The Role of Condensed Tannins in the In Vitro Rumen Fermentation Kinetics in Ruminant Species: Feeding Type Involved?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animals
2.2. Substrates
2.3. In Vitro Gas Production Assay
2.4. Short-Chain Fatty Acids Determination
2.5. Experimental Design and Statistical Analysis
3. Results
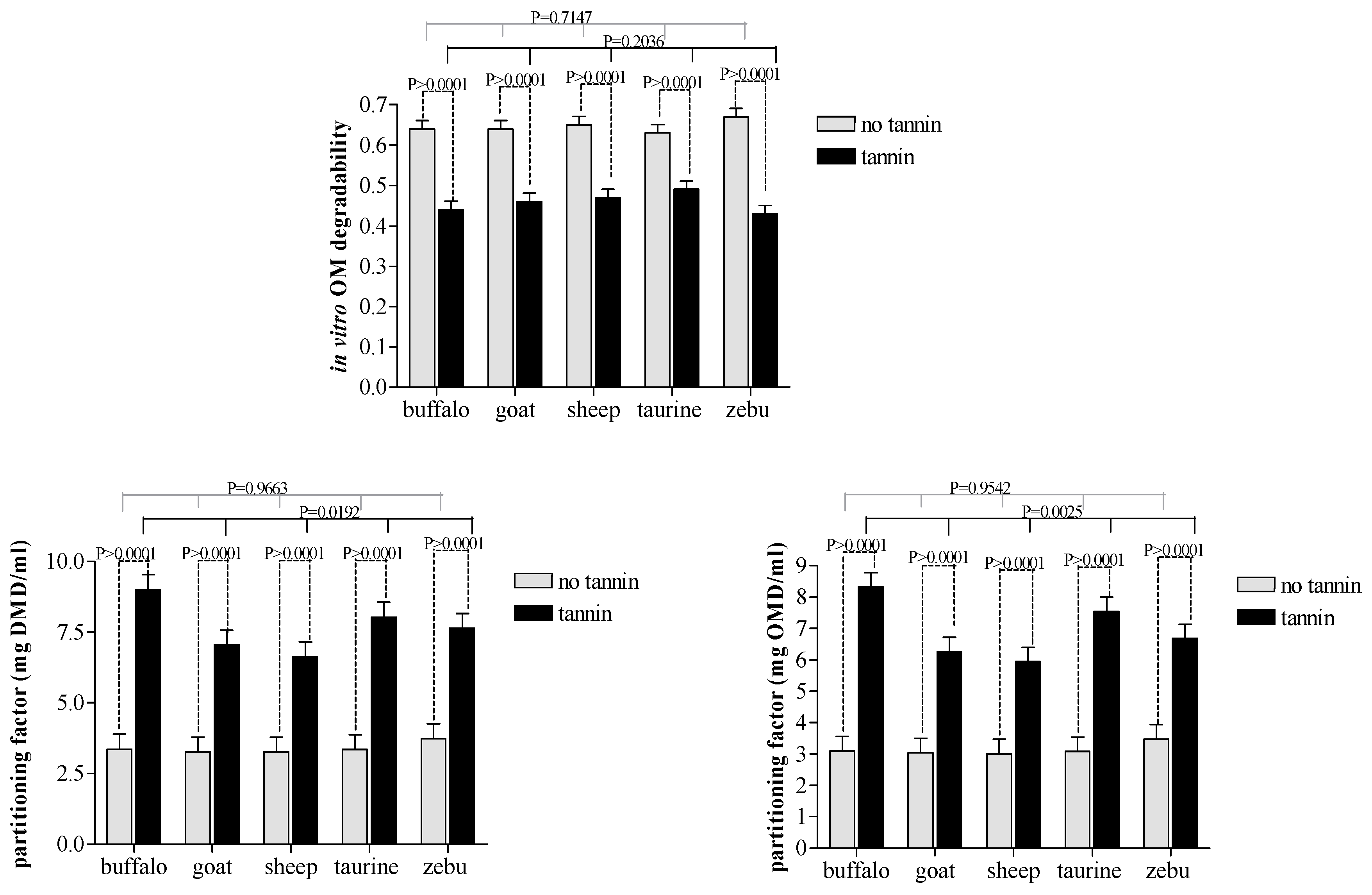
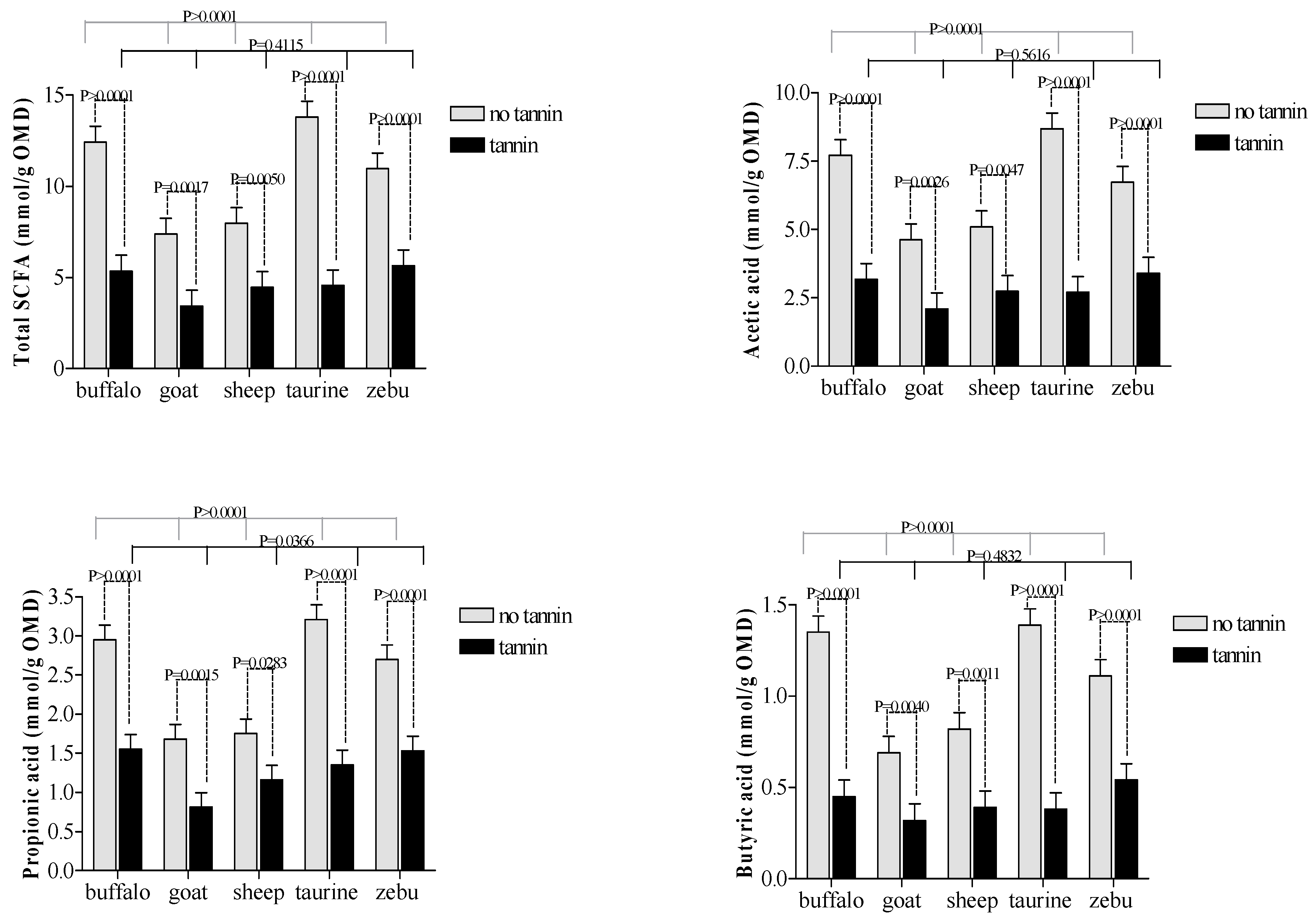
3.1. Tannin Extract Effects
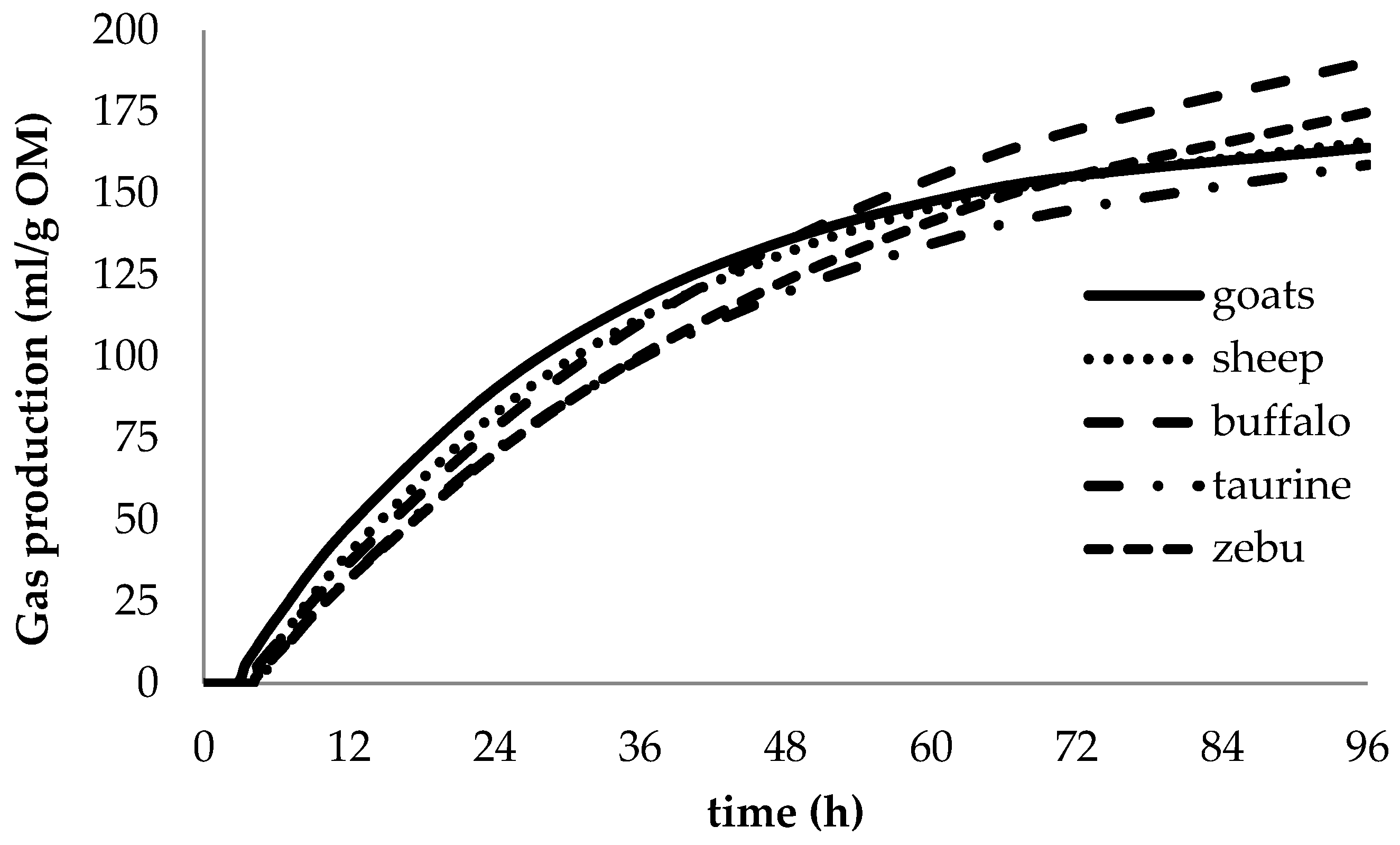
3.2. Animal Species Effects
3.3. Interactions
4. Discussion
4.1. Condensedtannin Extract Effects
4.2. Animal Species Effects
4.3. Interactions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fagundes, G.M.; Modesto, E.C.; Fonseca, C.E.M.; Lima, H.R.P.; Muir, J.P. Intake, digestibility and milk yield in goats fed Flemingia macrophylla with or without polyethylene glycol. Small Rumin. Res. 2014, 116, 88–93. [Google Scholar] [CrossRef]
- Van Soest, P.J. Nutritional Ecology of the Ruminant, 2nd ed.; Cornell University Press: Ithaca, NY, USA, 1994; 476p. [Google Scholar]
- Lamy, E.; Rawel, H.; Schweigert, F.J.; Capela e Silva, F.; Ferreira, A.; Costa, A.R.; Antunes, C.; Almeida, A.M.; Coelho, A.V.; Sales-Baptista, E. The effect of tannins on Mediterranean ruminant ingestive behavior: The role of the oral cavity. Molecules 2011, 16, 2766–2784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muir, J.P. The multi-faceted role of condensed tannins in the goat ecosystem. Small Rumin. Res. 2011, 98, 115–120. [Google Scholar] [CrossRef]
- Hofmann, R.R. Evolutionary steps of ecophysiological adaptation and diversification of ruminants: A comparative view of their digestive system. Oecologia 1989, 78, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Osawa, R.O.; Sly, L.I. Occurence of tannin-protein complex degrading Streptococcus sp. in feces of various animals. Syst. Appl. Microbiol. 1992, 15, 144–147. [Google Scholar] [CrossRef]
- Odenyo, A.A.; McSweeney, C.S.; Palmer, B.; Negassa, D.; Osuji, P.O. In vitro screening of rumen fluid samples from indigenous African ruminants provides evidence for rumen fluid with superior capacities to digest tannin-rich fodders. Aust. J. Agric. Res. 1999, 50, 1147–1157. [Google Scholar] [CrossRef]
- Jones, R.J.; Meyer, J.H.F.; Bechaz, F.M.; Stolzt, M.A.; Palmer, B.; van der Merwe, G. Comparison of rumen fluid from South African game species and from sheep to digest tanniniferous browse. Aust. J. Agric. Res. 2001, 52, 453–460. [Google Scholar] [CrossRef]
- Odenyo, A.A.; Bishop, R.; Asefa, G.; Jamnadass, R.; Odongo, D.; Osuji, P.O. Characterization of tannin-tolerant bacterial isolates from East African ruminants. Anaerobe 2001, 7, 5–156. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 16th ed.; AOAC: Arlington, VA, USA, 1995. [Google Scholar]
- Mertens, D.R. Gravimeric determination of amylase-treated neutral detergent fibre in feeds with refluxing beakers or crucibles: Collaborative study. J. Assoc. Off. Anal. Chem. 2002, 85, 1217–1240. [Google Scholar]
- Makkar, H.P. Quantification of Tannins in Tree and Shrub Foliage: A Laboratory Manual; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Makkar, H.P.S.; Blümmel, M.; Borowy, N.K.; Becker, K. Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. J. Sci. Food Technol. 1993, 61, 161–165. [Google Scholar] [CrossRef]
- Minho, A.P.; Bueno, I.C.S.; Louvandini, H.; Jackson, F.; Gennari, S.M.; Abdalla, A.L. Effect of Acacia molissima tannin extract on the control of gastrointestinal parasites in sheep. Anim. Feed Sci. Technol. 2008, 147, 172–181. [Google Scholar] [CrossRef]
- Bueno, I.C.S.; Cabral Filho, S.L.S.; Gobbo, S.P.; Louvandini, H.; Vitti, D.M.S.S.; Aballa, A.L. Influence of inoculum source in a gas production method. Anim. Feed Sci. Technol. 2005, 123–124, 96–105. [Google Scholar] [CrossRef]
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; McAllan, A.B.; France, J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim. Feed Sci. Technol. 1994, 48, 185–197. [Google Scholar] [CrossRef]
- Mauricio, R.M.; Mould, F.L.; Dhanoa, M.S.; Owen, E.; Channa, K.S.; Theodorou, M.K. A semi-automated in vitro gas production technnique for ruminant feedstuff evaluation. Anim. Feed Sci. Technol. 1999, 79, 321–330. [Google Scholar] [CrossRef]
- Blümmel, M.; Makkar, H.P.S.; Becker, K. In vitro gas production: A technique revisited. J. Anim. Physiol. Anim. Nutr. 1997, 77, 24–34. [Google Scholar] [CrossRef]
- Ørskov, E.R.; Mcdonald, I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Am. Sci. 1979, 92, 449–453. [Google Scholar] [CrossRef] [Green Version]
- Mcdonald, I. A revised model for estimation of protein degradability in the rumen. J. Agric. Sci. 1981, 96, 251–252. [Google Scholar] [CrossRef]
- Erwin, E.S.; Marco, G.J.; Emery, E.M. Volatile fatty acid analyses of blood and rumen fluid by gas chromatography. J. Dairy Sci. 1961, 44, 1768–1771. [Google Scholar] [CrossRef]
- Getachew, G.; Makkar, H.P.S.; Becker, K. Tropical browses: Contents of phenolic compounds, in vitro gas production and stoichiometric relationship between short chain fatty acid and in vitro gas production. J. Agric. Sci. 2002, 139, 341–352. [Google Scholar] [CrossRef]
- SAS INSTITUTE. The SAS System for Windows; Release 8.01; Sas Institute: Cary, NC, USA, 2000. [Google Scholar]
- Bueno, I.C.S.; Vitti, D.M.; Louvandini, H.; Abdalla, A.L. A new approach for in vitro bioassay to measure tannin biological effects based on a gas production technique. Anim. Feed Sci. Technol. 2008, 141, 153–170. [Google Scholar] [CrossRef]
- Patra, A.K.; Saxena, J. Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. J. Sci. Food Agric. 2011, 9, 24–37. [Google Scholar] [CrossRef]
- Tedeschi, L.O.; Ramírez-Restrepo, C.A.; Muir, J.P. Developing a conceptual model of possible benefits of condensed tannins for ruminant production. Animal 2014, 8, 1095–1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hervás, G.; Frutos, P.; Serrano, E.; Mantecón, A.R.; Giráldez, F.J. Effect of tannic acid on rumen degradation and intestinal digestion of treated soya bean meals in sheep. J. Agric. Sci. 2000, 135, 305–310. [Google Scholar]
- Tiemann, T.T.; Lascano, C.E.; Wettstein, H.R.; Mayer, A.C.; Kreuzer, M.; Hess, H.D. Effect of the tropical tannin-rich shrub legumes Calliandra calothyrsus and Flemingia macrophylla on methane emission and nitrogen and energy balance in growing lambs. Animal 2008, 2, 790–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hariadi, B.T.; Santoso, B. Evaluation of tropical plants containing tannin on in vitro methanogenesis and fermentation parameters using rumen fluid. J. Sci. Food Agric. 2010, 90, 456–461. [Google Scholar] [PubMed]
- Calabrò, S.; Moniello, G.; Piccolo, V.; Bovera, F.; Infascelli, F.; Tudisco, R.; Cutrignelli, M.I. Rumen fermentation and degradability in buffalo and cattle using the in vitro gas production technique. J. Anim. Physiol. Anim. Nutr. 2008, 92, 356–362. [Google Scholar]
- Bueno, I.C.; Brandi, R.A.; Franzolin, R.; Benetel, G.; Fagundes, G.M.; Abdalla, A.L.; Louvandini, H.; Muir, J.P. In vitro methane production and tolerance to condensed tannins in five ruminant species. Anim. Feed Sci. Technol. 2015, 205, 1–9. [Google Scholar] [CrossRef]
- Gordon, I.J. Browsing and grazing ruminants: Are they different beasts? Forest. Ecol. Manag. 2003, 181, 13–21. [Google Scholar] [CrossRef]
- Clauss, M.; Müller, K.; Fickel, J.; Streich, W.J.; Hatt, J.-M.; Südekum, K.-H. Macroecology of the host determines microecology of endobionts: Protozoal faunas vary with wild ruminant feeding type and body mass. J. Zool. 2011, 283, 169–185. [Google Scholar] [CrossRef] [Green Version]
- Salem, A.F.Z. Impact of season of harvest on in vitro gas production and dry matter degradability of Acacia saligna leaves with inoculum from three ruminant species. Anim. Feed Sci. Technol. 2005, 123, 67–79. [Google Scholar] [CrossRef]

| Composition | Substrates (1) | ||||
|---|---|---|---|---|---|
| ALF | ELE | TIF | SIL | ACA | |
| organic matter (2) | 916.82 | 897.75 | 936.45 | 964.64 | 978.86 |
| ether extract (2) | 84.04 | 46.72 | 57.92 | 62.98 | n.d. (5) |
| crude protein (2) | 278.97 | 60.28 | 158.02 | 82.02 | n.d. (5) |
| neutral-detergent fiber (2) | 735.62 | 770.03 | 795.29 | 563.28 | n.d. (5) |
| acid-detergent fiber (2) | 510.25 | 519.52 | 428.92 | 332.30 | n.d. (5) |
| acid-detergent lignin (2) | 126.69 | 121.63 | 133.08 | 71.35 | n.d. (5) |
| total phenols (3) | 13.60 | 5.47 | 5.32 | 10.18 | 558.63 |
| total tannins (3) | 8.14 | 3.05 | 2.82 | 6.58 | 519.58 |
| condensed tannins (4) | 0.25 | 0.10 | 0.10 | 0.15 | 235.87 |
| Variables | no CT | CT | SEM (1) | p-Value (2) |
|---|---|---|---|---|
| in vitro DM degradability | 0.646 a | 0.466 b | 0.0090 | *** |
| in vitro OM degradability | 0.644 a | 0.458 b | 0.0095 | *** |
| partitioning factor (mg DMD/mL) | 3.40 b | 7.68 a | 0.234 | *** |
| partitioning factor (mg OMD/mL) | 3.14 b | 6.94 a | 0.206 | *** |
| Model parameters (3) | ||||
| a | −27.39 b | −9.06 a | 0.816 | *** |
| b | 281.89 a | 133.47 b | 7.023 | *** |
| c | 0.0281 | 0.0258 | 0.00130 | ns |
| a + b | 254.50 a | 124.40 b | 7.097 | *** |
| t0 | 4.39 a | 3.06 b | 0.149 | *** |
| Variables | no CT | CT | SEM (1) | p-Value (2) |
|---|---|---|---|---|
| SCFA production (mmol/g OMD) | ||||
| acetic acid | 6.57 a | 2.83 b | 0.26 | *** |
| propionic acid | 2.46 a | 1.30 b | 0.09 | *** |
| iso-butyric acid | 0.11 a | 0.02 b | 0.01 | *** |
| butyric acid | 1.07 a | 0.42 b | 0.04 | *** |
| iso-valeric acid | 0.15 a | 0.05 b | 0.01 | *** |
| valeric acid | 0.19 a | 0.10 b | 0.01 | *** |
| total SCFA | 10.51 a | 4.69 b | 0.39 | *** |
| Variable (1) | Animal Species (2) | SEM (3) | p Value (4) | ||||
|---|---|---|---|---|---|---|---|
| Goats | Sheep | Buffalo | Taurine Cattle | Zebu Cattle | |||
| IVDMD | 0.556 | 0.565 | 0.543 | 0.564 | 0.554 | 0.014 | Ns |
| IVOMD | 0.551 | 0.560 | 0.538 | 0.559 | 0.547 | 0.015 | Ns |
| PF (mg DMD/mL) | 5.15 | 4.94 | 6.19 | 5.70 | 5.69 | 0.37 | Ns |
| PF (mg OMD/mL) | 4.65 | 4.48 | 5.70 | 5.31 | 5.07 | 0.33 | Ns |
| Model parameters (5) | |||||||
| A | −15.15 a | −22.01 c | −15.92 ab | −20.92 bc | −17.13 abc | 1.29 | *** |
| B | 185.82 b | 197.70 ab | 238.02 a | 194.83 ab | 222.00 ab | 11.10 | ** |
| C | 0.0348 a | 0.0314 a | 0.0210 b | 0.0267 ab | 0.0209 b | 0.0021 | *** |
| a + b | 170.67 b | 175.69 b | 222.10 a | 173.91 b | 204.88 ab | 11.22 | ** |
| t0 | 2.67 b | 3.42 ab | 4.24 a | 4.27 a | 4.03 a | 0.24 | *** |
| Variables | Animal Species (1) | SEM (2) | p-Value (3) | ||||
|---|---|---|---|---|---|---|---|
| Goats | Sheep | Buffalo | Taurine Cattle | Zebu Cattle | |||
| SCFA production (mmol/g OMD) | |||||||
| acetic acid | 3.37 c | 3.92 bc | 5.44 ab | 5.70 a | 5.06 ab | 0.41 | *** |
| propionic acid | 1.30 b | 1.45 b | 2.25 a | 2.28 a | 2.11 a | 0.14 | *** |
| iso-butyric acid | 0.09 a | 0.06 abc | 0.07 ab | 0.02 c | 0.04 bc | 0.01 | *** |
| butyric acid | 0.53 c | 0.61 bc | 0.90 a | 0.88 a | 0.82 ab | 0.07 | *** |
| iso-valeric acid | 0.10 | 0.10 | 0.10 | 0.12 | 0.10 | 0.01 | ns |
| valeric acid | 0.12 bc | 0.10c | 0.15 abc | 0.19 a | 0.17 ab | 0.02 | *** |
| total SCFA | 5.41 c | 6.21 bc | 8.89 a | 9.17 a | 8.30 ab | 0.61 | *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bueno, I.C.S.; Brandi, R.A.; Fagundes, G.M.; Benetel, G.; Muir, J.P. The Role of Condensed Tannins in the In Vitro Rumen Fermentation Kinetics in Ruminant Species: Feeding Type Involved? Animals 2020, 10, 635. https://doi.org/10.3390/ani10040635
Bueno ICS, Brandi RA, Fagundes GM, Benetel G, Muir JP. The Role of Condensed Tannins in the In Vitro Rumen Fermentation Kinetics in Ruminant Species: Feeding Type Involved? Animals. 2020; 10(4):635. https://doi.org/10.3390/ani10040635
Chicago/Turabian StyleBueno, Ives C. S., Roberta A. Brandi, Gisele M. Fagundes, Gabriela Benetel, and James Pierre Muir. 2020. "The Role of Condensed Tannins in the In Vitro Rumen Fermentation Kinetics in Ruminant Species: Feeding Type Involved?" Animals 10, no. 4: 635. https://doi.org/10.3390/ani10040635
APA StyleBueno, I. C. S., Brandi, R. A., Fagundes, G. M., Benetel, G., & Muir, J. P. (2020). The Role of Condensed Tannins in the In Vitro Rumen Fermentation Kinetics in Ruminant Species: Feeding Type Involved? Animals, 10(4), 635. https://doi.org/10.3390/ani10040635





