Root-Associated Microbiomes, Growth and Health of Ornamental Geophytes Treated with Commercial Plant Growth-Promoting Products
Abstract
:1. Introduction
2. Methods and Materials
2.1. Biological Material and Growth-Promoting Products
2.2. Bacterial Growth Media
2.3. Growing Conditions
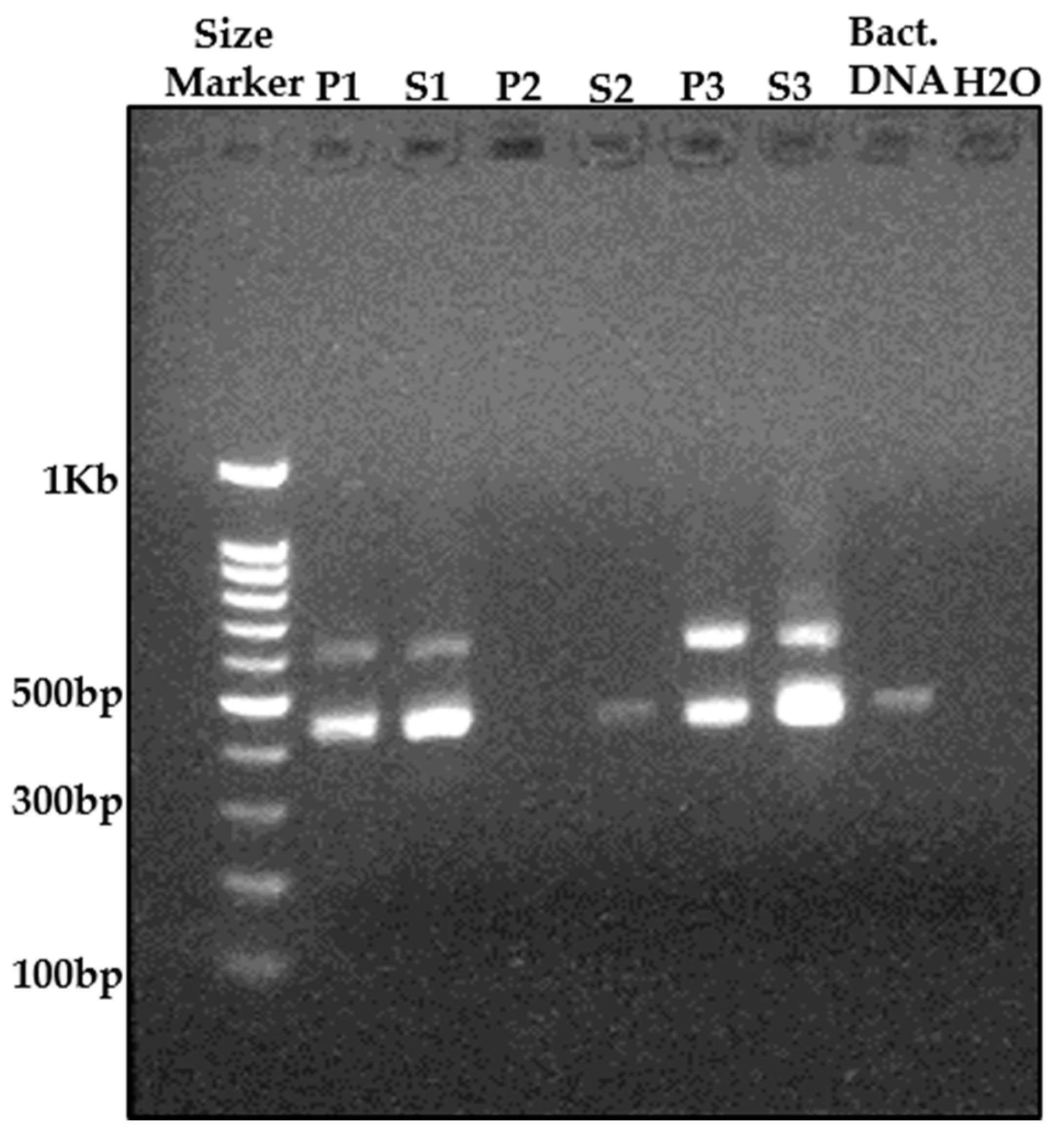
2.4. Molecular Analyses
2.5. Dual-Culture Assays
2.6. Statistical Analysis
3. Results
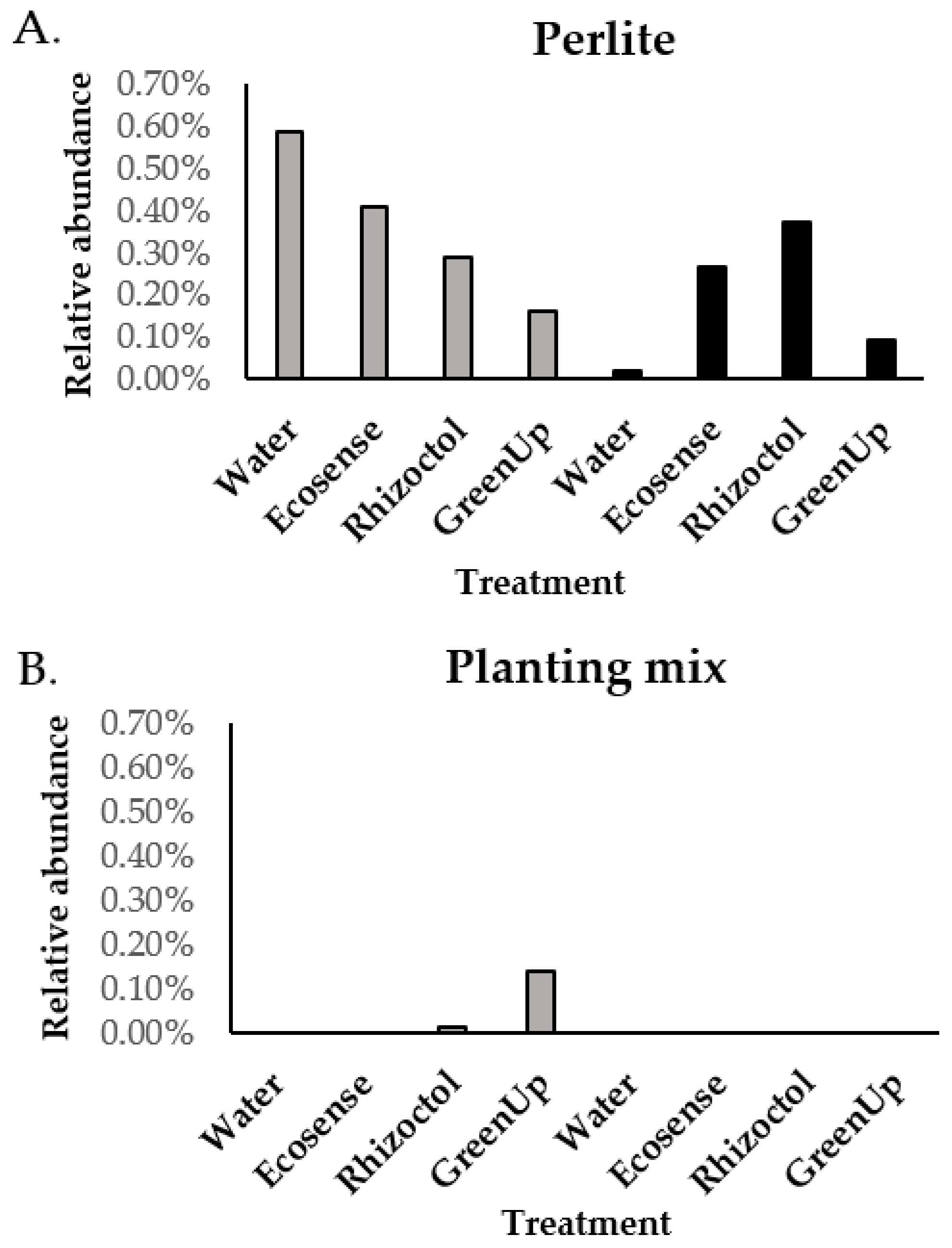
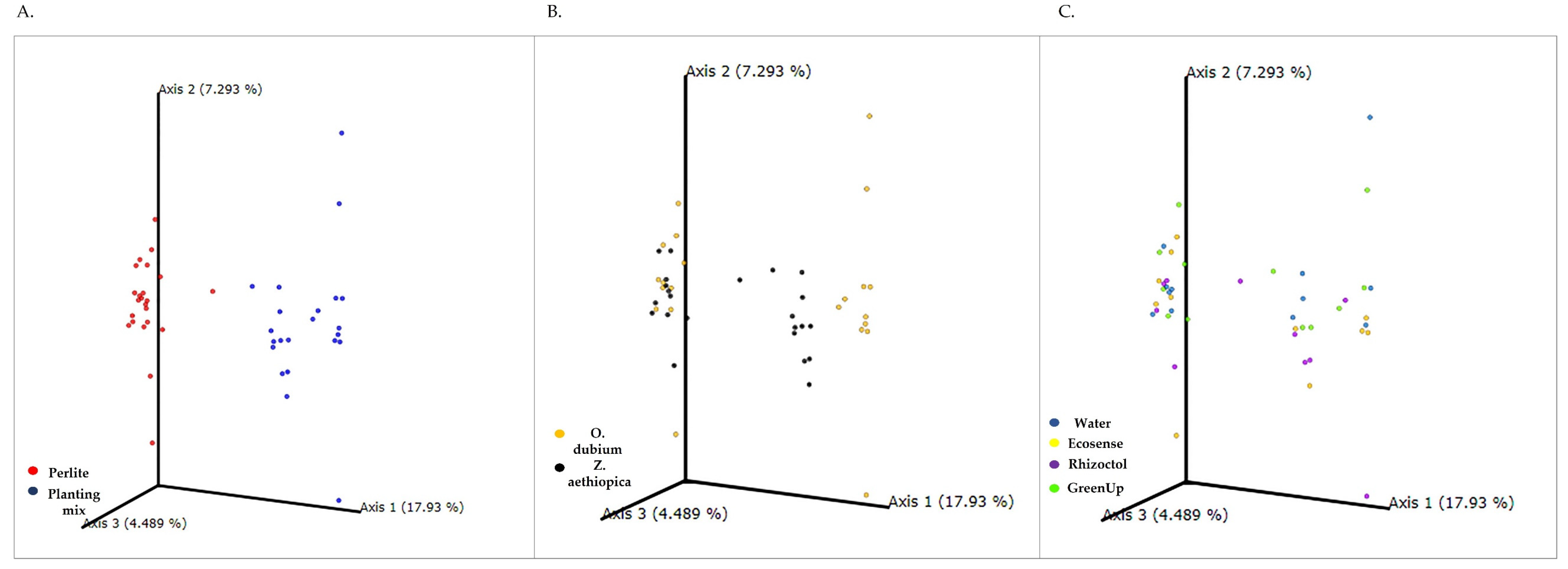
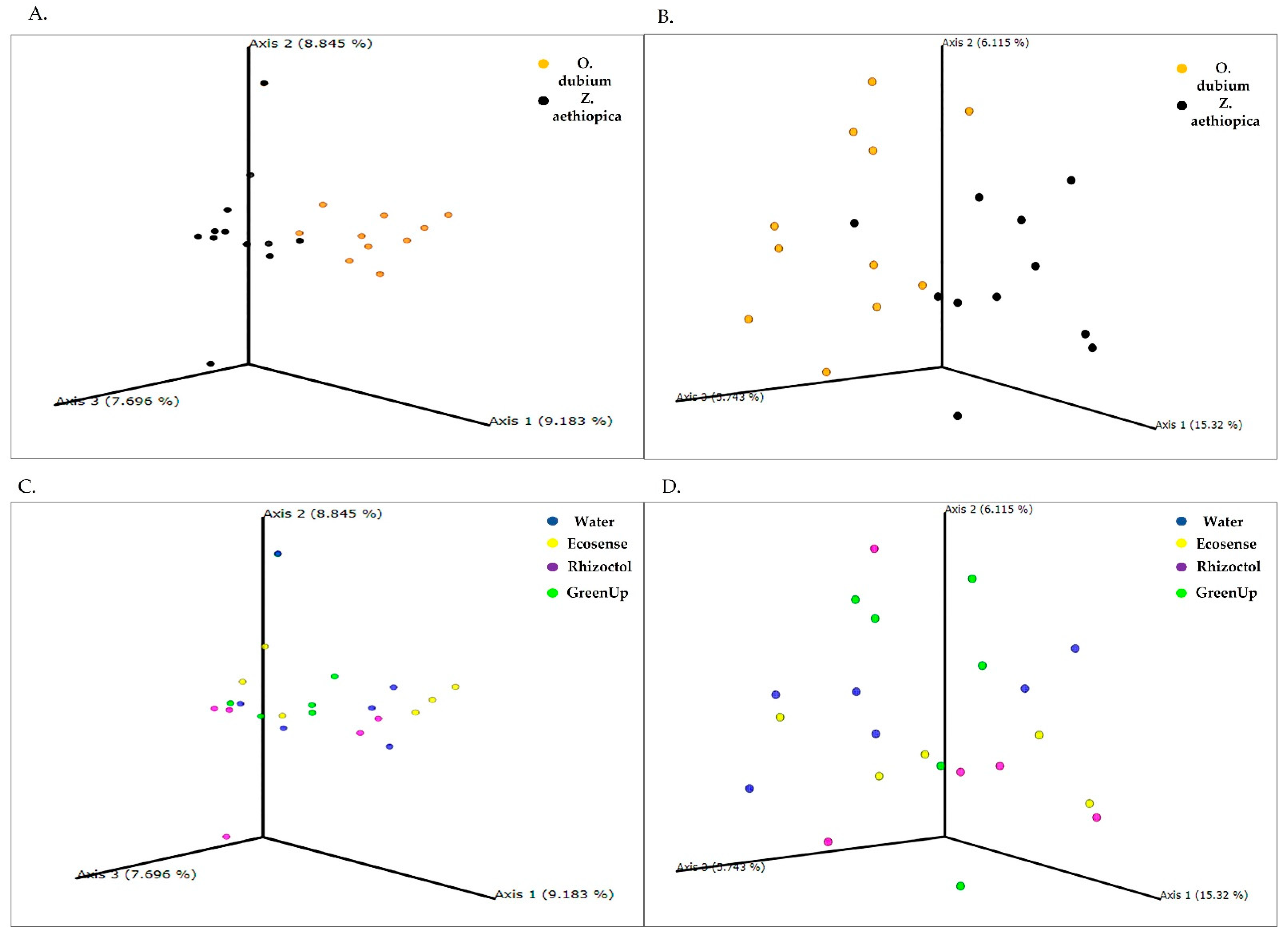
3.1. Root-Associated Microbiome Analysis
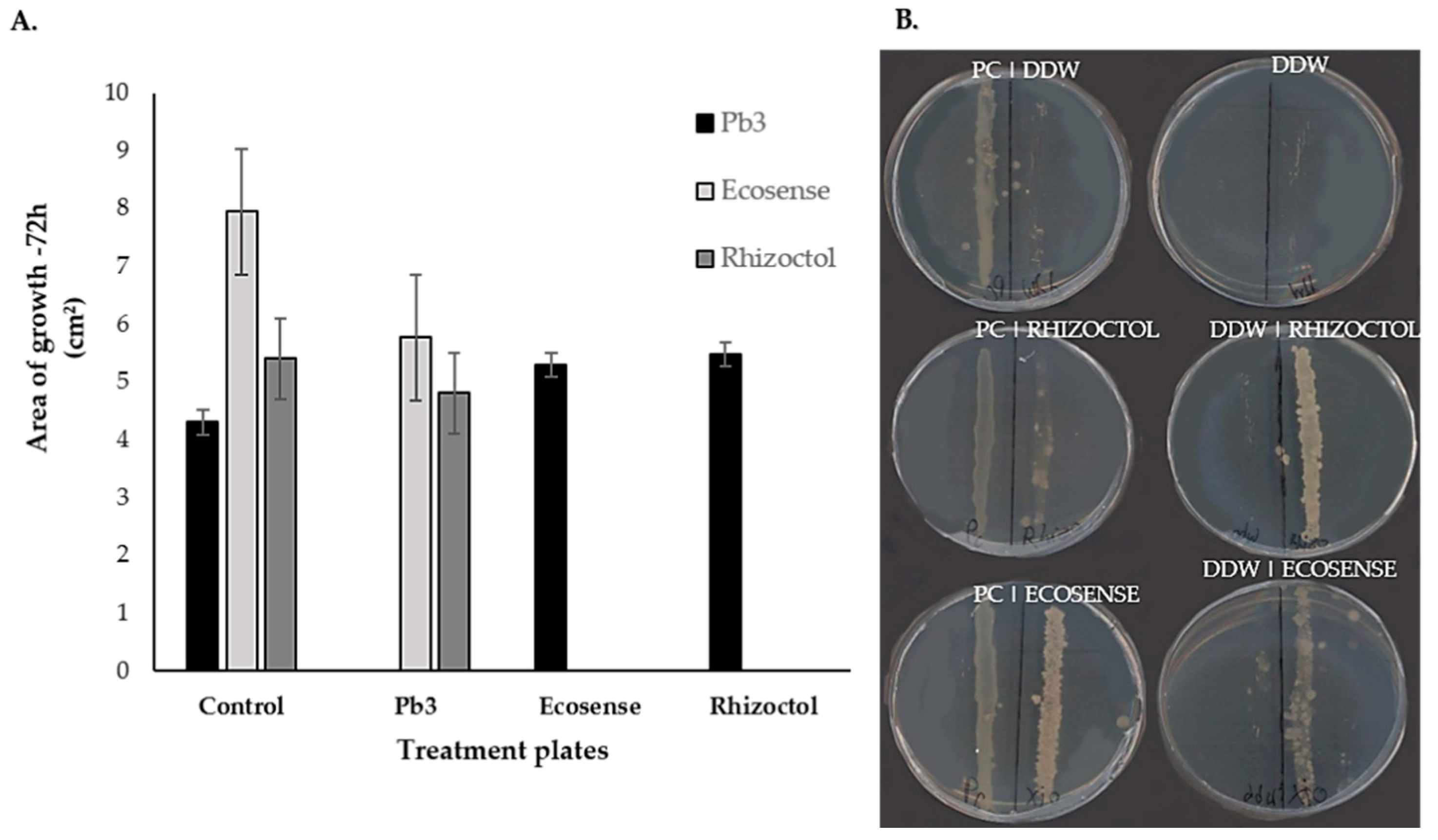
3.2. Dual-Culture Assay
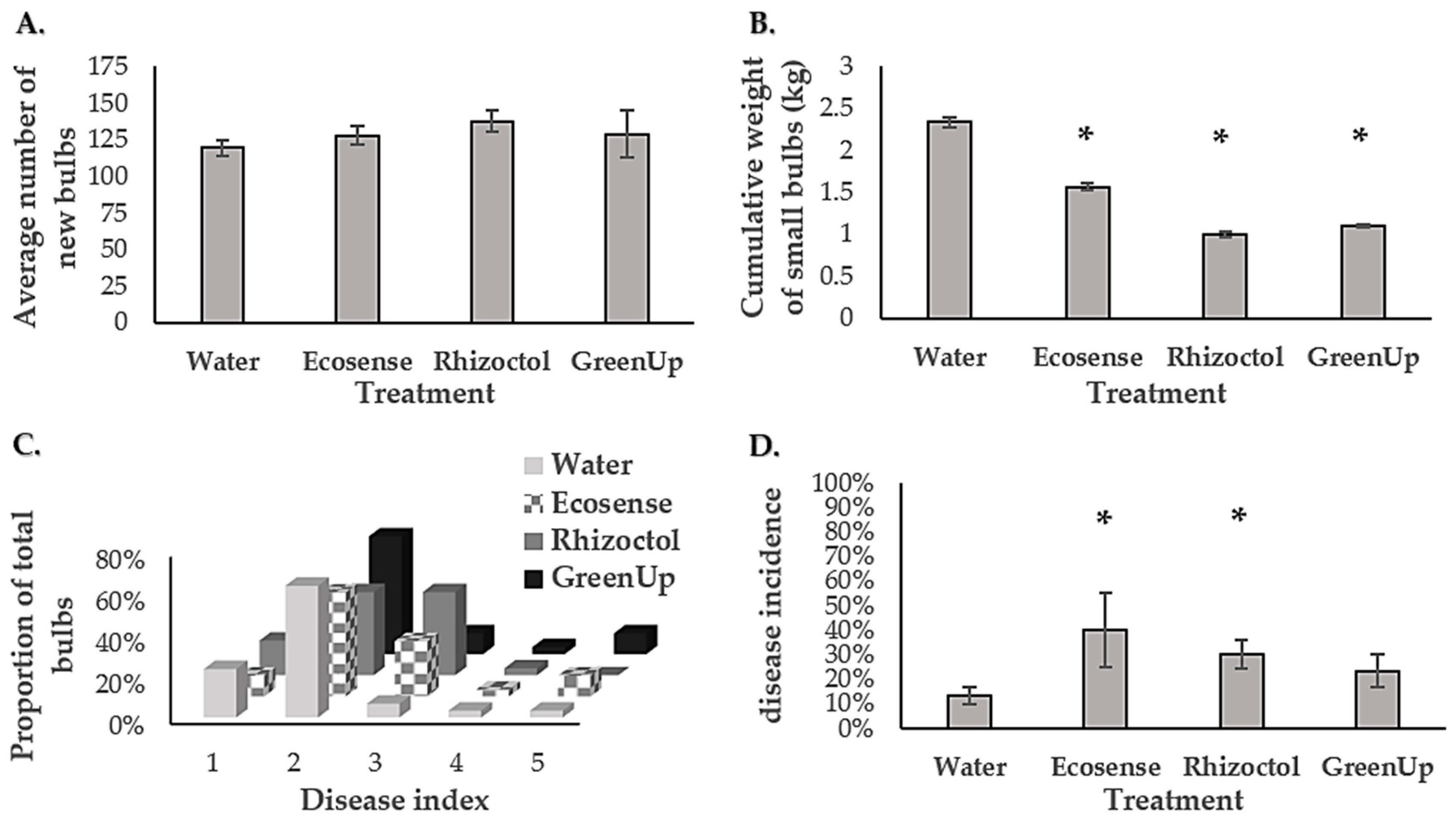
3.3. Geophytes’ Responses to the Growth-Promoting Products
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kamenetzky, R.; Miller, W.B. The global trade in ornamental geophytes. Chron. Horticult. 2010, 50, 27–30. [Google Scholar]
- Kamenetsky, R. Development and utilization of ornamental geophytes: Research challenges and sustainable production. In Proceedings of the Acta Horticulturae; International Society for Horticultural Science: Leuven, Belgium, 2017; Volume 1171, pp. 9–16. [Google Scholar]
- Lakshman, D.K.; Cloyd, R.A.; Chastagner, G.A. Integrated management of diseases and pests on ornamental geophytes: Challenges and progress. Acta Hortic. 2019, 1237, 13–32. [Google Scholar] [CrossRef]
- Chastagner, G.A.; van Tuyl, J.M.; Verbeek, M.; Miller, W.B.; Westerdahl, B.B. Diseases of lily. In Handbook of Florists’ Crops Diseases; McGovern, R., Elmer, W., Eds.; Springer: Cham, Switzerland; London, UK, 2018; pp. 1229–1288. [Google Scholar]
- Yedidia, I.; Ophir, R.; Yishay, M.; Ion, A.; Luzzatto, T.; Golan, A.; Burdman, S. A story of an old battle: Pectobacterium carotovorum and ornamental monocots. Acta Hortic. 2011, 886, 417–425. [Google Scholar] [CrossRef]
- Morris, E.K.; Fletcher, R.; Veresoglou, S.D. Effective methods of biofumigation: A meta-analysis. Plant Soil 2020, 446, 379–392. [Google Scholar] [CrossRef]
- Boetius, A. Global change microbiology—Big questions about small life for our future. Nat. Rev. Microbiol. 2019, 17, 331–332. [Google Scholar] [CrossRef] [PubMed]
- McGovern, R.; Wade, E. Handbook of Florists’ Crops Diseases; Springer International Publishing: Cham, Switzerland; London, UK, 2018. [Google Scholar]
- Thomas, F.; Cébron, A. Short-term rhizosphere effect on available carbon sources, phenanthrene degradation, and active microbiome in an aged-contaminated industrial soil. Front. Microbiol. 2016, 7, 92. [Google Scholar] [CrossRef]
- Edwards, J.; Johnson, C.; Santos-Medellín, C.; Lurie, E.; Podishetty, N.K.; Bhatnagar, S.; Eisen, J.A.; Sundaresan, V. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc. Natl. Acad. Sci. USA 2015, 112, E911–E920. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.J.; Jin, S.Y.; Cheong, D.C.; Kim, J.M.; Guak, S.H. Effect of different mulching materials on growth, flowering, bulb yields and soft rot incidence of calla lily (Zantedeschia aethiopica). In Proceedings of the Acta Horticulturae; International Society for Horticultural Science: Leuven, Belgium, 2019; Volume 1255, pp. 119–125. [Google Scholar]
- Tonelli, M.L.; Figueredo, M.S.; Rodríguez, J.; Fabra, A.; Ibañez, F. Induced systemic resistance-like responses elicited by rhizobia. Plant Soil 2020, 448, 1–14. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.; Pieterse, C.M.J.; Bakker, P.A.H.M.; Berendsen, R.L. Beneficial microbes going underground of root immunity. Plant Cell Environ. 2019, 42, 2860–2870. [Google Scholar] [CrossRef] [Green Version]
- Dunlap, C.A. Taxonomy of registered Bacillus spp. strains used as plant pathogen antagonists. Biol. Control 2019, 134, 82–86. [Google Scholar] [CrossRef] [Green Version]
- Gadhave, K.R.; Devlin, P.F.; Ebertz, A.; Ross, A.; Gange, A.C. Soil inoculation with Bacillus spp. modifies root endophytic bacterial diversity, evenness, and community composition in a context-specific manner. Microb. Ecol. 2018, 76, 741–750. [Google Scholar] [CrossRef] [Green Version]
- Gerayeli, N.; Baghaee-Ravari, S.; Tarighi, S. Evaluation of the antagonistic potential of Bacillus strains against Pectobacterium carotovorum subsp. carotovorum and their role in the induction of resistance to potato soft rot infection. Eur. J. Plant Pathol. 2018, 150, 1049–1063. [Google Scholar]
- Chen, X.H.; Koumoutsi, A.; Scholz, R.; Borriss, R. More than anticipated—Production of antibiotics and other secondary metabolites by Bacillus amyloliquefaciens FZB42. J. Mol. Microbiol. Biotechnol. 2008, 16, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Kurabachew, H.; Stahl, F.; Wydra, K. Global gene expression of rhizobacteria-silicon mediated induced systemic resistance in tomato (Solanum lycopersicum) against Ralstonia solanacearum. Physiol. Mol. Plant Pathol. 2013, 84, 44–52. [Google Scholar] [CrossRef]
- Etesami, H.; Jeong, B.R. Silicon (Si): Review and future prospects on the action mechanisms in alleviating biotic and abiotic stresses in plants. Ecotoxicol. Environ. Saf. 2018, 147, 881–896. [Google Scholar] [CrossRef] [PubMed]
- Grosch, R.; Koch, T.; Kofoet, A. Control of bottom rot on lettuce caused by Rhizoctonia solani with commercial biocontrol agents and a novel fungicide. J. Plant Dis. Prot. 2004, 111, 572–582. [Google Scholar]
- Lipsky, A.; Cohen, A.; Ion, A.; Yedidia, I. Genetic transformation of Ornithogalum via particle bombardment and generation of Pectobacterium carotovorum-resistant plants. Plant Sci. 2014, 228, 150–158. [Google Scholar] [CrossRef]
- Kersey, C.M.; Agyemang, P.A.; Dumenyo, C.K. CorA, the magnesium/nickel/cobalt transporter, affects virulence and extracellular enzyme production in the soft rot pathogen Pectobacterium carotovorum. Mol. Plant Pathol. 2012, 13, 58–71. [Google Scholar] [CrossRef]
- Angel, R.; Claus, P.; Conrad, R. Methanogenic archaea are globally ubiquitous in aerated soils and become active under wet anoxic conditions. ISME J. 2012, 6, 847–862. [Google Scholar] [CrossRef] [Green Version]
- Walters, W.; Hyde, E.R.; Berg-Lyons, D.; Ackermann, G.; Humphrey, G.; Parada, A.; Gilbert, J.A.; Jansson, J.K.; Caporaso, J.G.; Fuhrman, J.A.; et al. Improved bacterial 16S rRNA gene (V4 and V4-5) and fungal internal transcribed spacer marker gene primers for microbial community surveys. mSystems 2016, 1, 09–15. [Google Scholar] [CrossRef] [Green Version]
- Moonsamy, P.V.; Williams, T.; Bonella, P.; Holcomb, C.L.; Höglund, B.N.; Hillman, G.; Goodridge, D.; Turenchalk, G.S.; Blake, L.A.; Daigle, D.A.; et al. High-throughput HLA genotyping using 454 sequencing and the Fluidigm Access ArrayTM system for simplified amplicon library preparation. Tissue Antigens 2013, 81, 141–149. [Google Scholar] [CrossRef]
- Green, S.J.; Venkatramanan, R.; Naqib, A. Deconstructing the polymerase chain reaction: Understanding and correcting bias associated with primer degeneracies and primer-template mismatches. PLoS ONE 2015, 10, e0128122. [Google Scholar]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Orozco-Mosqueda, M.C.; Rocha-Granados, M.C.; Glick, B.R.; Santoyo, G. Microbiome engineering to improve biocontrol and plant growth-promoting mechanisms. Microbiol. Res. 2018, 208, 25–31. [Google Scholar] [CrossRef]
- Qiao, Q.; Wang, F.; Zhang, J.; Chen, Y.; Zhang, C.; Liu, G.; Zhang, H.; Ma, C.; Zhang, J. The variation in the rhizosphere microbiome of cotton with soil type, genotype and developmental stage. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Barnett, B.A.; Holm, D.G.; Koym, J.W.; Wilson, R.G.; Manter, D.K. Site and clone effects on the potato root-associated core microbiome and its relationship to tuber yield and nutrients. Am. J. Potato Res. 2015, 92, 1–9. [Google Scholar] [CrossRef]
- Kwak, M.J.; Kong, H.G.; Choi, K.; Kwon, S.K.; Song, J.Y.; Lee, J.; Lee, P.A.; Choi, S.Y.; Seo, M.; Lee, H.J.; et al. Rhizosphere microbiome structure alters to enable wilt resistance in tomato. Nat. Biotechnol. 2018, 36, 1100–1116. [Google Scholar] [CrossRef]
- Pfeiffer, S.; Mitter, B.; Oswald, A.; Schloter-Hai, B.; Schloter, M.; Declerck, S.; Sessitsch, A. Rhizosphere microbiomes of potato cultivated in the High Andes show stable and dynamic core microbiomes with different responses to plant development. FEMS Microbiol. Ecol. 2017, 93, fiw242. [Google Scholar] [CrossRef]
- Roquigny, R.; Novinscak, A.; Marcoux, N.; Joly, D.L.; Filion, M. Deciphering the rhizosphere and geocaulosphere microbiomes of potato following inoculation with the biocontrol agent Pseudomonas fluorescens strain LBUM223. Phytobiomes 2018, 2, 92–99. [Google Scholar] [CrossRef] [Green Version]
- Stringlis, I.A.; Proietti, S.; Hickman, R.; Van Verk, M.C.; Zamioudis, C.; Pieterse, C.M.J. Root transcriptional dynamics induced by beneficial rhizobacteria and microbial immune elicitors reveal signatures of adaptation to mutualists. Plant J. 2018, 93, 166–180. [Google Scholar] [CrossRef] [Green Version]
- Pepe-Ranney, C.; Keyser, C.; Trimble, J.K.; Bissinger, B. Surveying the sweetpotato rhizosphere, endophyte, and surrounding soil microbiomes at two North Carolina farms reveals underpinnings of sweetpotato microbiome community assembly. Phytobiomes 2019, 4, 75–89. [Google Scholar] [CrossRef] [Green Version]
- Bonilla, N.; Cazorla, F.M.; Martínez-Alonso, M.; Hermoso, J.M.; González-Fernández, J.J.; Gaju, N.; Landa, B.B.; de Vicente, A. Organic amendments and land management affect bacterial community composition, diversity and biomass in avocado crop soils. Plant Soil 2012, 357, 215–226. [Google Scholar] [CrossRef] [Green Version]
- Delgado-Baquerizo, M.; Reich, P.B.; Khachane, A.N.; Campbell, C.D.; Thomas, N.; Freitag, T.E.; Abu Al-Soud, W.; Sørensen, S.; Bardgett, R.D.; Singh, B.K. It is elemental: Soil nutrient stoichiometry drives bacterial diversity. Environ. Microbiol. 2017, 19, 1176–1188. [Google Scholar] [CrossRef]
- Gedion, B.W. Beneficial Microorganisms to Support. The Growth and Health of Ornamentals. Master’s Thesis, Hebrew University in Jerusalem, Robert H. Smith Faculty of Agriculture, Food and Environment, Rehovot, Israel, 2017. [Google Scholar]
- Edgar, R.C. Updating the 97% identity threshold for 16S ribosomal RNA OTUs. Bioinformatics 2018, 34, 2371–2375. [Google Scholar] [CrossRef]
- Tripathi, B.M.; Moroenyane, I.; Sherman, C.; Lee, Y.K.; Adams, J.M.; Steinberger, Y. Trends in taxonomic and functional composition of soil microbiome along a precipitation gradient in Israel. Microb. Ecol. 2017, 74, 168–176. [Google Scholar] [CrossRef]
- Ettenauer, J.D.; Piñar, G.; Lopandic, K.; Spangl, B.; Ellersdorfer, G.; Voitl, C.; Sterflinger, K. Microbes on building materials—Evaluation of DNA extraction protocols as common basis for molecular analysis. Sci. Total Environ. 2012, 439, 44–53. [Google Scholar] [CrossRef]
- Znój, A.; Grzesiak, J.; Gawor, J.; Gromadka, R.; Chwedorzewska, K.J.; Znój, C.; Grzesiak, A.; Gawor, J.; Gromadka, J.; Chwedorzewska, R.; et al. Bacterial communities associated with Poa annua roots in Central European (Poland) and Antarctic settings (King George Island). Microorganisms 2021, 9, 811. [Google Scholar] [CrossRef]
- Bonanomi, G.; Lorito, M.; Vinale, F.; Woo, S.L. Organic amendments, beneficial microbes, and soil microbiota: Toward a unified framework for disease suppression. Annu. Rev. Phytopathol. 2018, 56, 1–20. [Google Scholar] [CrossRef]
- Wei, Z.; Gu, Y.; Friman, V.P.; Kowalchuk, G.A.; Xu, Y.; Shen, Q.; Jousset, A. Initial soil microbiome composition and functioning predetermine future plant health. Sci. Adv. 2019, 5, 759–784. [Google Scholar] [CrossRef] [Green Version]
- Kuczynski, J.; Stombaugh, J.; Walters, W.A.; González, A.; Caporaso, J.G.; Knight, R. Using QIIME to analyze 16S rRNA gene sequences from microbial communities. Curr. Protoc. Microbiol. 2012, 27, 1E.5.1–1E.5.20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, J.R.; Yedidiaa, I. Breeding for resistance to soft rot disease in Ornithogalum. In Proceedings of the Acta Horticulturae; International Society for Horticultural Science: Leuven, Belgium, 2017; Volume 1171, pp. 279–283. [Google Scholar]
- Yedidia, I.; Schultz, K.; Golan, A.; Gottlieb, H.E.; Kerem, Z. Structural elucidation of three novel kaempferol otri-glycosides that are involved in the defense response of hybrid Ornithogalum to Pectobacterium carotovorum. Molecules 2019, 24, 2910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luzzatto-Knaan, T.; Yedidia, I. Induction of disease resistance in ornamental geophytes. Isr. J. Plant Sci. 2009, 57, 401–410. [Google Scholar] [CrossRef]
- Berg, G.; Smalla, K. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol. Ecol. 2009, 68, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trivedi, P.; Delgado-Baquerizo, M.; Anderson, I.C.; Singh, B.K. Response of soil properties and microbial communities to agriculture: Implications for primary productivity and soil health indicators. Front. Plant Sci. 2016, 7, 990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mhlongo, M.I.; Piater, L.A.; Madala, N.E.; Labuschagne, N.; Dubery, I.A. The chemistry of plant–microbe interactions in the rhizosphere and the potential for metabolomics to reveal signaling related to defense priming and induced systemic resistance. Front. Plant Sci. 2018, 9, 112. [Google Scholar] [CrossRef] [Green Version]
- Hanif, A.; Zhang, F.; Li, P.; Li, C.; Xu, Y.; Zubair, M.; Zhang, M.; Jia, D.; Zhao, X.; Liang, J.; et al. Fengycin produced by Bacillus amyloliquefaciens FZB42 inhibits Fusarium graminearum growth and mycotoxins biosynthesis. Toxins 2019, 11, 295. [Google Scholar] [CrossRef] [Green Version]
- Arizala, D.; Arif, M. Genome-wide analyses revealed remarkable heterogeneity in pathogenicity determinants, antimicrobial compounds, and CRISPR-cas systems of complex phytopathogenic genus Pectobacterium. Pathogens 2019, 8, 247. [Google Scholar] [CrossRef] [Green Version]
- Agrios, G.N. Environmental effects on the development of infectious plant disease. In Plant Pathology; Elsevier Academic Press: Burlington, MA, USA, 2005; pp. 347–375. [Google Scholar]
- Eo, J.; Park, K.C. Long-term effects of imbalanced fertilization on the composition and diversity of soil bacterial community. Agric. Ecosyst. Environ. 2016, 231, 176–182. [Google Scholar] [CrossRef]
- Dai, Z.; Su, W.; Chen, H.; Barberán, A.; Zhao, H.; Yu, M.; Yu, L.; Brookes, P.C.; Schadt, C.W.; Chang, S.X.; et al. Long-term nitrogen fertilization decreases bacterial diversity and favors the growth of Actinobacteria and Proteobacteria in agro-ecosystems across the globe. Glob. Chang. Biol. 2018, 24, 3452–3461. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Friesem, G.; Reznik, N.; Cohen, M.S.; Carmi, N.; Kerem, Z.; Yedidia, I. Root-Associated Microbiomes, Growth and Health of Ornamental Geophytes Treated with Commercial Plant Growth-Promoting Products. Microorganisms 2021, 9, 1785. https://doi.org/10.3390/microorganisms9081785
Friesem G, Reznik N, Cohen MS, Carmi N, Kerem Z, Yedidia I. Root-Associated Microbiomes, Growth and Health of Ornamental Geophytes Treated with Commercial Plant Growth-Promoting Products. Microorganisms. 2021; 9(8):1785. https://doi.org/10.3390/microorganisms9081785
Chicago/Turabian StyleFriesem, Gavriel, Noam Reznik, Michal Sharon Cohen, Nir Carmi, Zohar Kerem, and Iris Yedidia. 2021. "Root-Associated Microbiomes, Growth and Health of Ornamental Geophytes Treated with Commercial Plant Growth-Promoting Products" Microorganisms 9, no. 8: 1785. https://doi.org/10.3390/microorganisms9081785
APA StyleFriesem, G., Reznik, N., Cohen, M. S., Carmi, N., Kerem, Z., & Yedidia, I. (2021). Root-Associated Microbiomes, Growth and Health of Ornamental Geophytes Treated with Commercial Plant Growth-Promoting Products. Microorganisms, 9(8), 1785. https://doi.org/10.3390/microorganisms9081785





