Pigs’ Feed Fermentation Model with Antimicrobial Lactic Acid Bacteria Strains Combination by Changing Extruded Soya to Biomodified Local Feed Stock
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. LAB Strains Used for Feed Fermentation
2.2. Fermentation of the Local Feed Stock
2.3. Evaluation of Fermented Feed pH and Microbiological Parameters
2.4. Animals and Housing
2.5. Experimental Design and Diets
2.6. Metagenomics and Microbial Profiling Analysis
2.7. Microbiological Analysis of Fecal Samples
2.8. Blood Analysis
2.9. Evaluation of the Piglets’ Growth Performance
2.10. Analysis of Ammonia Emission
2.11. Statistical Analysis
3. Results and Discussion
3.1. Fermented Feed Characteristics
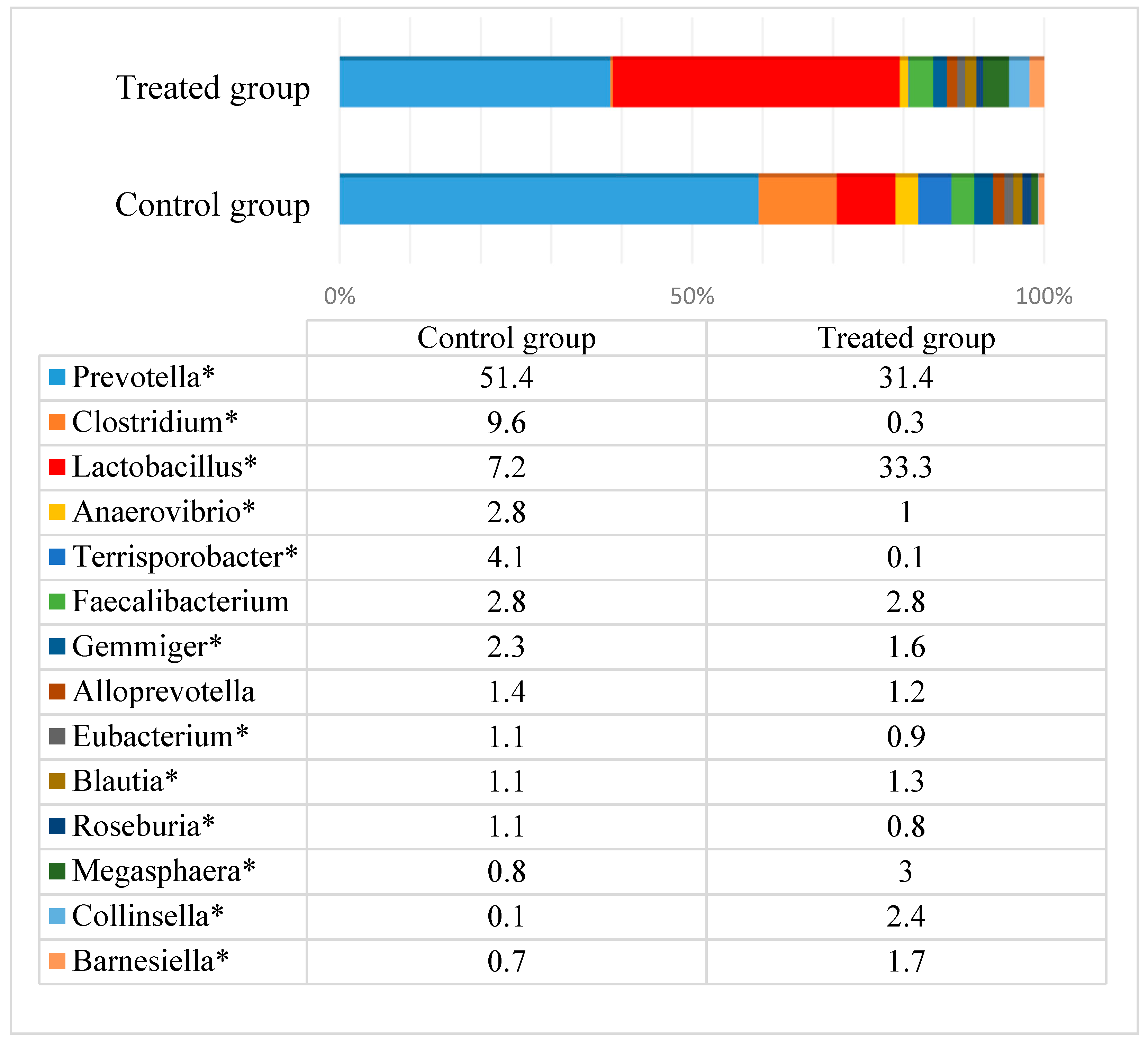
3.2. Microbial Profiles of Pig Feces
3.3. Influence of Fermented Feed on LAB, TEC, and Y/M Count in Piglets’ Feces
3.4. Piglet Blood Parameters
3.5. Piglets’ Growth Performance
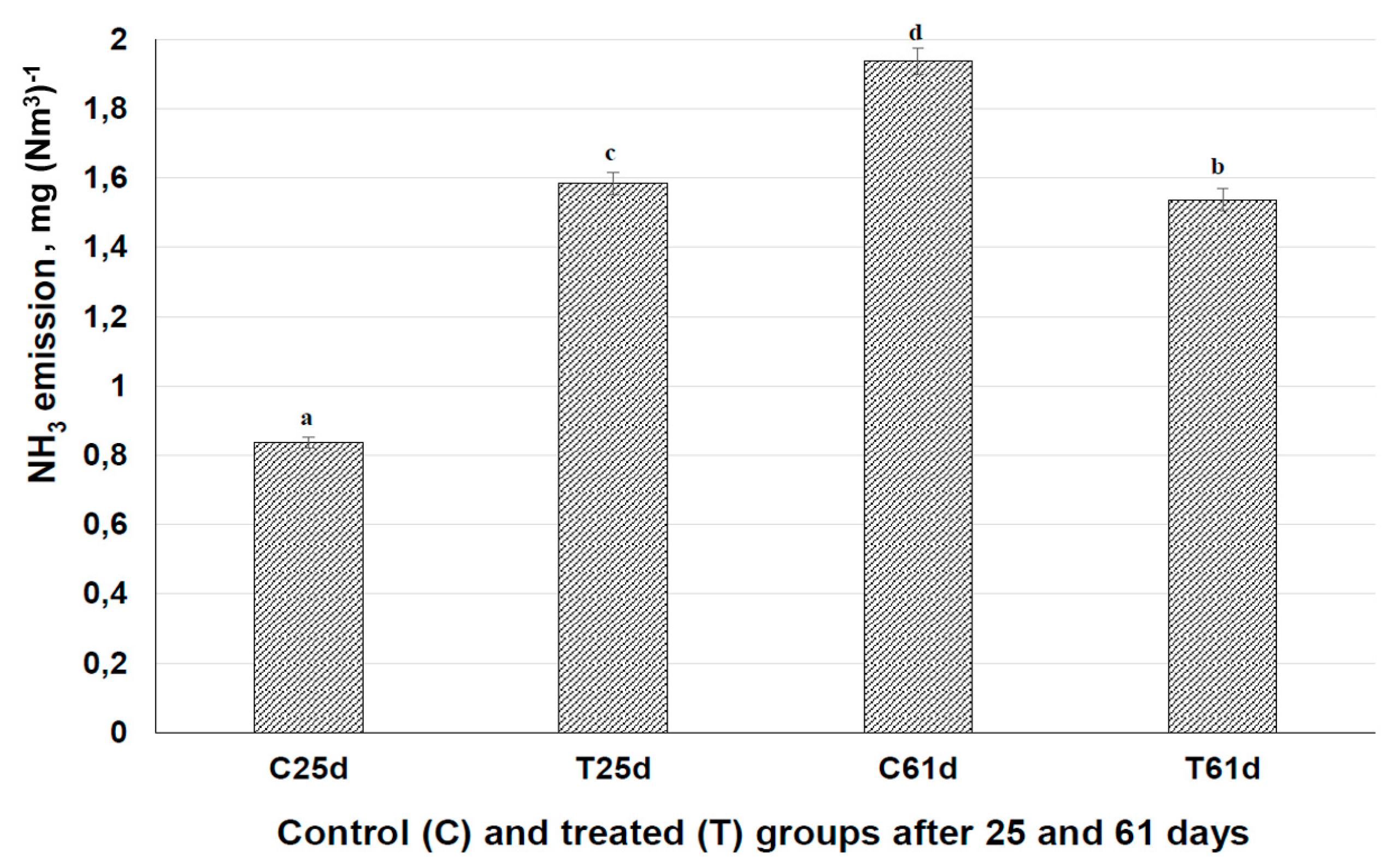
3.6. Influence of Fermented Feed on Ammonia Emission
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mostafa, E.; Selders, A.; Gates, R.S.; Buescher, W. Pig barns ammonia and greenhouse gas emission mitigation by slurry aeration and acid scrubber. Environ. Sci. Pollut. Res. 2020, 27, 9444–9453. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Downing, M.M.; Nejadhashemi, A.P.; Harrigan, T.; Woznicki, S.A. Climate change and livestock: Impacts, adaptation, and mitigation. Clim. Risk Manag. 2017, 16, 145–163. [Google Scholar] [CrossRef]
- Wang, H.; Long, W.; Chadwick, D.; Velthof, G.L.; Oenema, O.; Ma, W.; Wang, J.; Qin, W.; Hou, Y.; Zhang, F. Can dietary manipulations improve the productivity of pigs with lower environmental and economic cost? A global meta-analysis. Agric. Ecosyst. Environ. 2020, 289, 106748. [Google Scholar] [CrossRef]
- Dai, Z.; Cui, L.; Li, J.; Wang, B.; Guo, L.; Wu, Z.; Zhu, W.; Wu, G. In Animal Agriculture; Bazer, F.W., Lamb, G.C., Wu, G., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 407–429. [Google Scholar]
- Bartkiene, E.; Lele, V.; Sakiene, V.; Zavistanaviciute, P.; Ruzauskas, M.; Bernatoniene, J.; Jakstas, V.; Viskelis, P.; Zadeike, D.; Juodeikiene, G. Improvement of the antimicrobial activity of lactic acid bacteria in combination with berries/fruits and dairy industry by-products. J. Sci. Food Agric. 2019, 99, 3992–4002. [Google Scholar] [CrossRef]
- Bartkiene, E.; Lele, V.; Ruzauskas, M.; Domig, K.J.; Starkute, V.; Zavistanaviciute, P.; Bartkevics, V.; Pugajeva, I.; Klupsaite, D.; Juodeikiene, G.; et al. Lactic acid bacteria isolation from spontaneous sourdough and their characterization including antimicrobial and antifungal properties evaluation. Microorganisms 2020, 8, 64. [Google Scholar] [CrossRef] [Green Version]
- Fabà, L.; Litjens, R.; Allaart, J.G.; van den Hil, P.R. Feed additive blends fed to nursery pigs challenged with Salmonella. J. Anim. Sci. 2019, 98, skz382. [Google Scholar] [CrossRef]
- Pluske, J.R.; Hampson, D.J.; Williams, I.H. Factors influencing the structure and function of the small intestine in the weaned pig: A review. Livest. Prod. Sci. 1997, 51, 215–236. [Google Scholar] [CrossRef]
- Lallès, J.-P.; Boudry, G.; Favier, C.; Floc’h, N.L.; Luron, I.; Montagne, L.; Oswald, I.P.; Pié, S.; Piel, C.; Sève, B. Gut function and dysfunction in young pigs: Physiology. Anim. Res. 2004, 53, 301–316. [Google Scholar] [CrossRef] [Green Version]
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4, 19. [Google Scholar] [CrossRef] [Green Version]
- EMA Zinc Oxide. Available online: https://www.ema.europa.eu/en/medicines/veterinary/referrals/zinc-oxide (accessed on 10 March 2020).
- Liao, S.F.; Nyachoti, M. Using probiotics to improve swine gut health and nutrient utilization. Anim. Nutr. 2017, 3, 331–343. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, H.J.; Wu, S.G.; Yu, S.H.; Yoon, I.; Moore, D.; Gao, Y.P.; Yan, H.J.; Qi, G.H. Effect of Saccharomyces cerevisiae fermentation product on immune functions of broilers challenged with Eimeria tenella. Poult. Sci. 2009, 88, 2141–2151. [Google Scholar] [CrossRef] [PubMed]
- Sugiharto, S.; Ranjitkar, S. Recent advances in fermented feeds towards improved broiler chicken performance, gastrointestinal tract microecology and immune responses: A review. Anim. Nutr. 2019, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Canibe, N.; Jensen, B.B. Fermented liquid feed—Microbial and nutritional aspects and impact on enteric diseases in pigs. Anim. Feed Sci. Technol. 2012, 173, 17–40. [Google Scholar] [CrossRef]
- Missotten, J.A.; Michiels, J.; Degroote, J.; De Smet, S. Fermented liquid feed for pigs: An ancient technique for the future. J. Anim. Sci. Biotechnol. 2015, 6, 4. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Han, Y.; Zhao, J.; Zhou, Z.; Fan, H. Consuming fermented distillers’ dried grains with solubles (DDGS) feed reveals a shift in the faecal microbiota of growing and fattening pigs using 454 pyrosequencing. J. Integr. Agric. 2017, 16, 900–910. [Google Scholar]
- Wang, C.; Su, W.; Zhang, Y.; Hao, L.; Wang, F.; Lu, Z.; Zhao, J.; Liu, X.; Wang, Y. Solid-state fermentation of distilled dried grain with solubles with probiotics for degrading lignocellulose and upgrading nutrient utilization. AMB Express 2018, 8, 188. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Wei, F.; Liu, X.; Yi, C.; Zhang, Y. Improvement of the nutritional value, sensory properties and bioavailability of rapeseed meal fermented with mixed microorganisms. LWT 2019, 112, 108238. [Google Scholar] [CrossRef]
- Dubeňová, M.; Šima, T.; Gálik, R.; Mihina, Š.; Vagač, G.; Boďo, Š. Reduction of nitrous oxide and carbon dioxide in the pig barn piggery by different ventilation system intensities. Agron. Res. 2014, 12, 207–214. [Google Scholar]
- Velthof, G.L.; van Bruggen, C.; Groenestein, C.M.; de Haan, B.J.; Hoogeveen, M.W.; Huijsmans, J.F.M. A model for inventory of ammonia emissions from agriculture in the Netherlands. Atmos. Environ. 2012, 46, 248–255. [Google Scholar] [CrossRef]
- Bartkiene, E.; Sakiene, V.; Lele, V.; Bartkevics, V.; Rusko, J.; Wiacek, C.; Ruzauskas, M.; Braun, P.G.; Matusevicius, P.; Zdunczyk, Z.; et al. Perspectives of lupine wholemeal protein and protein isolates biodegradation. Int. J. Food Sci. Tech. 2019, 54, 1989–2001. [Google Scholar]
- Bartkiene, E.; Mozuriene, E.; Lele, V.; Zokaityte, E.; Gruzauskas, R.; Jakobsone, I.; Juodeikiene, G.; Ruibys, R.; Bartkevics, V. Changes of bioactive compounds in barley industry by-products during submerged and solid state fermentation with antimicrobial Pediococcus acidilactici strain LUHS29. Food Sci. Nutr. 2020, 8, 340–350. [Google Scholar] [CrossRef] [Green Version]
- Bartkiene, E.; Sakienė, V.; Bartkevics, V.; Juodeikiene, G.; Lele, V.; Wiacek, C.; Braun, P.G. Modulation of the nutritional value of lupine wholemeal and protein isolates using submerged and solid-state fermentation with Pediococcus pentosaceus strains. Int. J. Food Sci. Tech. 2018, 53, 1896–1905. [Google Scholar] [CrossRef]
- Bartkiene, E.; Bartkevics, V.; Krungleviciute, V.; Pugajeva, I.; Zadeike, D.; Juodeikiene, G. Lactic acid bacteria combinations for wheat sourdough preparation and their influence on wheat bread quality and acrylamide formation. J. Food Sci. 2017, 82, 2371–2378. [Google Scholar]
- Bartkiene, E.; Bartkevics, V.; Lele, V.; Pugajeva, I.; Zavistanaviciute, P.; Mickiene, R.; Zadeike, D.; Juodeikiene, G. A concept of mold spoilage prevention and acrylamide reduction in wheat bread: Application of lactobacilli in combination with a cranberry coating. Food Control 2018, 91, 284–293. [Google Scholar] [CrossRef]
- The European Parliament and of the Council EUR-Lex-32010L0063-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32010L0063 (accessed on 10 March 2020).
- Lithuanian Director of the State Food and Veterinary Service. B1-866 Dėl Mokslo ir mokymo tikslais naudojamų gyvūnų laikymo, priežiūros ir naudojimo reikalavimų patvirtinimo. Available online: https://e-seimas.lrs.lt/portal/legalAct/lt/TAD/TAIS.437081?positionInSearchResults=0&searchModelUUID=64a1f51f-6356-4b60-a21e-3a67ac3b1107 (accessed on 10 March 2020).
- National Research Council. Nutrient Requirements of Swine; 11th revised ed.; The National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- AOAC. Official Methods of Analysis, 21st ed.; AOAC International: Rockford, MD, USA, 2019. [Google Scholar]
- Merkeviciene, L.; Ruzauskaite, N.; Klimiene, I.; Siugzdiniene, R.; Dailidaviciene, J.; Virgailis, M.; Mockeliunas, R.; Ruzauskas, M. Microbiome and antimicrobial resistance genes in microbiota of cloacal samples from European herring gulls (Larus Argentatus). J. Vet. Res. 2017, 61, 27–35. [Google Scholar]
- Zavistanavičiūtė, P.; Poškienė, I.; Lėlė, V.; Antanaitis, R.; Kantautaitė, J.; Bartkienė, E. The influence of the newly isolated Lactobacillus plantarum LUHS135 and Lactobacillus paracasei LUHS244 strains on blood and faeces parameters in endurance horses. Pol. J. Vet. Sci. 2019, 22, 513–521. [Google Scholar]
- Lithuanian Minister of Environment. D1-862 Dėl Lietuvos Respublikos aplinkos apsaugos normatyvinio dokumento LAND 88-2009 "Amoniako koncentracijos nustatymas aplinkos ore spektrometriniu metodu“ patvirtinimo. Available online: https://e-seimas.lrs.lt/portal/legalAct/lt/TAD/TAIS.363222 (accessed on 10 March 2020).
- Engberg, R.M.; Hammershøj, M.; Johansen, N.F.; Abousekken, M.S.; Steenfeldt, S.; Jensen, B.B. Fermented feed for laying hens: Effects on egg production, egg quality, plumage condition and composition and activity of the intestinal microflora. Br. Poult. Sci. 2009, 50, 228–239. [Google Scholar] [CrossRef]
- Niba, A.T.; Beal, J.D.; Kudi, A.C.; Brooks, P.H. Potential of bacterial fermentation as a biosafe method of improving feeds for pigs and poultry. Afr. J. Biotechnol. 2009, 8, 1758–1767. [Google Scholar]
- Ranjitkar, S.; Karlsson, A.H.; Petersen, M.A.; Bredie, W.L.P.; Petersen, J.S.; Engberg, R.M. The influence of feeding crimped kernel maize silage on broiler production, nutrient digestibility and meat quality. Br. Poult. Sci. 2016, 57, 93–104. [Google Scholar] [CrossRef]
- Sugiharto, S.; Yudiarti, T.; Isroli, I. Performances and haematological profile of broilers fed fermented dried cassava (Manihot esculenta Crantz). Trop. Anim. Health Prod. 2016, 48, 1337–1341. [Google Scholar] [CrossRef] [Green Version]
- Juodeikiene, G.; Bartkiene, E.; Cernauskas, D.; Cizeikiene, D.; Zadeike, D.; Lele, V.; Bartkevics, V. Antifungal activity of lactic acid bacteria and their application for Fusarium mycotoxin reduction in malting wheat grains. LWT 2018, 89, 307–314. [Google Scholar] [CrossRef]
- Sugiharto, S.; Lauridsen, C.; Jensen, B.B. Gastrointestinal ecosystem and immunological responses in E. coli challenged pigs after weaning fed liquid diets containing whey permeate fermented with different lactic acid bacteria. Anim. Feed Sci. Techol. 2015, 207, 278–282. [Google Scholar] [CrossRef]
- Niu, Q.; Li, P.; Hao, S.; Kim, S.W.; Du, T.; Hua, J.; Huang, R. Characteristics of gut microbiota in sows and their relationship with apparent nutrient digestibility. Int. J. Mol. Sci. 2019, 20, 870. [Google Scholar] [CrossRef] [Green Version]
- Mølbak, L.; Thomsen, L.E.; Jensen, T.K.; Bach Knudsen, K.E.; Boye, M. Increased amount of Bifidobacterium thermacidophilum and Megasphaera elsdenii in the colonic microbiota of pigs fed a swine dysentery preventive diet containing chicory roots and sweet lupine. J. Appl. Microbiol. 2007, 103, 1853–1867. [Google Scholar] [CrossRef]
- Yang, F.; Hou, C.; Zeng, X.; Qiao, S. The use of lactic acid bacteria as a probiotic in swine diets. Pathogens 2015, 4, 34–45. [Google Scholar]
- Xiang, Q.; Wu, X.; Pan, Y.; Wang, L.; Cui, C.; Guo, Y.; Zhu, L.; Peng, J.; Wei, H. Early-life intervention using fecal microbiota combined with probiotics promotes gut microbiota maturation, regulates immune system development, and alleviates weaning stress in piglets. Int. J. Mol. Sci. 2020, 21, 503. [Google Scholar] [CrossRef] [Green Version]
- Nabhani, Z.A.; Eberl, G. GAPs in early life facilitate immune tolerance. Sci. Immunol. 2017, 2, eaar2465. [Google Scholar] [CrossRef]
- El Aidy, S.; Derrien, M.; Merrifield, C.A.; Levenez, F.; Doré, J.; Boekschoten, M.V.; Dekker, J.; Holmes, E.; Zoetendal, E.G.; van Baarlen, P.; et al. Gut bacteria–host metabolic interplay during conventionalisation of the mouse germfree colon. ISME J. 2013, 7, 743–755. [Google Scholar] [CrossRef] [Green Version]
- El Aidy, S.; Hooiveld, G.; Tremaroli, V.; Bäckhed, F.; Kleerebezem, M. The gut microbiota and mucosal homeostasis. Gut Microbes 2013, 4, 118–124. [Google Scholar] [CrossRef]
- Gensollen, T.; Iyer, S.S.; Kasper, D.L.; Blumberg, R.S. How colonization by microbiota in early life shapes the immune system. Science 2016, 352, 539–544. [Google Scholar]
- Konstantinov, S.R.; Awati, A.A.; Williams, B.A.; Miller, B.G.; Jones, P.; Stokes, C.R.; Akkermans, A.D.L.; Smidt, H.; de Vos, W.M. Post-natal development of the porcine microbiota composition and activities. Environ. Microbiol. 2006, 8, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Daly, K.; Darby, A.C.; Hall, N.; Nau, A.; Bravo, D.; Shirazi-Beechey, S.P. Dietary supplementation with lactose or artificial sweetener enhances swine gut Lactobacillus population abundance. Br. J. Nutr. 2014, 111 Suppl. 1, S30–S35. [Google Scholar] [CrossRef] [Green Version]
- Jiao, J.; Wu, J.; Zhou, C.; Tang, S.; Wang, M.; Tan, Z. Composition of ileal bacterial community in grazing goats varies across non-rumination, transition and rumination stages of life. Front. Microbiol. 2016, 7, 1364. [Google Scholar] [CrossRef] [Green Version]
- Shang, Q.; Shan, X.; Cai, C.; Hao, J.; Li, G.; Yu, G. Dietary fucoidan modulates the gut microbiota in mice by increasing the abundance of Lactobacillus and Ruminococcaceae. Food Funct. 2016, 7, 3224–3232. [Google Scholar] [CrossRef]
- Vujkovic-Cvijin, I.; Swainson, L.A.; Chu, S.N.; Ortiz, A.M.; Santee, C.A.; Petriello, A.; Dunham, R.M.; Fadrosh, D.W.; Lin, D.L.; Faruqi, A.A.; et al. Gut-resident Lactobacillus abundance associates with IDO1 inhibition and Th17 dynamics in SIV-infected macaques. Cell Rep. 2015, 13, 1589–1597. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.-J.; Lim, H.-J.; Shin, H.-S. Immunomodulatory effects of seven viable and sonicated Lactobacillus spp. and anti-bacterial activities of L. rhamnosus and L. helvetilus. Korean J. Microbiol. 2019, 55, 392–399. [Google Scholar]
- Morales-López, S.; Yepes, J.A.; Prada-Herrera, J.C.; Torres-Jiménez, A. Enterobacteria in the 21st century: A review focused on taxonomic changes. J. Infect. Dev. Ctries. 2019, 13, 265–273. [Google Scholar] [CrossRef]
- Duntas, L.H.; Brenta, G. A renewed focus on the association between thyroid hormones and lipid metabolism. Front. Endocrinol. 2018, 9, 511. [Google Scholar] [CrossRef]
- Czech, A.; Grela, E.R.; Kiesz, M.; Kłys, S. Biochemical and haematological blood parameters of sows and piglets fed a diet with a dried fermented rapeseed meal. Ann. Anim. Sci. 2019, 1, 211–223. [Google Scholar] [CrossRef]
- Vieco-Saiz, N.; Belguesmia, Y.; Raspoet, R.; Auclair, E.; Gancel, F.; Kempf, I.; Drider, D. Benefits and inputs from lactic acid bacteria and their bacteriocins as alternatives to antibiotic growth promoters during food-animal production. Front. Microbiol. 2019, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Weiner, M.L.; Ferguson, H.E.; Thorsrud, B.A.; Nelson, K.G.; Blakemore, W.R.; Zeigler, B.; Cameron, M.J.; Brant, A.; Cochrane, L.; Pellerin, M.; et al. An infant formula toxicity and toxicokinetic feeding study on carrageenan in preweaning piglets with special attention to the immune system and gastrointestinal tract. Food Chem. Toxicol. 2015, 77, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Song, P.; Huang, C.; Rezaei, A.; Farrar, S.; Brown, M.A.; Ma, X. Dietary grape seed proanthocyanidins (GSPs) improve weaned intestinal microbiota and mucosal barrier using a piglet model. Oncotarget 2016, 7, 80313–80326. [Google Scholar] [CrossRef] [Green Version]
- Le, M.H.A.; Galle, S.; Yang, Y.; Landero, J.L.; Beltranena, E.; Gänzle, M.G.; Zijlstra, R.T. Effects of feeding fermented wheat with Lactobacillus reuteri on gut morphology, intestinal fermentation, nutrient digestibility, and growth performance in weaned pigs. J. Anim. Sci. 2016, 94, 4677–4687. [Google Scholar] [CrossRef]
- Plumed-Ferrer, C.; von Wright, A. Fermented pig liquid feed: Nutritional, safety and regulatory aspects. J. Appl. Microbiol. 2009, 106, 351–368. [Google Scholar] [CrossRef]
- Valeriano, V.D.V.; Balolong, M.P.; Kang, D.-K. Probiotic roles of Lactobacillus sp. in swine: Insights from gut microbiota. J. Appl. Microbiol. 2017, 122, 554–567. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.; Zhang, Y.; Lu, Z.; Wang, Y. Solid-state fermentation of corn-soybean meal mixed feed with Bacillus subtilis and Enterococcus faecium for degrading antinutritional factors and enhancing nutritional value. J. Anim. Sci. Biotechnol. 2017, 8, 50. [Google Scholar] [CrossRef]
- Mukherjee, R.; Chakraborty, R.; Dutta, A. Role of fermentation in improving nutritional quality of soybean meal—A review. Asian-Australas. J. Anim. Sci. 2016, 29, 1523–1529. [Google Scholar] [CrossRef] [Green Version]
- Koo, B.; Bustamante-Garcia, D.; Nyachoti, C. 353 Effects of Lactobacillus-fermented barley on intestinal morphology, cytokine gene expression, and fecal microbiota in weaned pigs challenged with Escherichia coli K88+. J. Anim. Sci. 2018, 96, 176. [Google Scholar] [CrossRef]
- Cheng, S.-S.; Li, Y.; Geng, S.-J.; Hu, L.-S.; Fu, X.-F.; Han, X.-Y. Effects of dietary fresh fermented soybean meal on growth performance, ammonia and particulate matter emissions, and nitrogen excretion in nursery piglets. J. Zhejiang Univ. Sci. B 2017, 18, 1083–1092. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Li, Z.; Lu, Z.; Wang, Y. 358 Effects of fermented feed supplementation on pig growth performance: A meta-analysis. J. Anim. Sci. 2019, 97, 125–126. [Google Scholar] [CrossRef]
- Banhazi, T.M.; Rutley, D.L.; Pitchford, W.S. Pitchford identification of risk factors for sub-optimal housing conditions in Australian piggeries: Part 4. Emission factors and study recommendations. J. Agric. Saf. Health 2008, 14, 53–69. [Google Scholar] [CrossRef]
- Hoff, S.J.; Bundy, D.S.; Nelson, M.A.; Zelle, B.C.; Jacobson, L.D.; Heber, A.J.; Ni, J.; Zhang, Y.; Koziel, J.A.; Beasley, D.B. Emissions of ammonia, hydrogen sulfide, and odor before, during, and after slurry removal from a deep-pit swine finisher. J. Air Waste Manag. Assoc. 2006, 56, 581–590. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Hu, J.; Zhang, B.; Tan, Z. Gaseous emissions, growth performance and pork quality of pigs housed in deep-litter system compared to concrete-floor system. Anim. Sci. J. 2015, 86, 422–427. [Google Scholar] [CrossRef]
- Chiavegato, M.B.; Powers, W.; Palumbo, N. Ammonia and greenhouse gas emissions from housed Holstein steers fed different levels of diet crude protein. J. Anim. Sci. 2015, 93, 395–404. [Google Scholar] [CrossRef]




| Ingredients (%) | Control Group | Treated Group |
|---|---|---|
| Barley | 38.40 | 33.25 |
| Rapeseed meal | - | 25.00 |
| Wheat | 32.12 | 25.02 |
| Soya beans (extruded) | 9.30 | - |
| Potato protein | 5.00 | 2.00 |
| Soybean protein concentrate | 2.00 | - |
| Whey powder | 5.80 | 5.80 |
| Sunflower oil | 2.72 | 4.51 |
| Limestone | 1.48 | 1.1 |
| NaCl | 0.38 | 0.35 |
| Monocalcium phosphate | 0.33 | 0.41 |
| L-Lysine sulfate | 0.87 | 1.1 |
| DL-Methionine | 0.25 | 0.16 |
| Acidal NC (formic and acetic acids) | 0.30 | 0.30 |
| 1Vitamins and trace elements (premix) | 1.00 | 1.00 |
| Bredol 683 | 0.05 | 0.00 |
| Nutritional value | ||
| ME swine (MJ/kg) | 13.86 | 13.95 |
| Crude protein (%) | 19.00 | 19.00 |
| Crude fat (%) | 6.51 | 6.51 |
| Crude fiber (%) | 3.15 | 5.14 |
| Lysine (%) | 1.45 | 1.45 |
| Methionine (%) | 0.55 | 0.55 |
| Threonine (%) | 0.93 | 0.94 |
| Tryptophan (%) | 0.26 | 0.25 |
| Methionine + Cystine (%) | 0.87 | 0.88 |
| Ca (%) | 0.90 | 0.90 |
| Total P (%) | 0.59 | 0.62 |
| Available P (%) | 0.37 | 0.38 |
| Na (%) | 0.20 | 0.21 |
| Microbiological Parameters (log10 CFU/g) | Day | Treatments | p | |
|---|---|---|---|---|
| C | T | Day × Treatment Interaction | ||
| LAB | Baseline | 7.8 ± 0.3 Aa | 8.3 ± 0.1Ab | 0.0001 |
| 61 | 6.2 ± 0.1 Bb | 5.2 ± 0.1 Ba | ||
| TBC | Baseline | 7.1 ± 0.2 Aa | 8.4 ± 0.1 Ab | 0.0001 |
| 61 | 6.4 ± 0.1 Ba | 6.4 ± 0.1 Ba | ||
| TEC | Baseline | 7.2 ± 0.1 Ba | 7.4 ± 0.1 Ba | 0.081 |
| 61 | 6.4 ± 0.2 Aa | 6.9 ± 0.1 Ab | ||
| Y/F | Baseline | 6.7 ± 0.1 Bb | 6.2 ± 0.1 Ba | 0.122 |
| 61 | 6.4 ± 0.1 Ab | 5.7 ± 0.1 Aa | ||
| Blood Parameters | Day | Treatments | p | |
|---|---|---|---|---|
| C | T | Day × Treatment Interaction | ||
| Aspartate aminotransferase (AST), U/L | Baseline | 29.4 ± 3.4 Aa | 51.4 ± 11.2 Ab | 0.204 |
| 61 | 34.0 ± 6.1 Aa | 44.0 ± 7.2 Aa | ||
| Alanine aminotransferase (ALT), U/L | Baseline | 48.4 ± 6.8 Aa | 53.2 ± 11.6 Aa | 0.647 |
| 61 | 76.2 ± 11.8 Ba | 87.0 ± 12.5 Ba | ||
| Cholesterol (Chol), mmol/L | Baseline | 1.63 ± 0.21 Aa | 1.88 ± 0.54 Aa | 0.943 |
| 61 | 2.06 ± 0.21 Ba | 2.34 ± 0.35 Aa | ||
| High-density lipoprotein cholesterol (HDL-C), mmol/L | Baseline | 0.714 ± 0.134 Aa | 0.898 ± 0.201 Aa | 0.976 |
| 61 | 0.840 ± 0.134 Aa | 1.03 ± 0.18 Aa | ||
| Low-density lipoprotein cholesterol (LDL-C), mmol/L | Baseline | 0.758 ± 0.086Aa | 0.814 ± 0.329 Aa | 0.987 |
| 61 | 0.980 ± 0.123 Ba | 1.032 ± 0.173 Aa | ||
| Triglycerides (TG), mmol/L | Baseline | 0.360 ± 0.130 Aa | 0.366 ± 0.063 Aa | 0.245 |
| 61 | 0.466 ± 0.092 Aa | 0.620 ± 0.111 Ba | ||
| Total protein (TP), g/L | Baseline | 46.2 ± 2.3 Aa | 44.2 ± 2.1 Aa | 0.391 |
| 61 | 51.8 ± 2.8 Ba | 52.8 ± 3.9 Ba | ||
| Albumin (ALB), g/L | Baseline | 30.0 ± 2.1 Aa | 32.6 ± 3.1 Aa | 0.558 |
| 61 | 35.8 ± 3.9 Aa | 36.2 ± 3.1 Aa | ||
| Immunoglobulin IgG, g/L | Baseline | 2.64 ± 0.797Aa | 2.35 ± 0.705 Aa | 0.684 |
| 61 | 3.73 ± 1.10 Aa | 3.05 ± 0.467 Aa | ||
| Triiodothyronine (T3), nmol/L | Baseline | 1.21 ± 0.297 Aa | 1.30 ± 0.315 Aa | 0.046 |
| 61 | 2.14 ± 0.128 Bb | 1.59 ± 0.143 Aa | ||
| Thyroxine (T4), µ d/L | Baseline | 4.50 ± 0.424 Ab | 3.50 ± 0.346 Aa | 0.047 |
| 61 | 4.80 ± 0.230 Ab | 2.92 ± 0.268 Ab | ||
| Glucose (GLU), nmol/L | Baseline | 5.84 ± 0.737 Aa | 6.12 ± 0.259 Aa | 0.971 |
| 61 | 5.74 ± 0.503 Aa | 6.08 ± 0.286 Aa | ||
| Phosphorus (IP), mmol/L | Baseline | 2.94 ± 0.327 Aa | 2.61 ± 0.371 Aa | 0.737 |
| 61 | 3.50 ± 0.144 Ba | 3.28 ± 0.183 Ba | ||
| Magnesium (Mg), mmol/L | Baseline | 1.02 ± 0.117 Aa | 0.996 ± 0.106 Aa | 0.429 |
| 61 | 1.07 ± 0.054 Aa | 0.960 ± 0.0590 Aa | ||
| Potassium (K) | Baseline | 4.96 ± 0.427 Aa | 4.65 ± 0.298 Aa | 0.368 |
| 61 | 5.81 ± 0.35 Ba | 4.96 ± 0.747 Aa | ||
| Sodium (Na) | Baseline | 143.4 ± 3.05 Aa | 144.0 ± 1.0 Aa | 0.591 |
| 61 | 147.2 ± 0.837 Aa | 146.6 ± 1.67 Aa | ||
| Iron (Fe), µmol/L | Baseline | 23.6 ± 5.9 Aa | 31.5 ± 3.9 Aa | 0.195 |
| 61 | 28.1 ± 2.2 Aa | 47.1 ± 11.4 Bb | ||
| Calcium (Ca), nmol/L | Baseline | 2.60 ± 0.217 Aa | 2.71 ± 0.035 Aa | 0.261 |
| 61 | 2.87 ± 0.129 Aa | 2.79 ± 0.096 Aa | ||
| Vitamin B12, pmol/L | Baseline | 142.2 ± 32.32Ab | 78.2 ± 19.1 Aa | 0.270 |
| 61 | 214.6 ± 64.8 Ab | 94.2 ± 34.4 Aa | ||
| Creatinine (CREA), µmol/L | Baseline | 64.2 ± 11.7 Aa | 78.8 ± 17.5 Ba | 0.120 |
| 61 | 57.4 ± 3.7 Aa | 48.2 ± 10.2 Aa | ||
| Alkaline phosphatase (AP), U/L | Baseline | 336.2 ± 132.9 Aa | 408.6 ± 165.5 Aa | 0.502 |
| 61 | 263.6 ± 83.8 Aa | 242.6 ± 29.9 Aa | ||
| Urea, mmol/L | Baseline | 2.36 ± 0.49 Aa | 2.64 ± 0.624 Aa | 0.207 |
| 61 | 2.02 ± 0.14 Aa | 3.19 ± 0.778 Ab | ||
| Thyroid-stimulating hormone (TSH) | Baseline | 0.02 ± 0.005 Aa | 0.021 ± 0.002 Aa | 0.666 |
| 61 | 0.021 ± 0.01 Aa | 0.023 ± 0.012 Aa | ||
| Total bilirubin (pmol/L) | Baseline | ˂2 | ˂2 | - |
| 61 | ˂2 | ˂2 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vadopalas, L.; Ruzauskas, M.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Zokaityte, E.; Bartkevics, V.; Badaras, S.; Klupsaite, D.; Mozuriene, E.; et al. Pigs’ Feed Fermentation Model with Antimicrobial Lactic Acid Bacteria Strains Combination by Changing Extruded Soya to Biomodified Local Feed Stock. Animals 2020, 10, 783. https://doi.org/10.3390/ani10050783
Vadopalas L, Ruzauskas M, Lele V, Starkute V, Zavistanaviciute P, Zokaityte E, Bartkevics V, Badaras S, Klupsaite D, Mozuriene E, et al. Pigs’ Feed Fermentation Model with Antimicrobial Lactic Acid Bacteria Strains Combination by Changing Extruded Soya to Biomodified Local Feed Stock. Animals. 2020; 10(5):783. https://doi.org/10.3390/ani10050783
Chicago/Turabian StyleVadopalas, Laurynas, Modestas Ruzauskas, Vita Lele, Vytaute Starkute, Paulina Zavistanaviciute, Egle Zokaityte, Vadims Bartkevics, Sarunas Badaras, Dovile Klupsaite, Erika Mozuriene, and et al. 2020. "Pigs’ Feed Fermentation Model with Antimicrobial Lactic Acid Bacteria Strains Combination by Changing Extruded Soya to Biomodified Local Feed Stock" Animals 10, no. 5: 783. https://doi.org/10.3390/ani10050783
APA StyleVadopalas, L., Ruzauskas, M., Lele, V., Starkute, V., Zavistanaviciute, P., Zokaityte, E., Bartkevics, V., Badaras, S., Klupsaite, D., Mozuriene, E., Dauksiene, A., Sidlauskiene, S., Gruzauskas, R., & Bartkiene, E. (2020). Pigs’ Feed Fermentation Model with Antimicrobial Lactic Acid Bacteria Strains Combination by Changing Extruded Soya to Biomodified Local Feed Stock. Animals, 10(5), 783. https://doi.org/10.3390/ani10050783










