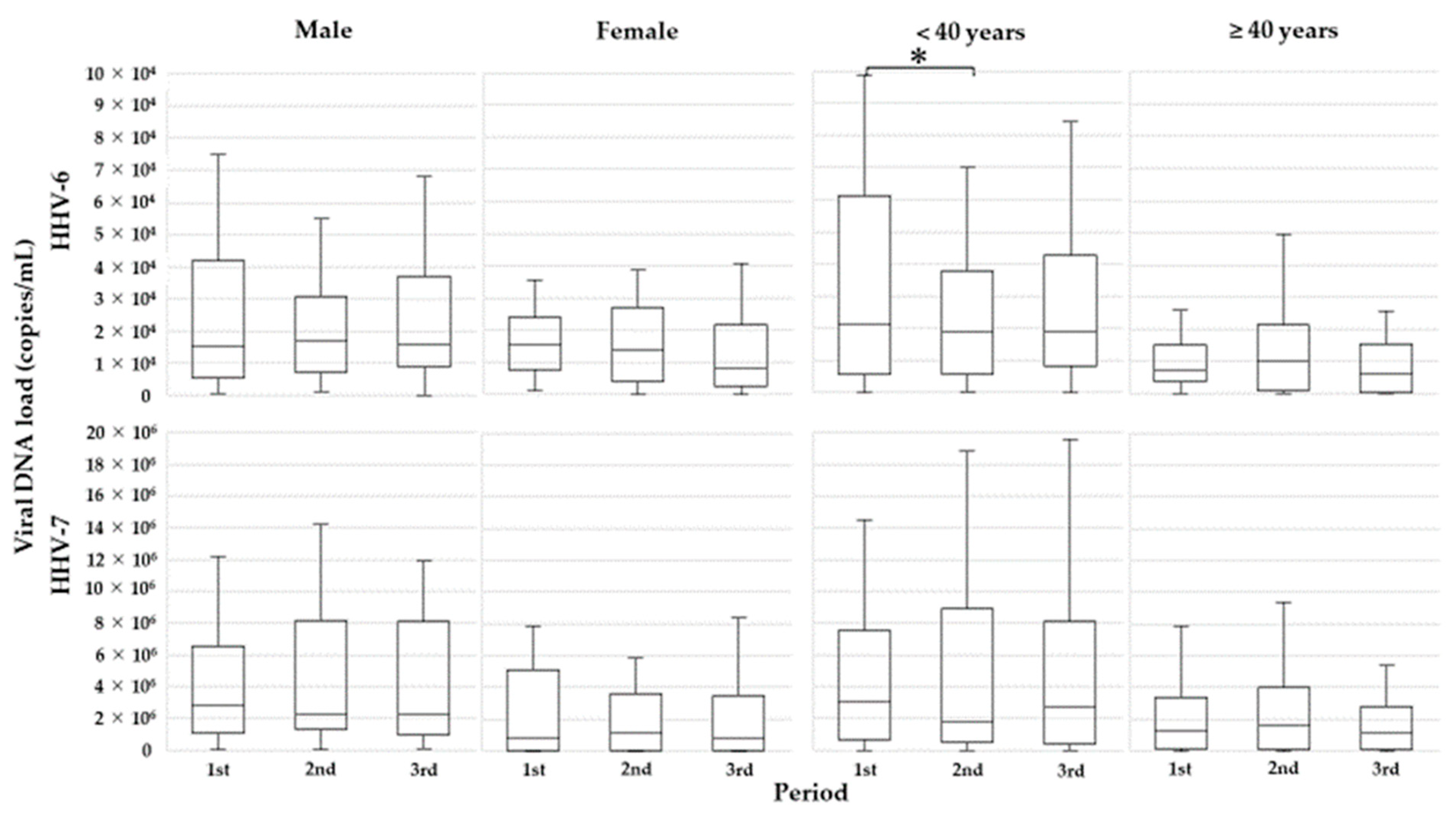
3.3. Effect of LC-Plasma Intake on Salivary Viral DNA Shedding
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yamanishi, K.; Shiraki, K.; Kondo, T.; Okuno, T.; Takahashi, M.; Asano, Y.; Kurata, T. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet 1988, 331, 1065–1067. [Google Scholar] [CrossRef]
- Asano, Y.; Suga, S.; Yoshikawa, T.; Urisu, A.; Yazaki, T. Human herpesvirus type 6 infection (exanthem subitum) without fever. J. Pediatr. 1989, 115, 264–265. [Google Scholar] [CrossRef]
- Suga, S.; Yoshikawa, T.; Nagai, T.; Asano, Y. Clinical Features and Virological Findings in Children with Primary Human Herpesvirus 7 Infection. Pediatrics 1997, 99, e4. [Google Scholar] [CrossRef]
- Sada, E.; Yasukawa, M.; Ito, C.; Takeda, A.; Shiosaka, T.; Tanioka, H.; Fujita, S. Detection of human herpesvirus 6 and human herpesvirus 7 in the submandibular gland, parotid gland, and lip salivary gland by PCR. J. Clin. Microbiol. 1996, 34, 2320–2321. [Google Scholar] [CrossRef]
- Harberts, E.; Yao, K.; Wohler, J.E.; Maric, D.; Ohayon, J.; Henkin, R.; Jacobson, S. Human herpesvirus-6 entry into the central nervous system through the olfactory pathway. Proc. Natl. Acad. Sci. USA 2011, 108, 13734–13739. [Google Scholar] [CrossRef]
- Kondo, K.; Yamanishi, K. HHV-6A, 6B, and 7: Molecular basis of latency and reactivation. In Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Arvin, A., Campadelli-Fiume, G., Mocarski, E., Eds.; Cambridge University Press: Cambridge, MA, USA, 2007; Chapter 47. [Google Scholar]
- Ihira, M.; Yoshikawa, T.; Ohashi, M.; Enomono, Y.; Akimoto, S.; Suga, S.; Saji, H.; Nishiyama, Y.; Asano, Y. Variation of Human Herpesvirus 7 Shedding in Saliva. J. Infect. Dis. 2003, 188, 1352–1354. [Google Scholar] [CrossRef]
- Kobayashi, N.; Nishiyama, T.; Yamauchi, T.; Shimada, K.; Suka, M.; Kondo, K.; Yanagisawa, H. Attenuation of human herpesvirus 6B reactivation by aging. J. Med. Virol. 2019, 91, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Collot, S.; Petit, B.; Bordessoule, D.; Alain, S.; Touati, M.; Denis, F.; Ranger-Rogez, S. Real-Time PCR for Quantification of Human Herpesvirus 6 DNA from Lymph Nodes and Saliva. J. Clin. Microbiol. 2002, 40, 2445–2451. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Namba, H.; Ohuchi, R.; Isomura, H.; Uno, F.; Yoshida, M.; Nii, S.; Yamada, M. Monitoring of human herpesvirus-6 and -7 genomes in saliva samples of healthy adults by competitive quantitative PCR. J. Med. Virol. 2000, 61, 208–213. [Google Scholar] [CrossRef]
- Lucht, E.; Brytting, M.; Bjerregaard, L.; Julander, I.; Linde, A. Shedding of cytomegalovirus and herpesviruses 6, 7, and 8 in saliva of human immunodeficiency virus type 1-infected patients and healthy controls. Clin. Infect. Dis. 1998, 27, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Ihira, M.; Taguchi, H.; Yoshida, S.; Asano, Y. Analysis of shedding of 3 beta-herpesviruses in saliva from patients with connective tissue diseases. J. Infect. Dis. 2005, 192, 1530–1536. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Namba, H.; Torigoe, S.; Watanabe, M.; Yamashita, N.; Ogawa, H.; Morishima, T.; Yamada, M. Monitoring of human herpesviruses-6 and -7 DNA in saliva samples during the acute and convalescent phases of exanthem subitum. J. Med. Virol. 2017, 89, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Aoki, R.; Kobayashi, N.; Suzuki, G.; Kuratsune, H.; Shimada, K.; Oka, N.; Takahashi, M.; Yamadera, W.; Iwashita, M.; Tokuno, S.; et al. Human herpesvirus 6 and 7 are biomarkers for fatigue, which distinguish between physiological fatigue and pathological fatigue. Biochem. Biophys. Res. Commun. 2016, 478, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Jounai, K.; Ikado, K.; Sugimura, T.; Ano, Y.; Braun, J.; Fujiwara, D. Spherical Lactic Acid Bacteria Activate Plasmacytoid Dendritic Cells Immunomodulatory Function via TLR9-Dependent Crosstalk with Myeloid Dendritic Cells. PLoS ONE 2012, 7, e32588. [Google Scholar] [CrossRef] [PubMed]
- Siegal, F.P.; Kadowaki, N.; Shodell, M.; Fitzgerald-Bocarsly, P.A.; Shah, K.; Ho, S.; Antonenko, S.; Liu, Y.-J. The Nature of the Principal Type 1 Interferon-Producing Cells in Human Blood. Science 1999, 284, 1835–1837. [Google Scholar] [CrossRef]
- Cervantes-Barragan, L.; Lewis, K.L.; Firner, S.; Thiel, V.; Hugues, S.; Reith, W.; Ludewig, B.; Reizis, B. Plasmacytoid dendritic cells control T-cell response to chronic viral infection. Proc. Natl. Acad. Sci. USA 2012, 109, 3012–3017. [Google Scholar] [CrossRef]
- Jounai, K.; Sugimura, T.; Ohshio, K.; Fujiwara, D. Oral Administration of Lactococcus lactis Subsp. lactis JCM5805 Enhances Lung Immune Response Resulting in Protection from Murine Parainfluenza Virus Infection. PLoS ONE 2015, 10, e0119055. [Google Scholar] [CrossRef]
- Jounai, K.; Sugimura, T.; Morita, Y.; Ohshio, K.; Fujiwara, D. Administration of Lactococcus lactis strain Plasma induces maturation of plasmacytoid dendritic cells and protection from rotavirus infection in suckling mice. Int. Immunopharmacol. 2018, 56, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Tsuji, R.; Sugamata, M.; Yamamoto, N.; Yamamoto, N.; Kanauchi, O. Administration of plasmacytoid dendritic cell-stimulative lactic acid bacteria is effective against dengue virus infection in mice. Int. J. Mol. Med. 2018, 43, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Wakai, S.; Fujiwara, D.; Kanauchi, O.; Jounai, K.; Ichikawa, H.; Takuma, M.; Kanaya, Y.; Shiraoka, R. Lactococcus lactis Strain Plasma Improves Subjective Physical State and Presenteeism: A Randomized, Open-Label Crossover Study among Healthy Office Workers. Prev. Nutr. Food Sci. 2020, 25, 140–145. [Google Scholar] [CrossRef]
- Komano, Y.; Shimada, K.; Naito, H.; Fukao, K.; Ishihara, Y.; Fujii, T.; Kokubo, T.; Daida, H. Efficacy of heat-killed Lactococcus lactis JCM 5805 on immunity and fatigue during consecutive high intensity exercise in male athletes: A randomized, placebo-controlled, double-blinded trial. J. Int. Soc. Sports Nutr. 2018, 15, 1–9. [Google Scholar] [CrossRef]
- Sugimura, T.; Jounai, K.; Ohshio, K.; Tanaka, T.; Suwa, M.; Fujiwara, D. Immunomodulatory effect of Lactococcus lactis JCM5805 on human plasmacytoid dendritic cells. Clin. Immunol. 2013, 149, 509–518. [Google Scholar] [CrossRef]
- Tanaka-Taya, K.; Kondo, T.; Mukai, T.; Miyoshi, H.; Yamamoto, Y.; Okada, S.; Yamanishi, K. Seroepidemiological study of human herpesvirus-6 and -7 in children of different ages and detection of these two viruses in throat swabs by polymerase chain reaction. J. Med. Virol. 1996, 48, 88–94. [Google Scholar] [CrossRef]
- Bartolini, L.; Piras, E.; Sullivan, K.; Gillen, S.; Bumbut, A.; Lin, C.-T.M.; Leibovitch, E.C.; Graves, J.S.; Waubant, E.L.; Chamberlain, J.M.; et al. Detection of HHV-6 and EBV and Cytokine Levels in Saliva From Children With Seizures: Results of a Multi-Center Cross-Sectional Study. Front. Neurol. 2018, 9, 834. [Google Scholar] [CrossRef]
- Tanaka, N.; Kimura, H.; Hoshino, Y.; Kato, K.; Yoshikawa, T.; Asano, Y.; Horibe, K.; Kojima, S.; Morishima, T. Monitoring four herpesviruses in unrelated cord blood transplantation. Bone Marrow Transpl. 2000, 26, 1193–1197. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Staheli, J.P.; Dyen, M.R.; Deutsch, G.H.; Basom, R.S.; Fitzgibbon, M.P.; Lewis, P.; Barcy, S. Complete Unique Genome Sequence, Expression Profile, and Salivary Gland Tissue Tropism of the Herpesvirus 7 Homolog in Pigtailed Macaques. J. Virol. 2016, 90, 6657–6674. [Google Scholar] [CrossRef]
- Zerr, D.M.; Corey, L.; Kim, H.W.; Huang, M.; Nguy, L.; Boeckh, M. Clinical Outcomes of Human Herpesvirus 6 Reactivation after Hematopoietic Stem Cell Transplantation. Clin. Infect. Dis. 2005, 40, 932–940. [Google Scholar] [CrossRef]
- Ogata, M.; Satou, T.; Kadota, J.-I.; Saito, N.; Yoshida, T.; Okumura, H.; Ueki, T.; Nagafuji, K.; Kako, S.; Uoshima, N.; et al. Human Herpesvirus 6 (HHV-6) Reactivation and HHV-6 Encephalitis After Allogeneic Hematopoietic Cell Transplantation: A Multicenter, Prospective Study. Clin. Infect. Dis. 2013, 57, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Rooney, B.V.; Crucian, B.E.; Pierson, D.L.; Laudenslager, M.L.; Mehta, S.K. Herpes Virus Reactivation in Astronauts During Spaceflight and Its Application on Earth. Front. Microbiol. 2019, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Vora, R.; Bernardo, D.; Durant, L.; Reddi, D.; Hart, A.L.; Fell, J.M.E.; Al-Hassi, H.O.; Knight, S.C. Age-related alterations in blood and colonic dendritic cell properties. Oncotarget 2016, 7, 11913–11922. [Google Scholar] [CrossRef]
- Sakata, K.; Sasaki, Y.; Jounai, K.; Fujii, T.; Fujiwara, D. Preventive Effect of Lactococcus lactis subsp. lactis JCM 5805 Yogurt Intake on Influenza Infection among Schoolchildren. Health 2017, 09, 756–762. [Google Scholar] [CrossRef]
- Yamamoto, S.; Ando, M.; Suzuki, E. High-Temperature Effects on Antibody Response to Viral Antigen in Mice. Exp. Anim. 1999, 48, 9–14. [Google Scholar] [CrossRef][Green Version]
- Hu, Y.; Jin, H.; Du, X.; Xiao, C.; Luo, D.; Wang, B.; She, R. Effects of Chronic Heat Stress on Immune Responses of the Foot-and-Mouth Disease DNA Vaccination. DNA Cell Biol. 2007, 26, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Osaki, T.; Morikawa, T.; Kajita, H.; Kobayashi, N.; Kondo, K.; Maeda, K. Caregiver burden and fatigue in caregivers of people with dementia: Measuring human herpesvirus (HHV)-6 and -7 DNA levels in saliva. Arch. Gerontol. Geriatr. 2016, 66, 42–48. [Google Scholar] [CrossRef] [PubMed]
| HHV-6 | HHV-7 | |||||
|---|---|---|---|---|---|---|
| Overall n (%) | DNA Load (Copies/mL) Median/SD | p-Value | DNA Load (Copies/mL) Median/SD | p-Value | ||
| Sex | Male | 27 (50.0) | 14,888/5494–32,356 | 0.170 | 2,676,363/1,425,588–5,941,663 | 0.018 |
| Female | 27 (50.0) | 9300/582–18,925 | 552,575/50,469–3,915,838 | |||
| Age | <40 years | 31 (57.4) | 19,738/5519–45,049 | 0.018 | 2,676,363/436,688–6,437,419 | 0.059 |
| ≥40 years | 23 (42.6) | 6638/3231–14,869 | 881,688/104,869–2,808,094 | |||
| Night shift | Yes | 20 (37.0) | 14,369/3391–30,722 | 0.667 | 2,656,950/1,311,269–5,520,622 | 0.234 |
| No | 34 (63.0) | 9506/3850–22,609 | 1,045,719/44,197–4,445,647 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miura, H.; Ihira, M.; Kozawa, K.; Kawamura, Y.; Higashimoto, Y.; Hattori, F.; Yoshikawa, T. Effect of Lactococcus lactis Strain Plasma on HHV-6 and HHV-7 Shedding in Saliva: A Prospective Observational Study. Microorganisms 2021, 9, 1683. https://doi.org/10.3390/microorganisms9081683
Miura H, Ihira M, Kozawa K, Kawamura Y, Higashimoto Y, Hattori F, Yoshikawa T. Effect of Lactococcus lactis Strain Plasma on HHV-6 and HHV-7 Shedding in Saliva: A Prospective Observational Study. Microorganisms. 2021; 9(8):1683. https://doi.org/10.3390/microorganisms9081683
Chicago/Turabian StyleMiura, Hiroki, Masaru Ihira, Kei Kozawa, Yoshiki Kawamura, Yuki Higashimoto, Fumihiko Hattori, and Tetsushi Yoshikawa. 2021. "Effect of Lactococcus lactis Strain Plasma on HHV-6 and HHV-7 Shedding in Saliva: A Prospective Observational Study" Microorganisms 9, no. 8: 1683. https://doi.org/10.3390/microorganisms9081683
APA StyleMiura, H., Ihira, M., Kozawa, K., Kawamura, Y., Higashimoto, Y., Hattori, F., & Yoshikawa, T. (2021). Effect of Lactococcus lactis Strain Plasma on HHV-6 and HHV-7 Shedding in Saliva: A Prospective Observational Study. Microorganisms, 9(8), 1683. https://doi.org/10.3390/microorganisms9081683






