Seasonal Dynamics of Fungi Associated with Healthy and Diseased Pinus sylvestris Needles in Northern Europe
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites and Sampling
2.2. Molecular Analysis
2.3. Bioinformatics
2.4. Statistical Analyses
3. Results
3.1. Fungal Taxonomic Richness and Dominant Species
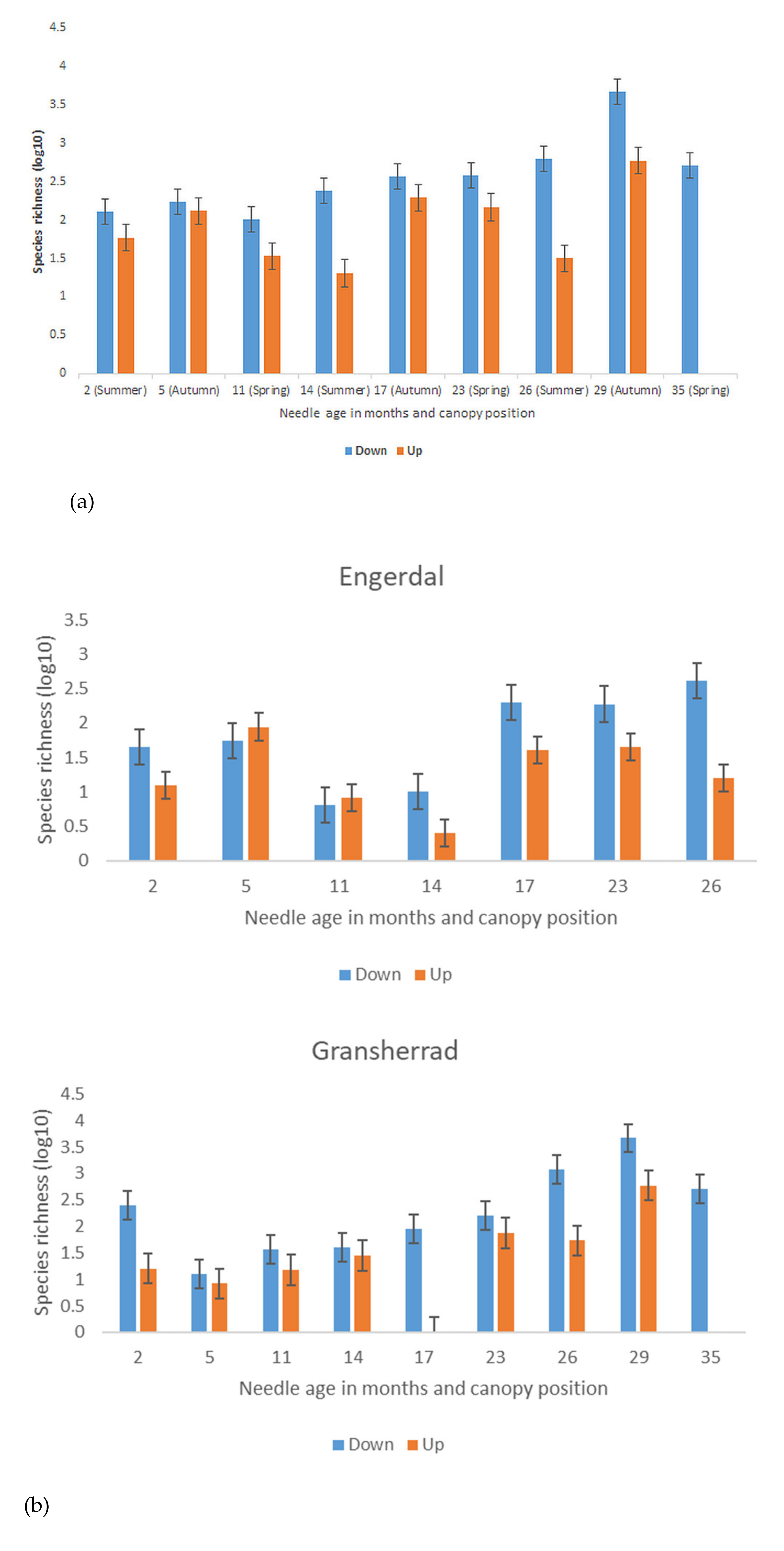
3.2. Seasonal Differences in Fungal Species Richness and Composition
3.3. Factors Affecting Relative Abundance of Pathogens D. septosporum and L. conigenum
3.4. Fungal Species Composition
4. Discussion
4.1. Fungal Taxonomic Richness
4.2. Season-, Needle Age-, and Canopy Position-Related Differences in Fungal Species Richness
4.3. Relations between Sampling Date, Needle Age, and Location in the Canopy and Relative Abundance of D. septosporum and L. conigenum
4.4. The Presence of Other Lophodermium Species in Estonia and Norway
4.5. Fungal Species Composition
4.6. Co-Occurrence and Pathogen Effects
4.7. Technical Notes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Terhonen, E.; Marco, T.; Sun, H.; Jalkanen, R.; Kasanen, R.; Vuorinen, M.; Asiegbu, F. The Effect of Latitude, Season and Needle Age on the Mycota of Scots Pine (Pinus sylvestris) in Finland. Silva. Fennica 2011, 45, 301–317. [Google Scholar] [CrossRef] [Green Version]
- Millberg, H.; Boberg, J.; Stenlid, J. Changes in Fungal Community of Scots Pine (Pinus sylvestris) Needles along a Latitudinal Gradient in Sweden. Fungal Ecol. 2015, 17, 126–139. [Google Scholar] [CrossRef]
- Taudière, A.; Bellanger, J.-M.; Carcaillet, C.; Hugot, L.; Kjellberg, F.; Lecanda, A.; Lesne, A.; Moreau, P.-A.; Scharmann, K.; Leidel, S.; et al. Diversity of Foliar Endophytic Ascomycetes in the Endemic Corsican Pine Forests. Fungal Ecol. 2018, 36, 128–140. [Google Scholar] [CrossRef] [Green Version]
- Hyde, K.; Soytong, K. The Fungal Endophyte Dilemma. Fungal Diversity 2008, 33, 163–173. [Google Scholar]
- Petrini, O. Fungal Endophytes of Tree Leaves. In Microbial Ecology of Leaves; Andrews, J.H., Hirano, S.S., Eds.; Brock/Springer Series in Contemporary Bioscience; Springer: New York, NY, USA, 1991; pp. 179–197. [Google Scholar] [CrossRef]
- Hane, J.K.; Paxman, J.; Jones, D.A.B.; Oliver, R.P.; de Wit, P. “CATAStrophy,” a Genome-Informed Trophic Classification of Filamentous Plant Pathogens—How Many Different Types of Filamentous Plant Pathogens Are There? Front. Microbiol. 2020, 10, 3088. [Google Scholar] [CrossRef] [Green Version]
- Clay, K. Endophytes as Antagonists of Plant Pests. In Microbial Ecology of Leaves; Andrews, J.H., Hirano, S.S., Eds.; Brock/Springer Series in Contemporary Bioscience; Springer: New York, NY, USA, 1991; pp. 331–357. [Google Scholar] [CrossRef]
- Carrol, G. Forest endophytes: Pattern and process. Can. J. Bot. 1995, 73. [Google Scholar] [CrossRef]
- Faeth, S.H.; Wilson, D. Induced Responses in Trees: Mediators of Interactions among Macro- and Micro-Herbivores? In Multitrophic Interactions in Terrestrial Systems; Gange, A.C., Ed.; Blackwell Science: Oxford, UK, 1997; pp. 201–215. [Google Scholar]
- Stone, J.; Petrini, O. Endophytes of Forest Trees: A Model for Fungus-Plant Interactions. In Plant Relationships Part B; Carroll, G.C., Tudzynski, P., Eds.; The Mycota; Springer: Berlin, Heidelberg, 1997; pp. 129–140. [Google Scholar] [CrossRef]
- Wilson, D.; Carroll, G.C. Avoidance of High-Endophyte Space by Gall-Forming Insects. Ecology 1997, 78, 2153–2163. [Google Scholar] [CrossRef]
- Sieber, T.N. Endophytic Fungi in Forest Trees: Are They Mutualists? Fungal Biol. Rev. 2007, 21, 75–89. [Google Scholar] [CrossRef]
- Delaye, L.; García-Guzmán, G.; Heil, M. Endophytes versus Biotrophic and Necrotrophic Pathogens—Are Fungal Lifestyles Evolutionarily Stable Traits? Fungal Divers. 2013, 60, 125–135. [Google Scholar] [CrossRef]
- Gaeumann, E. Die Rostpilze Mitteleuropas; Buchdruckerei Buchler &, Co.: Berne, Switzerland, 1959. [Google Scholar]
- Johnson, J.A.; Whitney, N.J. Isolation of Fungal Endophytes from Black Spruce (Picea Mariana) Dormant Buds and Needles from New Brunswick, Canada. Can. J. Bot. 1992, 70, 1754–1757. [Google Scholar] [CrossRef]
- Magan, N.; Smith, M.K.; Kirkwood, I.A. Effects of Atmospheric Pollutants on Phyllosphere and Endophytic Fungi. Fungi and environmental change: Symposium of the British Mycological Society, held at Cranfield University; Cambridge University Press: Bedford, UK, 1994. [Google Scholar]
- Deckert, R.J.; Peterson, R.L. Distribution of Foliar Fungal Endophytes of Pinus Strobus between and within Host Trees. Can. J. For. Res. 2000, 30, 1436–1442. [Google Scholar] [CrossRef]
- Barnes, I.; Crous, P.W.; Wingfield, B.D.; Wingfield, M.J. Multigene Phylogenies Reveal That Red Band Needle Blight of Pinus Is Caused by Two Distinct Species of Dothistroma, D. Septosporum and D. Pini. Stud.Mycol. 2004, 50, 551–565. [Google Scholar]
- Drenkhan, R.; Tomešová-Haataja, V.; Fraser, S.; Bradshaw, R.E.; Vahalík, P.; Mullett, M.S.; Martín-García, J.; Bulman, L.S.; Wingfield, M.J.; Kirisits, T.; et al. Global Geographic Distribution and Host Range of Dothistroma Species: A Comprehensive Review. For. Pathol. 2016, 46, 408–442. [Google Scholar] [CrossRef]
- Doroguine, M. A Cryptogamic Disease of Pines [In French]. Bull. Trimest. Société Mycol. Fr. 1911, 27, 105–106. [Google Scholar]
- Barnes, I.; Walla, J.A.; Bergdahl, A.; Wingfield, M.J. Four New Host and Three New State Records of Dothistroma Needle Blight Caused by Dothistroma Pini in the United States. Plant Dis. 2014, 98, 1443. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, R.E. Dothistroma (Red-Band) Needle Blight of Pines and the Dothistromin Toxin: A Review. For. Pathol. 2004, 34, 163–185. [Google Scholar] [CrossRef]
- Hanso, M.; Drenkhan, R. First Observations of Mycosphaerella Pini in Estonia. Plant. Pathol. 2008, 57, 1177. [Google Scholar] [CrossRef]
- Solheim, H.; Vuorinen, M. First Report of Mycosphaerella Pini Causing Red Band Needle Blight on Scots Pine in Norway. Plant. Dis. 2011, 95, 875. [Google Scholar] [CrossRef]
- Drenkhan, R.; Kurkela, T.; Hanso, M. The Relationship between the Needle Age and the Growth Rate in Scots Pine (Pinus sylvestris): A Retrospective Analysis by Needle Trace Method (NTM). Eur. J. For. Res. 2006, 125, 397–405. [Google Scholar] [CrossRef]
- Kurkela, T.; Drenkhan, R.; Vuorinen, M.; Hanso, M. Growth Response of Young Scots Pines to Needle Loss Assessed from Productive Foliage. For. Stud. Metsanduslikud Uurim. 2009, 50, 5–22. [Google Scholar] [CrossRef]
- Reignoux, S.N.A.; Green, S.; Ennos, R.A. Molecular Identification and Relative Abundance of Cryptic Lophodermium Species in Natural Populations of Scots Pine, Pinus sylvestris, L. Fungal Biol. 2014, 118, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.K.; Bacon, C.W.; White, J.F., Jr. An Overview of Endophytic Microbes: Endophytism Defined. In Microbial Endophytes; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Minter, D.W.; Millar, C.S. Ecology and biology of three Lophodermium species on secondary needles of Pinus sylvestris. Eur. J. For. Res. 1980, 10, 169–181. [Google Scholar] [CrossRef]
- Diwani, S.A.; Millar, C.S. Pathogenicity of Three Lophodermium Species on Pinus sylvestris, L. Eur. J. For. Res. 1987, 17, 53–58. [Google Scholar] [CrossRef]
- Hanso, M.; Drenkhan, R. Lophodermium Needle Cast, Insect Defoliation and Growth Responses of Young Scots Pines in Estonia. For. Pathol. 2012, 42, 124–135. [Google Scholar] [CrossRef]
- Dietrich, H.A. Blicke in die cryptogamenwelt der Ostseeprovinzen. Heinrich Laakmann. 1856. [Google Scholar]
- Jansons, Ā.; Zeltiņš, P.; Donis, J.; Neimane, U. Long-Term Effect of Lophodermium Needle Cast on The Growth of Scots Pine and Implications for Financial Outcomes. Forests 2020, 11, 718. [Google Scholar] [CrossRef]
- Woods, A.; Coates, K.D.; Hamann, A. Is an Unprecedented Dothistroma Needle Blight Epidemic Related to Climate Change? BioScience 2005, 55, 761–769. [Google Scholar] [CrossRef] [Green Version]
- Lõhmus, E. Eesti Metsakasvukohatüübid [In Estonian]. Teine, Täiendatud Trükk. Tartu. 2004. [Google Scholar]
- Tedersoo, L.; Anslan, S. Towards PacBio-based pan-eukaryote metabarcoding using full-length ITS sequences. Environ. Microbiol. Rep. 2019, 11, 659–668. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global Diversity and Geography of Soil Fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef] [Green Version]
- Agan, A.; Drenkhan, R.; Adamson, K.; Tedersoo, L.; Solheim, H.; Børja, I.; Matsiakh, I.; Timmermann, V.; Nagy, N.E.; Hietala, A.M. The Relationship between Fungal Diversity and Invasibility of a Foliar Niche—The Case of Ash Dieback. J. Fungi 2020, 6, 150. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing Mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME Improves Sensitivity and Speed of Chimera Detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bengtsson-Palme, J.; Ryberg, M.; Hartmann, M.; Branco, S.; Wang, Z.; Godhe, A.; De Wit, P.; Sánchez-García, M.; Ebersberger, I.; de Sousa, F.; et al. Improved software detection and extraction of ITS1 and ITS2 from ribosomal ITS sequences of fungi and other eukaryotes for analysis of environmental sequencing data. Methods Ecol. Evol. 2013, 4, 914–919. [Google Scholar] [CrossRef]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT: Accelerated for Clustering the next-Generation Sequencing Data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef]
- Anslan, S.; Bahram, M.; Hiiesalu, I.; Tedersoo, L. PipeCraft: Flexible Open-Source Toolkit for Bioinformatics Analysis of Custom High-Throughput Amplicon Sequencing Data. Mol Ecol Resour. 2017, 17, e234–e240. [Google Scholar] [CrossRef]
- Loit, K.; Adamson, K.; Bahram, M.; Puusepp, R.; Anslan, S.; Kiiker, R.; Drenkhan, R.; Tedersoo, L. Relative Performance of Oxford Nanopore MinION vs. Pacific Biosciences Sequel Third-Generation Sequencing Platforms in Identification of Agricultural and Forest Pathogens. Appl. Environ. Microbiol. 2019, 85, e01368-19. [Google Scholar] [CrossRef]
- Kõljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.S.; Bahram, M.; Bates, S.T.; Bruns, T.D.; Bengtsson-Palme, J.; Callaghan, T.M.; et al. Larsson, K.-H. Towards a Unified Paradigm for Sequence-Based Identification of Fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tedersoo, L.; Drenkhan, R.; Anslan, S.; Morales-Rodriguez, C.; Cleary, M. High-throughput identification and diagnostics of pathogens and pests: Overview and practical recommendations. Mol. Ecol. Resour. 2019, 19, 47–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontologia Electronica 2001, 4, 9. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2014, 67, 1–48. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2006; p. 192. [Google Scholar]
- Anderson, M.; Gorley, R.N.; Clarke, R.K. Permanova+ for Primer: Guide to Software and Statistical Methods; Primer-E Limited: Plymouth, UK, 2008. [Google Scholar]
- Bray, J.R.; Curtis, J.T. An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 326–349. [Google Scholar] [CrossRef]
- Lazarević, J.; Menkis, A. Fungal Diversity in the Phyllosphere of Pinus Heldreichii, H. Christ—An Endemic and High-Altitude Pine of the Mediterranean Region. Diversity 2020, 12, 172. [Google Scholar] [CrossRef]
- Minter, D.W. Possible Biological Control of Lophodermium Seditiosum. In Current Research on Conifer Needle Diseases, Proceedings of IUFRO WP on Needle Diseases, Sarajevo, 1980; Aberdeen University Forest Department: Aberdeen, UK, 1981; pp. 75–80. [Google Scholar]
- Müller, M.M.; Hantula, J.; Vuorinen, M. First Observations of Mycosphaerella Pini on Scots Pine in Finland. Plant. Dis. 2009, 93, 322. [Google Scholar] [CrossRef] [PubMed]
- Millberg, H.; Hopkins, A.J.M.; Boberg, J.; Davydenko, K.; Stenlid, J. Disease development of Dothistroma needle blight in seedlings of Pinus sylvestris and Pinus contorta under Nordic conditions. For. Pathol. 2016, 46, 515–521. [Google Scholar] [CrossRef]
- Liu, C.M.; Kachur, S.; Dwan, M.G.; Abraham, A.G.; Aziz, M.; Hsueh, P.-R.; Huang, Y.-T.; Busch, J.D.; Lamit, L.J.; Gehring, C.A.; et al. FungiQuant: A Broad-Coverage Fungal Quantitative Real-Time PCR Assay. BMC Microbiol. 2012, 12, 255. [Google Scholar] [CrossRef] [Green Version]
- Jumpponen, A.; Jones, K.L. Seasonally Dynamic Fungal Communities in the Quercus Macrocarpa Phyllosphere Differ between Urban and Nonurban Environments. New Phytol. 2010, 186, 496–513. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, M.; Queloz, V.; Sieber, T.N. The Endophytic Mycobiome of European Ash and Sycamore Maple Leaves—Geographic Patterns, Host Specificity and Influence of Ash Dieback. Front. Microbiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Peršoh, D. Plant-Associated Fungal Communities in the Light of Meta’omics. Fungal Divers. 2015, 75, 1–25. [Google Scholar] [CrossRef]
- Zamora, P.; Martínez-Ruiz, C.; Diez, J.J. Fungi in Needles and Twigs of Pine Plantations from Northern Spain. Fungal Divers. 2008, 30, 171–184. [Google Scholar]
- Kabir, M.S.; Ganley, R.J.; Bradshaw, R.E. The Hemibiotrophic Lifestyle of the Fungal Pine Pathogen Dothistroma Septosporum. For. Pathol. 2015, 45, 190–202. [Google Scholar] [CrossRef]
- Gibson, I.A.S. Dothistroma Blight of Pinus Radiata. Annu. Rev. Phytopathol. 1972, 10, 51–72. [Google Scholar] [CrossRef]
- Karadžič, D. Scirrhia Pini Funk et Parker. Life Cycle of the Fungus in Plantations of Pinus Nigra Arn. in Serbia. Eur. J. Plant Pathol. 1989, 19, 231–236. [Google Scholar] [CrossRef]
- Stachowicz, J.; Tilman, D. Species Invasions and the Relationships between Species Diversity, Community Saturation, and Ecosystem Functioning 2. Species Invasions: Insights into Ecology, Evolution, and Biogeography; Springer: Berlin, Germany, 2005; pp. 41–64. [Google Scholar]
- Lynikienė, J.; Marčiulynienė, D.; Marčiulynas, A.; Gedminas, A.; Vaičiukynė, M.; Menkis, A. Managed and Unmanaged Pinus sylvestris Forest Stands Harbour Similar Diversity and Composition of the Phyllosphere and Soil Fungi. Microorganisms 2020, 8, 259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golubev, W.I. Capsules. In The Yeasts, 2nd ed.; Rose, A.H., Harrison, J.S., Eds.; Academic: London, UK, 1991; Volume 4, pp. 175–197. [Google Scholar]
- Arnold, A.E.; Herre, E.A. Canopy Cover and Leaf Age Affect Colonization by Tropical Fungal Endophytes: Ecological Pattern and Process in Theobroma Cacao (Malvaceae). Mycologia 2003, 95, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Kovalchuk, A.; Mukrimin, M.; Zeng, Z.; Raffaello, T.; Liu, M.; Kasanen, R.; Sun, H.; Asiegbu, F.O. Mycobiome Analysis of Asymptomatic and Symptomatic Norway Spruce Trees Naturally Infected by the Conifer Pathogens Heterobasidion spp. Environ. Microbiol. Rep. 2018, 10, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Adamson, K.; Mullett, M.S.; Solheim, H.; Barnes, I.; Müller, M.M.; Hantula, J.; Vuorinen, M.; Kačergius, A.; Markovskaja, S.; Musolin, D.L.; et al. Looking for Relationships between the Populations of Dothistroma Septosporum in Northern Europe and Asia. Fungal Genet. Biol. 2018, 110, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Mullett, M.S.; Drenkhan, R.; Adamson, K.; Boroń, P.; Lenart-Boroń, A.; Barnes, I.; Tomšovský, M.; Jánošíková, Z.; Adamčíková, K.; Ondrušková, E.; et al. Worldwide Genetic Structure Elucidates the Eurasian Origin and Invasion Pathways of Dothistroma Septosporum, Causal Agent of Dothistroma Needle Blight. J. Fungi 2021, 7, 111. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agan, A.; Solheim, H.; Adamson, K.; Hietala, A.M.; Tedersoo, L.; Drenkhan, R. Seasonal Dynamics of Fungi Associated with Healthy and Diseased Pinus sylvestris Needles in Northern Europe. Microorganisms 2021, 9, 1757. https://doi.org/10.3390/microorganisms9081757
Agan A, Solheim H, Adamson K, Hietala AM, Tedersoo L, Drenkhan R. Seasonal Dynamics of Fungi Associated with Healthy and Diseased Pinus sylvestris Needles in Northern Europe. Microorganisms. 2021; 9(8):1757. https://doi.org/10.3390/microorganisms9081757
Chicago/Turabian StyleAgan, Ahto, Halvor Solheim, Kalev Adamson, Ari Mikko Hietala, Leho Tedersoo, and Rein Drenkhan. 2021. "Seasonal Dynamics of Fungi Associated with Healthy and Diseased Pinus sylvestris Needles in Northern Europe" Microorganisms 9, no. 8: 1757. https://doi.org/10.3390/microorganisms9081757






