Comparative Genome Analysis Provides Molecular Evidence for Reclassification of the Photosynthetic Bacterium Rhodobacter sphaeroides EBL0706 as a Strain of Luteovulum azotoformans
Abstract
1. Introduction
2. Materials and Methods
2.1. Medium and Growth Conditions
2.2. Sequencing and Genome Assembly
2.3. Phylogenetic Tree
2.4. Sequence-Based Methods for Species Circumscription
2.5. Comparative Genomics
3. Results and Discussion
3.1. Genome Assemblies and Features
3.2. Photosynthetic Genes
3.3. Phylogenetic Analysis
3.4. ANI, TETRA, and dDDH Analyses
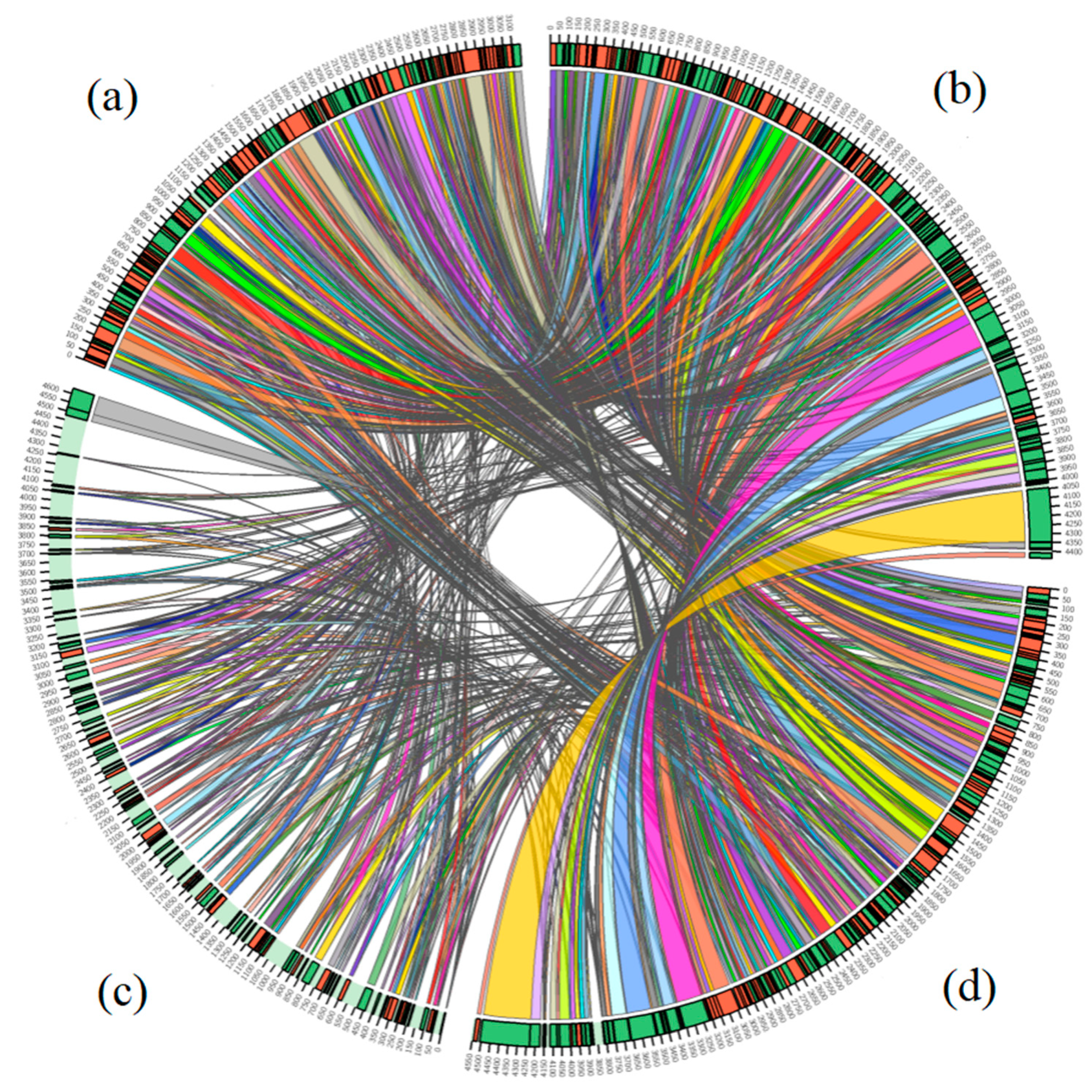
3.5. Comparative Genome Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pemberton, J.; Horne, I.M.; McEwan, A. Regulation of photosynthetic gene expression in purple bacteria. Microbiology 1998, 144, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Licht, M.; Nuss, A.; Volk, M.; Konzer, A.; Beckstette, M.; Berghoff, B.; Klug, G. Adaptation to photooxidative stress: Common and specialstrategies of the Alphaproteobacteria Rhodobacter sphaeroides and Rhodobacter capsulatus. Microorganisms 2020, 8, 283. [Google Scholar] [CrossRef]
- Yamaoka, Y.; Takeno, K.; Shinkawa, H.; Noparatnarapopn, N.; Sasaki, K. Isolation of a thermotolerant photosynthetic bacterium, Rhodobacter sphaeroides strain, NAT, and its capacity for oil and chemical oxygen demand removal at high temperatures. Biosci. Biotechnol. Biochem. 2008, 72, 1601–1603. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, C.; Eraso, J.M.; Choudhary, M.; Roh, J.; Zeng, X.; Bruscella, P.; Puskas, A.; Kaplan, S. Postgenomic adventures with Rhodobacter sphaeroides. Annu Rev. Microbiol. 2007, 61, 283–307. [Google Scholar] [CrossRef]
- Gabrielyan, L.; Hakobyan, L.; Trchounian, A. The Rhodobacter sphaeroides F0F1-ATPase activity: Effects of heavy metal ions and relationship with hydrogen photoproduction. Energy 2012, 1817, S133. [Google Scholar] [CrossRef][Green Version]
- Suresh, G.; Lodha, T.; Indu, B.; Sasikala, C.; Ramana, C. Taxogenomics resolves conflict in the genus Rhodobacter: A two and half decades pending thought to reclassify the genus Rhodobacter. Front. Microbiol. 2020, 11, 1111. [Google Scholar] [CrossRef]
- Nie, X.; Remes, B.; Klug, G. Multiple sense and antisense promoters contribute to the regulated expression of the isc-suf operon for iron-sulfur cluster assembly in Rhodobacter. Microorganisms 2019, 7, 671. [Google Scholar] [CrossRef]
- Masuda, S.; Bauer, C. AppA is a blue light photoreceptor that antirepresses photosynthesis gene expression in Rhodobacter sphaeroides. Cell 2002, 110, 613–623. [Google Scholar] [CrossRef]
- Gomelsky, M.; Horne, I.; Lee, H.; Pemberton, J.; McEwan, A.; Kaplan, S. Domain structure, oligomeric state, and mutational analysis of PpsR, the Rhodobacter sphaeroides repressor of photosystem gene expression. J. Bacteriol. 2000, 182, 2253–2261. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, A.; Liu, Z. Supramolecular structural basis of the Light-Harvesting process in plants. Prog. Biochem. Biophys. 2018, 45, 935–946. [Google Scholar]
- Li, Z.; Kong, L.; Hui, B.; Shang, X.; Gao, L.; Luan, N.; Zhuang, X.; Wang, D.; Bai, Z. Identification and antioxidant activity of carotenoids from superfine powder of Rhodobacter sphaeroides. Emir. J. Food Agric. 2017, 29, 833–845. [Google Scholar] [CrossRef]
- An, J.; Yang, C.; Li, Z.; Finn, P.; Perkins, D.; Sun, J.; Bai, Z.; Gao, L.; Zhang, M.; Ren, D. In vitro antioxidant activities of Rhodobacter sphaeroides and protective effect on Caco-2 cell line model. Appl. Microbiol. Biotechnol. 2019, 103, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, M.; Zhang, J.; Gao, L.; Hui, B.; Yang, W.; Wang, D.; Bai, Z. Conditions optimization of ultrasonic assisted with HCl extraction of carotenoid from Rhodobacter sphaeroides. J. Microbiol. 2014, 34, 19–24. [Google Scholar]
- Kong, L.; Li, Z.; Gao, Z.; Yang, W.; Wang, D.; Bai, Z. Optimization of fermentation conditions for carotenoids production by Rhodobacter sphaeroides. Indust. Microbiol. 2015, 45, 25–31. [Google Scholar]
- Ipekoglu, E.; Gocmen, K.; Oz, M.; Gurgan, M.; Yucel, M. Cloning and heterologous expression of chlorophyll a synthase in Rhodobacter sphaeroides. J. Basic Microbiol. 2017, 57, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lin, Y.; Qing, F.; Gao, L.; Hui, B.; Yang, W.; Wang, D.; Bai, Z. Optimization of fermentation technology for coenzyme Q10 production by Rhodobacter sphaeroides 3757. J. China Indust. Food Sci. Technol. 2014, 14, 124–129. [Google Scholar]
- Li, Z.; An, J.; Hui, B.; Gao, L.; Bai, Z.; Yang, W.; Wang, D. Effect of various extraction methods on the antioxidantactivity of superoxide dismutase from Rhodobacter sphaeroides. Mod. Food Sci. Technol. 2015, 31, 205–210. [Google Scholar]
- Pollich, M.; Wersig, C.; Klug, G. The bluF gene of Rhodobacter capsulatus is involved in conversion of cobinamide to cobalamin (vitamin B-12). J. Bacteriol. 1996, 178, 7308–7310. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, K.; Han, Q.; Jang, D.; Wang, D.; Li, Z.; Huang, G.; Bai, Z. Degradation of dichlorvos by Rhodobacter sphaeroides. Environ. Sci. 2009, 30, 1199–1204. [Google Scholar]
- Jiao, H.; Xu, S.; Wang, K.; Huang, Z.; Bai, Z. Effect of Rhodobacter Sphaeroides biofertilizer on the carcinogenic risk of the polycyclic aromatic hydrocarbons contaminated soil. China Agric. Sci. Bull. 2014, 30, 177–183. [Google Scholar]
- Aharon, O.; George, M. List of new names and new combinations that have appeared in effective publications outside of the IJSEM and are submitted for valid publication. Int. J. Syst. Evol. Microbiol. 2020, 70, 5596–5601. [Google Scholar]
- Luo, R.B.; Liu, B.H.; Xie, Y.L.; Li, Z.Y.; Huang, W.H.; Yuan, J.Y.; He, G.Z.; Chen, Y.X.; Pan, Q.; Liu, Y.J.; et al. SOAPdenovo2: An empirically improved memory-efficient short-read de novo assembler. Giga Sci. 2012, 1, 1–6. [Google Scholar] [CrossRef]
- Li, R.Q.; Zhu, H.M.; Ruan, J.; Qian, W.B.; Fang, X.D.; Shi, Z.B.; Li, Y.R.; Li, S.T.; Shan, G.; Kristiansen, K.; et al. De novo assembly of human genomes with massively parallel short read sequencing. Genome Res. 2010, 20, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Parks, D.; Imelfort, M.; Skennerton, C.; Hugenholtz, P.; Tyson, G. CheckM assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015, 25, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Parks, D.; Chuvochina, M.; Chaumeil, P.; Rinke, C.; Mussig, A.; Hugenholtz, P. A complete domain-to-species taxonomy for Bacteria and Archaea. Nat. Biotechnol. 2020, 38, 1079. [Google Scholar] [CrossRef] [PubMed]
- Tindall, B.; Rosselló, R.; Busse, H.; Ludwig, W.; Kämpfer, P. Notes on the characterization of prokaryote strains for taxonomic purposes. Int. J. Syst. Evol. Microbiol. 2010, 60, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Oh, H.; Park, S.; Chun, J. Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int. J. Syst. Evol. Microbiol. 2014, 64, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Colston, S.; Fullmer, M.S.; Beka, L.; Lamy, B.; Gogarten, J. Bioinformatic genome comparisons for taxonomic and phylogenetic assignments using Aeromonas as a test case. mBio 2014, 5, e02136. [Google Scholar] [CrossRef]
- Meier, J.; Auch, A.; Klenk, H.; Göker, M. Genome sequence base species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 2013, 14, 60. [Google Scholar]
- Gomila, M.; Peña, A.; Mulet, M.; Lalucat, J.; García, E. Phylogenomics and systematics in Pseudomonas. Front. Microbiol. 2015, 6, 214. [Google Scholar] [CrossRef] [PubMed]
- Darling, A.; Mau, B.; Blattner, F.; Perna, N. Mauve: Multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 2004, 14, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, G.; Singh, S. Comparative genome analysis provides shreds of molecular evidence for reclassification of Leuconostoc mesenteroides MTCC 10508 as a strain of Leu. suionicum. Genomics 2020, 112, 4023–4031. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Krishnan, K.; Kurian, P. Complete genome sequence and comparative genome analysis of Alcanivorax sp. IO_7, a marine alkane-degrading bacterium isolated from hydrothermally influenced deep seawater of southwest Indian ridge. Genomics 2021, 113, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Strnad, H.; Lapidus, A.; Paces, J.; Ulbrich, P.; Vlcek, C.; Paces, V.; Haselkorn, R. Complete genome sequence of the photosynthetic purple nonsulfur bacterium Rhodobacter capsulatus SB 1003. J. Bacteriol. 2010, 192, 3545–3546. [Google Scholar] [CrossRef]
- Park, J.; Kim, B.; Kim, Y.; Min, J. Whole-genome sequence of purple non-sulfur bacteria, Rhodobacter sphaeroides strain MBTLJ-8 with improved CO2 reduction capacity. J. Biotechnol. 2018, 288, 9–14. [Google Scholar] [CrossRef]
- Porter, S.; Wilkinson, D.; Byles, E.; Wadhams, G.; Taylor, S.; Saunders, N.; Armitage, J. Genome sequence of Rhodobacter sphaeroides Strain WS8N. J. Bacteriol. 2011, 193, 4027–4028. [Google Scholar] [CrossRef]
- Sychantha, D.; Brott, A.; Jones, C.; Clarke, A. Mechanistic pathways for peptidoglycan O-acetylation and De-O-acetylation. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Kleinheinz, K.; Joensen, K.; Larsen, M. Applying the ResFinder and VirulenceFinder web-services for easy identification of acquired antibiotic resistance and E. coli virulence genes in bacteriophage and prophage nucleotide sequences. Bacteriophage 2014, 4, 27943. [Google Scholar] [CrossRef]
- Wendling, C.; Refardt, D.; Hall, A. Fitness benefits to bacteria of carrying prophages and prophage encoded antibiotic resistance genes peak in different environments. Evolution 2021, 75, 515–528. [Google Scholar] [CrossRef]
- Shao, H.; Chen, M.; Fei, X.; Zhang, R.; Zhong, Y.; Ni, W.; Tao, X.; He, X.; Zhang, E.; Yong, B.; et al. Complete genome sequence and characterization of a polyethylene biodegradation strain, Streptomyces albogriseolus LBX-2. Microorganisms 2019, 7, 379. [Google Scholar] [CrossRef] [PubMed]
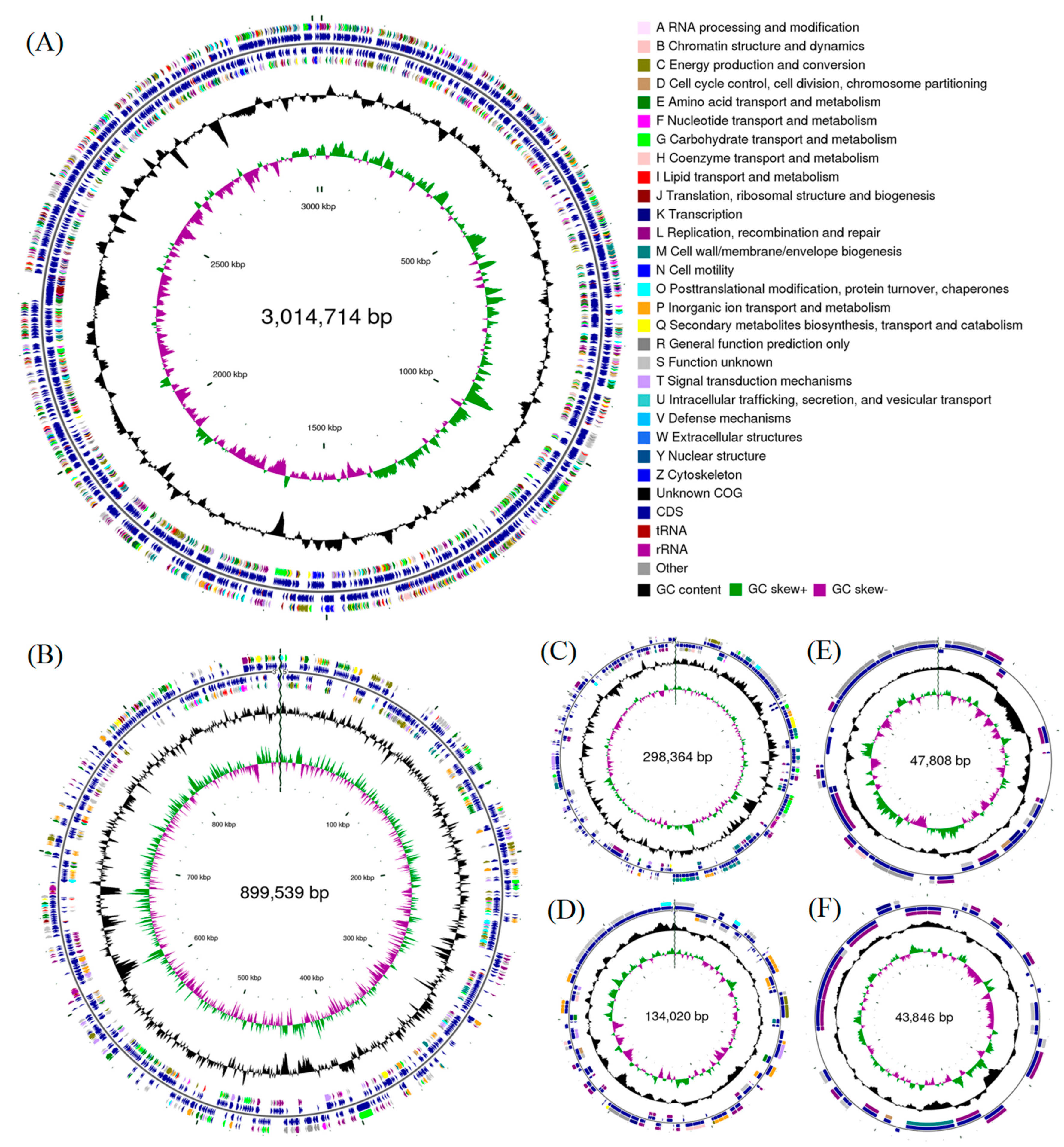


| Genome Features | Strain EBL0706 | L. azotoformans ATCC 17025 | L. azotoformans KA25T | L. sphaeroides 2.4.1T |
|---|---|---|---|---|
| Genome size (bp) | 4,438,291 | 4,557,127 | 4,414,500 | 4,629,754 |
| G + C content (%) | 68.4% | 68.2% | 68.4% | 68.8% |
| Contigs | 6 | 6 | 80 | 6 |
| Scaffold N50 (bp) | 3,014,714 | 3,217,726 | 186,783 | 3,188,530 |
| Total number of CDS | 4484 | 4503 | 4282 | 4382 |
| tRNA | 55 | 54 | 48 | 53 |
| rRNA | 12 | 12 | 3 | 9 |
| Strain EBL0706 | L. azotoformans ATCC 17025 | L. azotoformans KA25T | L. sphaeroides 2.4.1T | L. sphaeroides AB25 | L. sphaeroides ATCC 17029 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ANI | TETRA | dDDH | ANI | TETRA | dDDH | ANI | TETRA | dDDH | ANI | TETRA | dDDH | ANI | TETRA | dDDH | ANI | TETRA | dDDH | |
| strain EBL0706 | * | * | * | 98.15 | 0.9997 | 94.67 | 99.5 | 0.9999 | 97.96 | 84.93 | 0.9750 | 0.12 | 84.99 | 0.9756 | 0.12 | 85.04 | 0.9765 | 0.13 |
| L. azotoformans ATCC 17025 | 98.13 | 0.9997 | 94.67 | * | * | * | 98.03 | 0.9997 | 94.63 | 85.06 | 0.9751 | 0.12 | 85.05 | 0.9755 | 0.12 | 85 | 0.9765 | 0.14 |
| L. azotoformans KA25T | 99.56 | 0.9998 | 97.96 | 98.19 | 0.9997 | 94.63 | * | * | * | 84.98 | 0.9756 | 0.12 | 85.13 | 0.9762 | 0.12 | 85.08 | 0.9771 | 0.13 |
| L. sphaeroides 2.4.1T | 84.84 | 0.9749 | 0.12 | 84.71 | 0.9751 | 0.12 | 84.88 | 0.9756 | 0.12 | * | * | * | 97.78 | 0.9994 | 92.03 | 97.73 | 0.9996 | 92.74 |
| L. sphaeroides AB25 | 84.7 | 0.9756 | 0.12 | 84.69 | 0.9755 | 0.12 | 84.79 | 0.9762 | 0.12 | 97.49 | 0.9994 | 92.03 | * | * | * | 97.59 | 0.9998 | 92.28 |
| L. sphaeroides ATCC 17029 | 85 | 0.9765 | 0.13 | 84.92 | 0.9765 | 0.14 | 84.97 | 0.9771 | 0.13 | 97.8 | 0.9996 | 92.74 | 97.87 | 0.9998 | 92.28 | * | * | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Sha, X.; Li, R.; Li, Y.; Khaleque, H.N.; Zhang, Y.; Bohu, T.; Bai, Z.; Zhuang, X. Comparative Genome Analysis Provides Molecular Evidence for Reclassification of the Photosynthetic Bacterium Rhodobacter sphaeroides EBL0706 as a Strain of Luteovulum azotoformans. Microorganisms 2021, 9, 1754. https://doi.org/10.3390/microorganisms9081754
Wang H, Sha X, Li R, Li Y, Khaleque HN, Zhang Y, Bohu T, Bai Z, Zhuang X. Comparative Genome Analysis Provides Molecular Evidence for Reclassification of the Photosynthetic Bacterium Rhodobacter sphaeroides EBL0706 as a Strain of Luteovulum azotoformans. Microorganisms. 2021; 9(8):1754. https://doi.org/10.3390/microorganisms9081754
Chicago/Turabian StyleWang, Haoyu, Xiaoling Sha, Rui Li, Yijing Li, Himel Nahreen Khaleque, Yuxiu Zhang, Tsing Bohu, Zhihui Bai, and Xuliang Zhuang. 2021. "Comparative Genome Analysis Provides Molecular Evidence for Reclassification of the Photosynthetic Bacterium Rhodobacter sphaeroides EBL0706 as a Strain of Luteovulum azotoformans" Microorganisms 9, no. 8: 1754. https://doi.org/10.3390/microorganisms9081754
APA StyleWang, H., Sha, X., Li, R., Li, Y., Khaleque, H. N., Zhang, Y., Bohu, T., Bai, Z., & Zhuang, X. (2021). Comparative Genome Analysis Provides Molecular Evidence for Reclassification of the Photosynthetic Bacterium Rhodobacter sphaeroides EBL0706 as a Strain of Luteovulum azotoformans. Microorganisms, 9(8), 1754. https://doi.org/10.3390/microorganisms9081754









