Separate and Synergic Effects of Lactobacillus uvarum LUHSS245 and Arabinogalactan on the In Vitro Antimicrobial Properties as Well as on the Fecal and Metabolic Profile of Newborn Calves
Abstract
Simple Summary
Abstract
1. Introduction
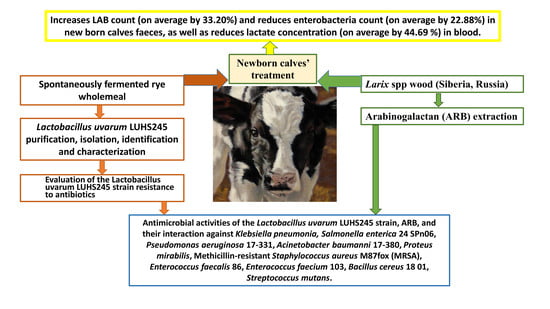
2. Materials and Methods
2.1. Lactobacillus uvarum LUHS245 Purification, Isolation, Identification, and Characterization
2.2. Evaluation of Lactobacillus uvarum LUHS245 Strain, Arabinogalactan, and their Combination Antimicrobial Activities
2.3. Evaluation of the Lactobacillus uvarum LUHS245 Strain Resistance to Antibiotics
2.4. In Vivo Experiment with Newborn Calves
2.5. In Vivo Experiment Ethical Guidelines
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of the Lactobacillus uvarum LUHS245 Strain
3.2. Antimicrobial Properties of Arabinogalactan, the Lactobacillus uvarum LUHS245 Strain, and their Combination
3.3. Lactobacillus uvarum LUHS245 Resistance to Antibiotics
3.4. The Influence of Arabinogalactan, the Lactobacillus uvarum LUHS245 Strain, and their Combination on Newborn Calves’ Health Parameters
3.5. Influence of the Lactobacillus uvarum LUHS245 Strain, Arabinogalactan, and their Combination on Newborn Calves’ Blood Parameters
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Romanowski, R.; Culbert, R.; Alkemade, S.; Medellin-Peña, M.J.; Bugarski, D.; Milovanovic, A.; Nesic, S.; Masic, A. Mycobacterium cell wall fraction immunostimulant (AMPLIMUNETM) efficacy in the reduction of the severity of ETEC induced diarrhea in neonatal calves. Acta Vet. 2017, 67, 222–237. [Google Scholar] [CrossRef]
- Berchtold, J. Treatment of Calf Diarrhea: Intravenous Fluid Therapy. Vet. Clin. North Am.: Food Anim. Pract. 2009, 25, 73–99. [Google Scholar] [CrossRef] [PubMed]
- Perales-Adán, J.; Rubiño, S.; Martínez-Bueno, M.; Valdivia, E.; Montalbán-López, M.; Cebrián, R.; Maqueda, M. LAB Bacteriocins Controlling the Food Isolated (Drug-Resistant) Staphylococci. Front. Microbiol. 2018, 9, 1143. [Google Scholar] [CrossRef] [PubMed]
- Ledina, T.; Mohar-Lorbeg, P.; Golob, M.; Djordjevic, J.; Bogovič-Matijašić, B.; Bulajic, S. Tetracycline resistance in lactobacilli isolated from Serbian traditional raw milk cheeses. J. Food. Sci. Technol. 2018, 55, 1426–1434. [Google Scholar] [CrossRef]
- Froehlich, K.A.; Abdelsalam, K.W.; Chase, C.; Koppien-Fox, J.; Casper, D.P. Evaluation of essential oils and prebiotics for newborn dairy calves. J. Anim. Sci. 2017, 95, 3772–3782. [Google Scholar] [CrossRef]
- Vieco-Saiz, N.; Belguesmia, Y.; Raspoet, R.; Auclair, E.; Gancel, F.; Kempf, I.; Drider, D. Benefits and Inputs From Lactic Acid Bacteria and Their Bacteriocins as Alternatives to Antibiotic Growth Promoters During Food-Animal Production. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Ricke, S.C. Impact of Prebiotics on Poultry Production and Food Safety. Yale J. Biol. Med. 2018, 91, 151–159. [Google Scholar]
- Dion, C.; Chappuis, E.; Ripoll, C. Does larch arabinogalactan enhance immune function? A review of mechanistic and clinical trials. Nutr. Metab. 2016, 13, 28. [Google Scholar] [CrossRef]
- Fitzpatrick, A.; Roberts, A.; Witherly, S. Larch arabinogalactan: A novel and multifunctional natural product. Agro Food Ind. Hi-Tech 2004, 15, 30–32. [Google Scholar]
- Marzorati, M.; Verhelst, A.; Luta, G.; Sinnott, R.; Verstraete, W.; De Wiele, T.V.; Possemiers, S. In vitro modulation of the human gastrointestinal microbial community by plant-derived polysaccharide-rich dietary supplements. Int. J. Food Microbiol. 2010, 139, 168–176. [Google Scholar] [CrossRef]
- Kiss, H.; Kögler, B.; Petricevic, L.; Sauerzapf, I.; Klayraung, S.; Domig, K.; Viernstein, H.; Kneifel, W. Vaginal Lactobacillus microbiota of healthy women in the late first trimester of pregnancy. BJOG: Int. J. Obstet. Gynaecol. 2007, 114, 1402–1407. [Google Scholar] [CrossRef] [PubMed]
- Versalovic, J.; Schneider, M.; Bruijn, F.J.D.; Lupski, J.R. Genomic fingerprinting of bacteria using repetitive sequence-based polymerase chain reaction. Methods Mol. Cell Biol. 1994, 5, 25–40. [Google Scholar]
- Song, Y.; Kato, N.; Liu, C.; Matsumiya, Y.; Kato, H.; Watanabe, K. Rapid identification of 11 human intestinal Lactobacillus species by multiplex PCR assays using group- and species-specific primers derived from the 16S-23S rRNA intergenic spacer region and its flanking 23S rRNA. FEMS Microbiol. Lett. 2000, 187, 167–173. [Google Scholar] [CrossRef]
- Lee, Y.; Yun, H.S.; Cho, K.W.; Oh, S.; Kim, S.T.; Chun, T.; Kim, B.; Whang, K.Y. Evaluation of probiotic characteristics of newly isolated Lactobacillus spp.: Immune modulation and longevity. Int. J. Food Microbiol. 2011, 148, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Cizeikiene, D.; Juodeikiene, G.; Paskevicius, A.; Bartkiene, E. Antimicrobial activity of lactic acid bacteria against pathogenic and spoilage microorganism isolated from food and their control in wheat bread. Food Control 2013, 31, 539–545. [Google Scholar] [CrossRef]
- EFSA. Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J. 2012, 10, 2740. [Google Scholar]
- Law Republic of Lithuania Amending the Law on the Carem Keeping and Use of Animal. 2012, pp. 1–47. Available online: https://e-seimas.lrs.lt/portal/legalAct/lt/TAD/TAIS.434660 (accessed on 3 February 2020).
- Kobierecka, P.A.; Wyszyńska, A.K.; Aleksandrzak-Piekarczyk, T.; Kuczkowski, M.; Tuzimek, A.; Piotrowska, W.; Górecki, A.; Adamska, I.; Wieliczko, A.; Bardowski, J.; et al. In vitro characteristics of Lactobacillus spp. strains isolated from the chicken digestive tract and their role in the inhibition of Campylobacter colonization. Microbiologyopen 2017, 6. [Google Scholar] [CrossRef]
- Amado, L.; Berends, H.; Leal, L.N.; Wilms, J.; Van Laar, H.; Gerrits, W.J.J.; Martín-Tereso, J. Effect of energy source in calf milk replacer on performance, digestibility, and gut permeability in rearing calves. J. Dairy Sci. 2019, 102, 3994–4001. [Google Scholar] [CrossRef]
- Bull, M.; Plummer, S.; Marchesi, J.; Mahenthiralingam, E. The life history of Lactobacillus acidophilus as a probiotic: A tale of revisionary taxonomy, misidentification and commercial success. FEMS Microbiol. Lett. 2013, 349, 77–87. [Google Scholar] [CrossRef]
- Bengoa, A.A.; Zavala, L.; Carasi, P.; Trejo, S.A.; Bronsoms, S.; Serradell, M.; De los, Á.; Garrote, G.L.; Abraham, A.G. Simulated gastrointestinal conditions increase adhesion ability of Lactobacillus paracasei strains isolated from kefir to Caco-2 cells and mucin. Food Res. Int. 2018, 103, 462–467. [Google Scholar] [CrossRef]
- Drackley, J.K. Calf Nutrition from Birth to Breeding. Vet. Clin. North Am. Food Anim. Pract. 2008, 24, 55–86. [Google Scholar] [CrossRef] [PubMed]
- Ghazanfar, S.; Riaz, A.; Tahir, M.N.; Maqbool, S.; Ali, G.M.; Tariq, F.; Arif, I. Probiotic Supplement Improves the Health Status and Lactation Performance in Dairy Animals. In Lactation in Farm Animals—Biology, Physiological Basis, Nutritional Requirements, and Modelization; IntechOpen: London, UK, 2019. [Google Scholar]
- Georgieva, R.; Yocheva, L.; Tserovska, L.; Zhelezova, G.; Stefanova, N.; Atanasova, A.; Danguleva, A.; Ivanova, G.; Karapetkov, N.; Rumyan, N.; et al. Antimicrobial activity and antibiotic susceptibility of Lactobacillus and Bifidobacterium spp. intended for use as starter and probiotic cultures. Biotechnol. Biotechnol. Equip. 2015, 29, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Bartkiene, E.; Lele, V.; Sakiene, V.; Zavistanaviciute, P.; Ruzauskas, M.; Bernatoniene, J.; Jakstas, V.; Viskelis, P.; Zadeike, D.; Juodeikiene, G. Improvement of the antimicrobial activity of lactic acid bacteria in combination with berries/fruits and dairy industry by-products. J. Sci. Food Agr. 2019, 99, 3992–4002. [Google Scholar] [CrossRef] [PubMed]
- Todorov, S.D.; De Melo Franco, B.D.G.; Tagg, J.R. Bacteriocins of Gram-positive bacteria having activity spectra extending beyond closely-related species. Benef. Microbes 2019, 10, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Grube, B.; Stier, H.; Riede, L.; Gruenwald, J. Tolerability of a Proprietary Larch Arabinogalactan Extract: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial in Healthy Subjects. FNS 2012, 3, 1533–1538. [Google Scholar] [CrossRef]
- Nazareth, M.R.; Kennedy, C.E.; Bhatia, V.N. Studies on larch arabogalactan I. J. Pharm. Sci. 1961, 50, 560–563. [Google Scholar] [CrossRef]
- Sharon, N.; Ofek, I. Safe as mother’s milk: Carbohydrates as future anti-adhesion drugs for bacterial diseases. Glycoconj. J. 2000, 17, 659–664. [Google Scholar] [CrossRef]
- Mousavifar, L.; Touaibia, M.; Roy, R. Development of Mannopyranoside Therapeutics against Adherent-Invasive Escherichia coli Infections. Acc. Chem. Res. 2018, 51, 2937–2948. [Google Scholar] [CrossRef]
- Qiao, M.; Ying, G.-G.; Singer, A.C.; Zhu, Y.-G. Review of antibiotic resistance in China and its environment. Environ. Int. 2018, 110, 160–172. [Google Scholar] [CrossRef]
- Alrubaye, H.H.; Fakhry, S.S.; Jebur, Z.A.; Farhan, F. Biochemical and Molecular Characterization of Lactobacillus spp. Isolated from Dairy Products. JSM Microbiol. 2018, 6, 1–7. [Google Scholar]
- Kawakami, S.I.; Yamada, T.; Nakanishi, N.; Cai, Y. Feeding of lactic acid bacteria and yeast affects fecal flora of Holstein calves. J. Anim. Vet. Adv. 2011, 10, 269–271. [Google Scholar] [CrossRef][Green Version]
- Dowarah, R.; Verma, A.K.; Agarwal, N. The use of Lactobacillus as an alternative of antibiotic growth promoters in pigs: A review. Anim. Nutr. 2017, 3, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Trofimova, N.N.; Medvedeva, E.N.; Ivanova, N.V.; Malkov, Y.A.; Babkin, V.A. Polysaccharides from Larch Biomass. In The Complex World of Polysaccharides; IntechOpen: London, UK, 2012; pp. 153–194. [Google Scholar]
- Den Besten, G.; Van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; De los Reyes-Gavilán, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front. Microbiol. 2016, 7. [Google Scholar]
- Bodera, P. Influence of Prebiotics on the Human Immune System (GALT). Recent Pat. Inflamm. Allergy Drug Discov. 2008, 2, 149–153. [Google Scholar] [CrossRef]
- Patel, S.; Goyal, A. The current trends and future perspectives of prebiotics research: A review. 3 Biotech. 2012, 2, 115–125. [Google Scholar] [CrossRef]
- Ramaiah, S.K. A toxicologist guide to the diagnostic interpretation of hepatic biochemical parameters. Food Chem. Toxicol. 2007, 45, 1551–1557. [Google Scholar] [CrossRef]
- Huang, X.-J.; Choi, Y.-K.; Im, H.-S.; Yarimaga, O.; Yoon, E.; Kim, H.-S. Aspartate Aminotransferase (AST/GOT) and Alanine Aminotransferase (ALT/GPT) Detection Techniques. Sensors (Basel) 2006, 6, 756–782. [Google Scholar] [CrossRef]
- Bleul, U.; Götz, E. The effect of lactic acidosis on the generation and compensation of mixed respiratory-metabolic acidosis in neonatal calves. Vet. Rec. 2013, 172, 528. [Google Scholar] [CrossRef]
- Lorenz, I. Influence of d-lactate on Metabolic Acidosis and on Prognosis in Neonatal Calves with Diarrhoea. J. Vet. Med. A 2004, 51, 425–428. [Google Scholar] [CrossRef]
- Bartkiene, E.; Krungleviciute, V.; Antanaitis, R.; Kantautaite, J.; Kucinskas, A.; Ruzauskas, M.; Vaskeviciute, L.; Siugzdiniene, R.; Kucinskiene, J.; Damasius, J.; et al. Antimicrobial activity of lactic acid bacteria multiplied in an alternative substrate and their influence on physiological parameters of new-born calves. Vet. Med.-Czech 2016, 61, 653–662. [Google Scholar] [CrossRef]
- Lettat, A.; Nozière, P.; Silberberg, M.; Morgavi, D.P.; Berger, C.; Martin, C. Rumen microbial and fermentation characteristics are affected differently by bacterial probiotic supplementation during induced lactic and subacute acidosis in sheep. BMC Microbiol. 2012, 12, 142. [Google Scholar] [CrossRef] [PubMed]

| Carbohydrate | Interpretation of LAB Growth in API 50 CH System | |
|---|---|---|
| Glycerol | - | |
| Erythritol | - | |
| D-arabinose | - | |
| L-arabinose | - | |
| D-ribose | - | |
| D-xylose | - | |
| L-xylose | - | |
| D-adonitol | - | |
| Methyl-ßd-xYlopiranoside | - | |
| D-galactose | - | |
| D-glucose | +++ | |
| D-fructose | +++ | |
| D-mannose | +++ | |
| L-sorbose | + | |
| L-rhamnose | - | |
| Dulcitol | - | |
| Inositol | - | |
| D-mannitol | +++ | |
| D-sorbitol | - | |
| Methyl-αD-mannopyranoside | - | |
| Methyl-αD-glucopyranoside | +++ | |
| N-acetylglucosamine | +++ | |
| Amigdalin | +++ | |
| Arbutin | +++ | |
| Esculin | +++ | |
| Salicin | +++ | |
| D-cellobiose | +++ | |
| D-maltose | +++ | |
| D-lactose | - | |
| D-melibiose | - | |
| D-sucrose | +++ | |
| D-trehalose | +++ | |
| Inulin | - | |
| D-melezitose | - | |
| D-raffinose | - | |
| Starch | - | |
| Glycogen | - | |
| Xylitol | - | |
| Gentiobiose | +++ | |
| D-turanose | +++ | |
| D-lyxose | - | |
| D-tagatose | - | |
| D-fucose | - | |
| L-fucose | - | |
| D-arabitol | - | |
| L-arabitol | - | |
| Potassium gluconate | - | |
| Potassium 2-ketogluconate | - | |
| Potassium 5-ketogluconate | - | |
| Gas production (+/-) | - | |
| Tolerance to temperature | 10 °C 30 °C 37 °C 45 °C | - |
| ++ | ||
| ++ | ||
| - | ||
| pH 2.5 | After 0 h, LAB count, log10 CFU mL−1 After 2 h, LAB count, log10 CFU mL−1 | 9.03 ± 0.2 |
| 7.55 ± 0.1 | ||
| Microorganisms | Zone of Inhibition (mm) | Antibiotics | Resistance to Antibiotics | |||
|---|---|---|---|---|---|---|
| L. uvarum | L. uvarum and Arabinogalactan Mix | Arabino-galactan | L. uvarum | FEEDAP Breakpoint (mg mL −1) | ||
| MIC (mg mL−1) | ||||||
| Klebsiella pneumoniae | 14.0 ± 0.2 | - | - | |||
| Salmonella enterica | 13.0 ± 0.3 | 9.0 ± 0.1 | - | GEN | 16.0 ± 0.2 | 16 |
| Pseudomonas aeruginosa 17-331 | 16.2 ± 0.3 | - | - | |||
| Acinetobacter baumanni 17-380 | 15.0 ± 0.4 | 14.0 ± 0.2 | - | TET | 0.75 ± 0.4 | 8 |
| Proteus mirabilis | 15.0 ± 0.1 | - | 17.0 ± 0.3 | |||
| MRSA M87fox | 16.0 ± 0.2 | 9.0 ± 0.2 | 11.0 ± 0.1 | ERY | 0.0016 ± 0.3 | 1 |
| Enterococcus faecalis 86 | 16.0 ± 0.3 | - | - | |||
| Enterococcus faecium 103 | 20.0 ± 0.2 | 14.0 ± 0.3 | AML | 0.016 ± 0.1 | n.r. | |
| Bacillus cereus 18 01 | 21.3 ± 0.5 | - | 10.0 ±0.2 | |||
| Streptococcus mutans | 15.0 ± 0.1 | - | - | TM | 0.75 ± 0.2 | n.r. |
| Variable | Day | Treatments | p-Value | |||
|---|---|---|---|---|---|---|
| CON | LUH | ARA | BOTH | Day × Treat. Int. | ||
| TCM | Baseline | 6.15 ± 0.02 Ab | 7.73 ± 0.02 Ad | 6.92 ± 0.01 Ac | 5.26 ± 0.01 Aa | 0.0001 |
| 14 | 8.21 ± 0.01 Bb | 8.34 ± 0.03 Bc | 8.46 ± 0.03 Bd | 7.80 ± 0.05 Ba | ||
| LAB | Baseline | 3.66 ± 0.01 Aa | 4.08 ± 0.03 Ab | 5.84 ± 0.02 Ad | 4.13 ± 0.01 Abc | 0.0001 |
| 14 | 6.15 ± 0.04 Ba | 7.53 ± 0.04 Bd | 6.48 ± 0.02 Bb | 7.02 ± 0.03 Bc | ||
| TCE | Baseline | 4.81 ± 0.01 Aa | 6.55 ± 0.01 Ac | 6.78 ± 0.03 Bd | 6.46 ± 0.03 Bb | 0.0001 |
| 14 | 6.92 ± 0.02 Bd | 5.68 ± 0.06 Bc | 4.94 ± 0.03 Ab | 4.65 ± 0.04 Aa | ||
| Y/F | Baseline | 3.45 ± 0.02 Aa | 6.97 ± 0.03 Ad | 5.89 ± 0.01 Bc | 3.94 ± 0.01 Ab | 0.0001 |
| 14 | 5.54 ± 0.03 Ba | 5.87 ± 0.03 Ab | 5.65 ± 0.02 Aa | 6.19 ± 0.03 Bc | ||
| Variable | Day | Treatments | p-Value | |||
|---|---|---|---|---|---|---|
| CON | LUH | ARA | BOTH | Day × Treat. Int. | ||
| Alb. | Baseline | 26.55 ± 0.50 Aa | 27.13 ± 0.11 Aa | 26.40 ± 0.82 Aa | 34.85 ± 0.73 Aa | 0.0001 |
| 14 | 26.84 ± 0.93 Aa | 27.83 ± 0.72 Aa | 36.81 ± 0.74 Bc | 38.92 ± 0.95 Bd | ||
| Urea | Baseline | 3.35 ± 0.02 Ba | 2.82 ± 0.07 Aa | 3.63 ± 0.01 Ba | 3.34 ± 0.09 Bb | 0.0001 |
| 14 | 2.26 ± 0.03 Ab | 3.11 ± 0.02 Bc | 2.21 ± 0.01 Ad | 2.52 ± 0.03 Aa | ||
| AST | Baseline | 54.67 ± 1.01 Ab | 59.50 ± 1.02 Bc | 79.33 ± 1.47 Ba | 37.34 ± 1.97 Ab | 0.0001 |
| 14 | 50.67 ± 1.44 Ad | 44.00 ± 1.55 Ac | 28.51 ± 1.72 Aa | 39.00 ± 1.93 Aa | ||
| pH | Baseline | 7.45 ± 0.02 Aa | 7.41 ± 0.05 Aa | 7.42 ± 0.07 Aa | 7.43 ± 0.08 Aa | 0.956 |
| 14 | 7.40 ± 0.08 Aa | 7.39 ± 0.04 Aa | 7.37 ± 0.02 Aa | 7.39 ± 0.01 Aa | ||
| PCO2 mmHg | Baseline | 41.00 ± 0.91 Ab | 50.15 ± 1.24 Ac | 43.20 ± 1.58 Ab | 37.75 ± 1.65 Aa | 0.056 |
| 14 | 52.28 ± 0.53 Bb | 48.25 ± 0.61 Aa | 57.85 ± 0.22 Bc | 51.48 ± 0.41 Bb | ||
| PO2 mmHg | Baseline | 165.23 ± 2.72 Bc | 133.95 ± 3.42 Ba | 158.97 ± 3.61 Ba | 135.97 ± 4.64 Bc | 0.0001 |
| 14 | 103.90 ± 2.91 Ad | 76.53 ± 3.22 Ab | 66.9 ± 2.23 Aa | 82.53 ± 2.34 Aa | ||
| HCO3 mmol/L | Baseline | 27.85 ± 0.93 Aa | 31.20 ± 0.88 Ab | 28.00 ± 0.51 Aa | 30.13 ± 0.78 Ab | 0.0001 |
| 14 | 32.40 ± 0.94 Bab | 29.13 ± 0.83 Aa | 33.03 ± 0.82 Bc | 31.18 ± 0.91 Ba | ||
| BE (ecf) | Baseline | 3.77 ± 0.05 Ab | 6.48 ± 0.02 Ba | 3.58 ± 0.03 Ac | 3.11 ± 0.02 Ab | 0.0001 |
| 14 | 7.55 ± 0.01 Bb | 4.15 ± 0.03 Ab | 7.68 ± 0.02 Ba | 6.25 ± 0.05 Ba | ||
| O2 saturation | Baseline | 92.65 ± 1.62 A;a | 86.72 ± 1.51 Aa | 98.42 ± 2.35 Ba | 94.56 ± 2.64 Ba | 0.0001 |
| 14 | 93.60 ± 2.48 A;b | 91.52 ± 3.63 Bb | 88.12 ± 1.93 Aa | 87.88 ± 2.92 Aa | ||
| Na, mmol/L | Baseline | 139.67 ± 4.51 A;a | 137.50 ± 3.92 Aa | 139.00 ± 3.23 Aa | 136.54 ± 2.91 Aa | 0.438 |
| 14 | 137.83 ± 3.73 A;a | 138.50 ± 3.43 Aa | 137.00 ± 2.54 Aa | 141.00 ± 5.39 Aa | ||
| K, mmol/L | Baseline | 5.07 ± 0.03 A;c | 4.57 ± 0.03 Aa | 4.80 ± 0.02 Ab | 11.17 ± 0.04 Bd | 0.0001 |
| 14 | 5.52 ± 0.09 Bb | 4.90 ± 0.01 Bb | 5.07 ± 0.07 Bb | 4.75 ± 0.01 Ab | ||
| iCa, mmol/L | Baseline | 1.24 ± 0.01 Aa | 1.27 ± 0.02 Ab | 1.28 ± 0.03 Ab | 1.81 ± 0.02 Bd | 0.0001 |
| 14 | 1.25 ± 0.02 Aa | 1.26 ± 0.01 Aa | 1.26 ± 0.03 Aa | 1.26 ± 0.02 Aa | ||
| TCO2, mmol/L | Baseline | 29.10 ± 1.21 ab | 32.72 ± 2.07 b | 29.33 ± 2.61 ab | 23.98 ± 3.65 a | 0.154 |
| 14 | 34.02 ± 2.03 a | 30.60 ± 3.61 a | 34.80 ± 2.45 a | 32.75 ± 2.36 a | ||
| Hct fraction | Baseline | 26.33 ± 3.15 Aa | 27.00 ± 4.03 Aab | 26.83 ± 2.01 Aa | 26.09 ± 1.01 Aa | 0.604 |
| 14 | 25.50 ± 2.30 Aa | 23.83 ± 1.23 Aa | 25.83 ± 1.53 Aa | 26.50 ± 1.25 Aa | ||
| Hb, g/L | Baseline | 8.92 ± 0.01 Ba | 9.23 ± 0.02 Bc | 9.18 ± 0.04 Bb | 9.11 ± 0.03 Ab | 0.0001 |
| 14 | 8.67 ± 0.03 Ab | 8.08 ± 0.05 Aa | 8.73 ± 0.04 Ab | 9.08 ± 0.02 Ac | ||
| BE(b) | Baseline | 3.40 ± 0.02 Ab | 5.72 ± 0.03 Bd | 3.17 ± 0.05 Aa | 5.11 ± 0.04 Ac | 0.0001 |
| 14 | 6.58 ± 0.03 Bc | 3.62 ± 0.04 Aa | 6.63 ± 0.05 Bc | 5.40 ± 0.04 Bb | ||
| Glu, mmol/L | Baseline | 6.37 ± 0.01 Ab | 7.17 ± 0.03 Bb | 7.42 ± 0.04 Bd | 6.16 ± 0.05 Ba | 0.031 |
| 14 | 6.05 ± 0.01 Abc | 5.92 ± 0.03 Ab | 5.93 ± 0.04 Ad | 5.21 ± 0.06 Ab | ||
| Lactate, mmol/L | Baseline | 6.19 ± 0.04 Bc | 4.25 ± 0.03 Ba | 6.24 ± 0.02 Bb | 5.88 ± 0.04 B | 0.0001 |
| 14 | 3.24 ± 0.06 Ab | 3.83 ± 0.03 Ac | 2.02 ± 0.04 Aa | 3.21 ± 0.01 Ab | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zavistanaviciute, P.; Lele, V.; Antanaitis, R.; Televičius, M.; Ruzauskas, M.; Zebeli, Q.; Bartkiene, E. Separate and Synergic Effects of Lactobacillus uvarum LUHSS245 and Arabinogalactan on the In Vitro Antimicrobial Properties as Well as on the Fecal and Metabolic Profile of Newborn Calves. Animals 2020, 10, 593. https://doi.org/10.3390/ani10040593
Zavistanaviciute P, Lele V, Antanaitis R, Televičius M, Ruzauskas M, Zebeli Q, Bartkiene E. Separate and Synergic Effects of Lactobacillus uvarum LUHSS245 and Arabinogalactan on the In Vitro Antimicrobial Properties as Well as on the Fecal and Metabolic Profile of Newborn Calves. Animals. 2020; 10(4):593. https://doi.org/10.3390/ani10040593
Chicago/Turabian StyleZavistanaviciute, Paulina, Vita Lele, Ramūnas Antanaitis, Mindaugas Televičius, Modestas Ruzauskas, Qendrim Zebeli, and Elena Bartkiene. 2020. "Separate and Synergic Effects of Lactobacillus uvarum LUHSS245 and Arabinogalactan on the In Vitro Antimicrobial Properties as Well as on the Fecal and Metabolic Profile of Newborn Calves" Animals 10, no. 4: 593. https://doi.org/10.3390/ani10040593
APA StyleZavistanaviciute, P., Lele, V., Antanaitis, R., Televičius, M., Ruzauskas, M., Zebeli, Q., & Bartkiene, E. (2020). Separate and Synergic Effects of Lactobacillus uvarum LUHSS245 and Arabinogalactan on the In Vitro Antimicrobial Properties as Well as on the Fecal and Metabolic Profile of Newborn Calves. Animals, 10(4), 593. https://doi.org/10.3390/ani10040593