Characterization of Proteolytic Activity of Artichoke (Cynara scolymus L.) Flower Extracts on Bovine Casein to Obtain Bioactive Peptides
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Lyophilized C. scolymus L. Extract Preparation
2.2. Caseinolytic Activity Assay
2.3. Temperature, pH, and Extract Concentration Optimization
2.4. Vmax and Km Estimation and 24-h Enzymatic Kinetic Evaluation
2.5. Casein Hydrolysate Preparation
2.6. Angiotensin Converting Enzyme- I (ACE-I) Inhibitory Activity
2.7. Antioxidant Activity
2.8. Statistical Analysis
3. Results
3.1. Temperature Effect on Proteolysis
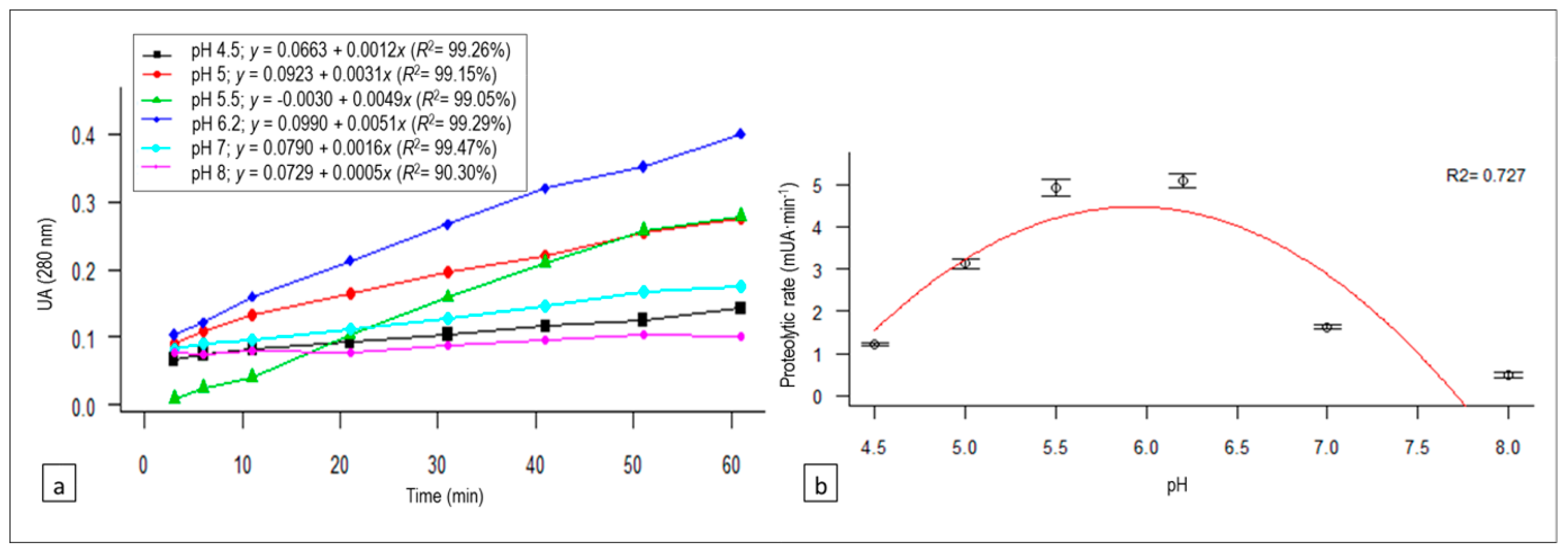
3.2. pH Effect on Proteolysis
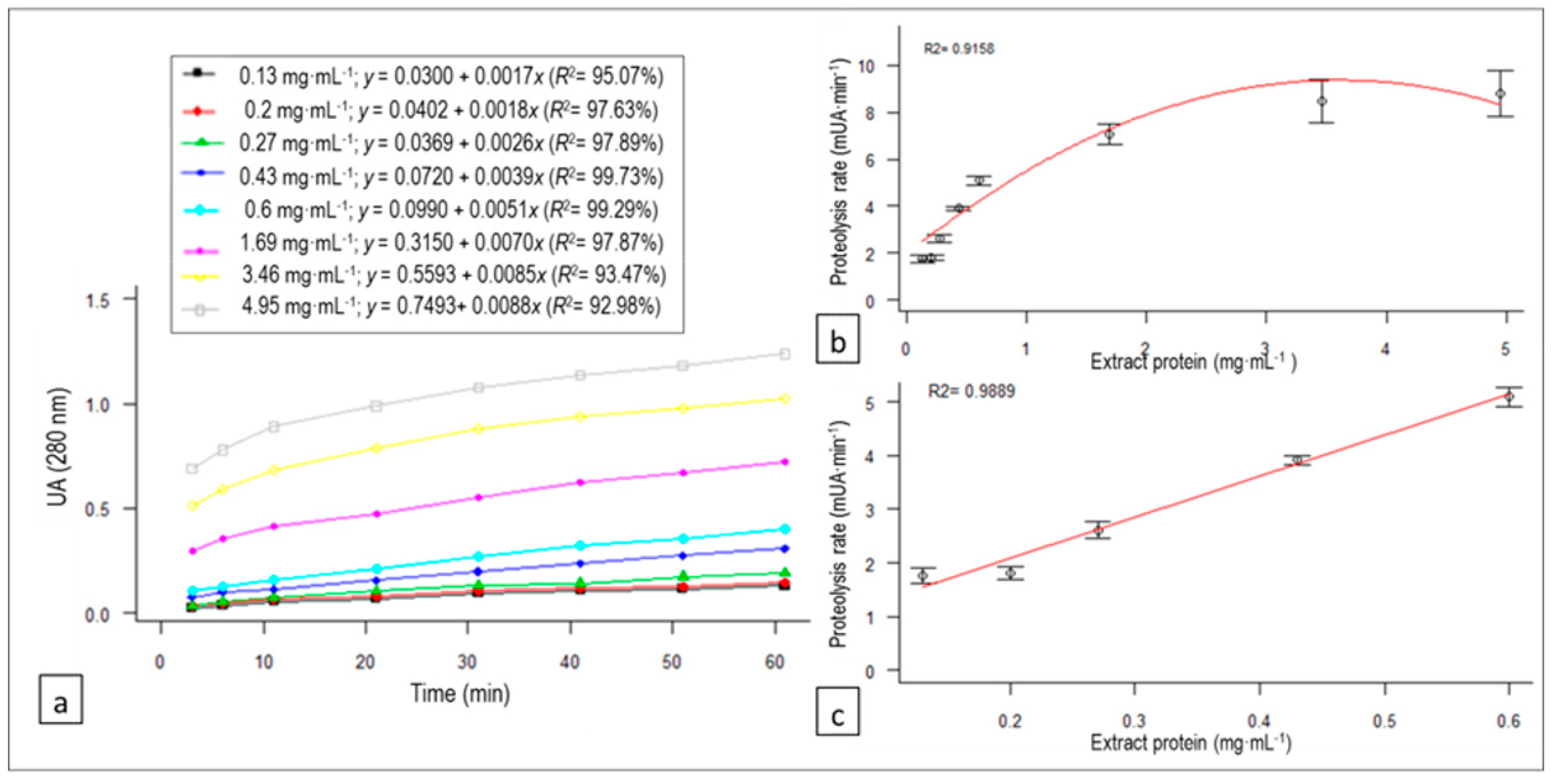
3.3. C. scolymus L. Flower Extract Concentration Effect on Proteolysis
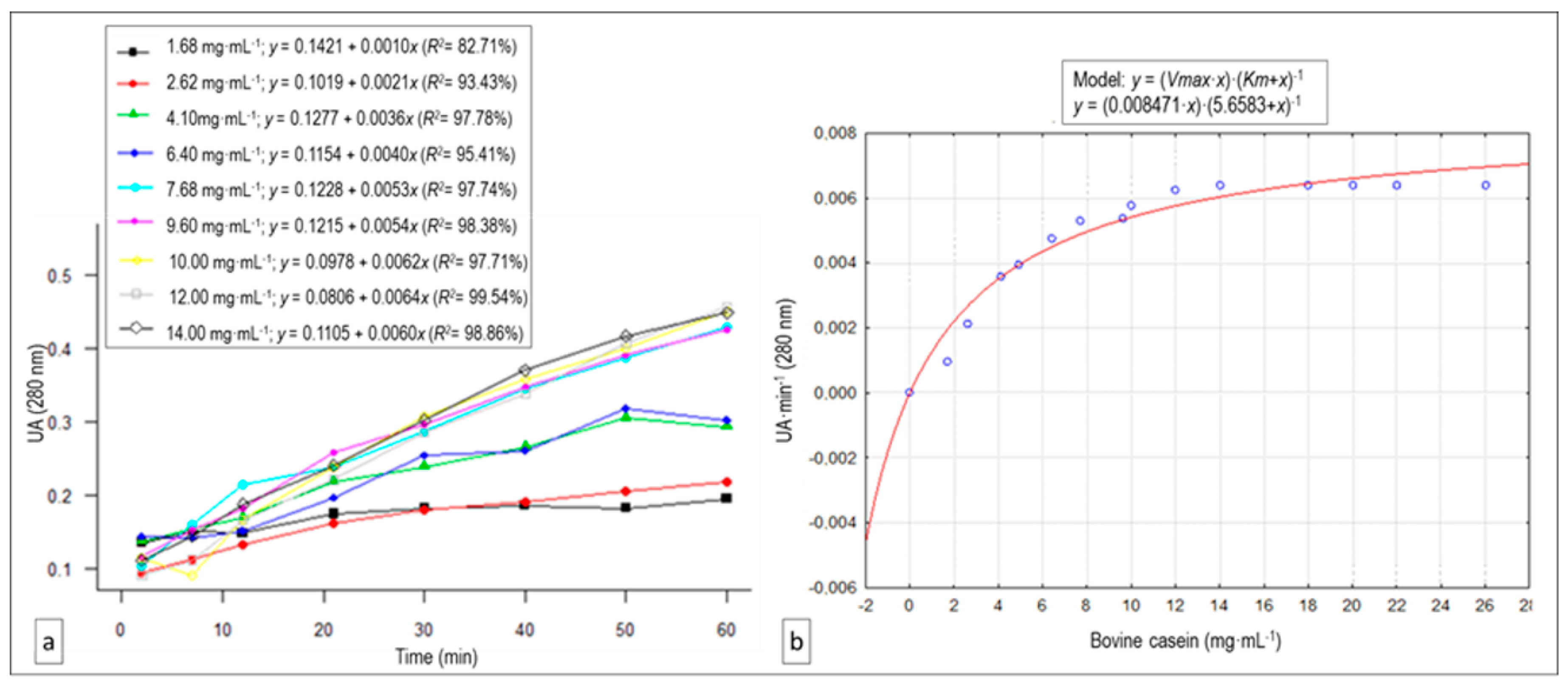
3.4. Substrate Concentration Effect: Vmax and Km Estimation
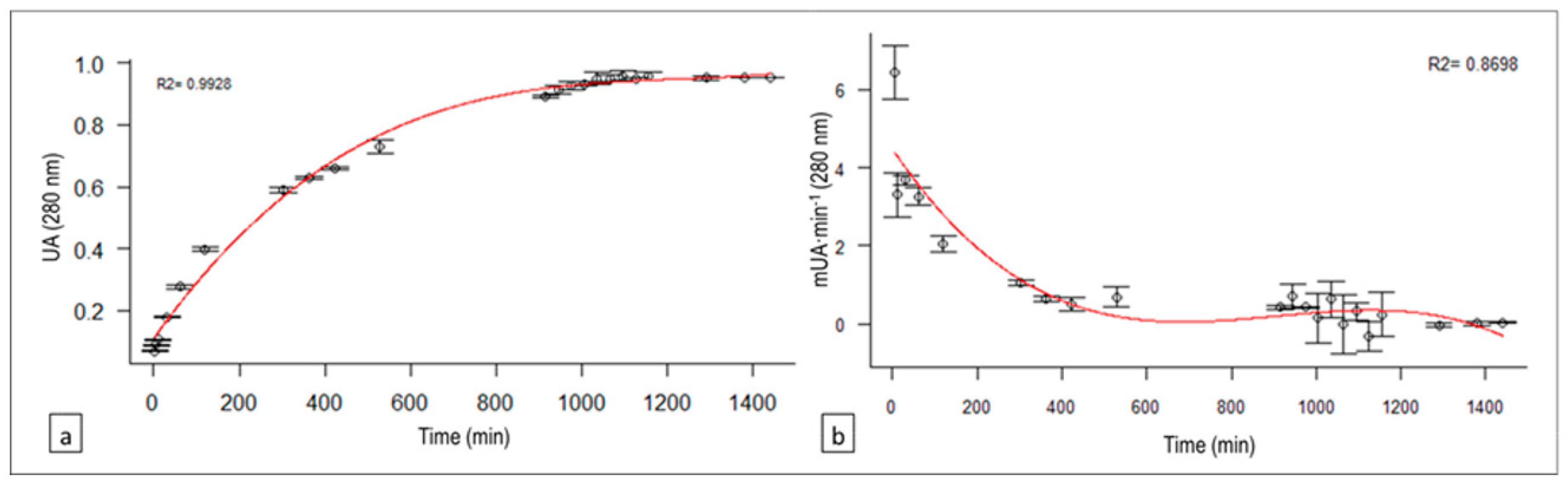
3.5. Hydrolysis Time Effect on Caseinolytic Rate: Enzymatic Activity for 24 h
3.6. ACE-I Inhibition and Antioxidant Activity of the Hydrolysates
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tidona, F.; Criscione, A.; Guastella, A.M.; Zuccaro, A.; Bordonaro, S.; Marletta, D. Bioactive peptides in dairy products. Ital. J. Anim. Sci. 2009, 8, 315–340. [Google Scholar] [CrossRef]
- Gobbetti, M.; Stepaniak, L.; Angelis, M.D.; Corsetti, A.; Cagno, R.D. Latent bioactive peptides in milk proteins: Proteolytic activation and significance in dairy processing. Crit. Rev. Food Sci. Nutr. 2002, 42, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, H.; Pihlanto, A. Bioactive peptides: Production and functionality. Int. Dairy J. 2006, 16, 945–960. [Google Scholar] [CrossRef]
- Sibel Akalın, A. Dairy-derived antimicrobial peptides: Action mechanisms, pharmaceutical uses and production proposals. Trends Food Sci. Technol. 2014, 36, 79–95. [Google Scholar] [CrossRef]
- De Gobba, C.; Tompa, G.; Otte, J. Bioactive peptides from caseins released by cold active proteolytic enzymes from Arsukibacterium ikkense. Food Chem. 2014, 165, 205–215. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; García-Nebot, M.J.; Fernández-Tomé, S.; Amigo, L.; Recio, I. Dairy protein hydrolysates: Peptides for health benefits. Int. Dairy J. 2014, 38, 82–100. [Google Scholar] [CrossRef]
- Cardinali, F.; Taccari, M.; Milanović, V.; Osimani, A.; Polverigiani, S.; Garofalo, C.; Foligni, R.; Mozzon, M.; Zitti, S.; Raffaelli, N.; et al. Yeast and mould dynamics in Caciofiore della Sibilla cheese coagulated with an aqueous extract of Carlina acanthifolia All. Yeast and mould dynamics in a specialty cheese. Yeast 2016, 33, 403–414. [Google Scholar] [CrossRef]
- Macedo, A.C.; Malcata, F.X.; Oliveira, J.C. The technology, chemistry, and microbiology of Serra cheese: A review. J. Dairy Sci. 1993, 76, 1725–1739. [Google Scholar] [CrossRef]
- Tejada, L.; Fernández-Salguero, J. Chemical and microbiological characteristics of ewe cheese (Los Pedroches) made with a powdered vegetable coagulant or calf rennet. Ital. J. Food Sci. 2003, 15, 125–131. [Google Scholar]
- Tejada, L.; Abellán, A.; Cayuela, J.M.; Martínez-Cachá, A. Sensorial characteristics during ripening of the Murcia al Vino goat"s milk cheese. The effect of the type of coagulant used and the size of the cheese. J. Sens. Stud. 2006, 21, 333–347. [Google Scholar] [CrossRef]
- Tejada, L.; Abellán, A.; Prados, F.; Cayuela, J. Compositional characteristics of Murcia al Vino goat’s cheese made with calf rennet and plant coagulant. Int. J. Dairy Technol. 2008, 61, 119–125. [Google Scholar] [CrossRef]
- Cardinali, F.; Osimani, A.; Taccari, M.; Milanović, V.; Garofalo, C.; Clementi, F.; Polverigiani, S.; Zitti, S.; Raffaelli, N.; Mozzon, M.; et al. Impact of thistle rennet from Carlina acanthifolia All. subsp. acanthifolia on bacterial diversity and dynamics of a specialty Italian raw ewes’ milk cheese. Int. J. Food Microbiol. 2017, 255, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Llorente, B.E.; Brutti, C.B.; Caffini, N.O. Purification and characterization of a milk-clotting aspartic proteinase from globe artichoke (Cynara scolymus L.). J. Agric. Food Chem. 2004, 52, 8182–8189. [Google Scholar] [CrossRef] [PubMed]
- Sidrach, L.; García-Cánovas, F.; Tudela, J.; Rodríguez-López, J.N. Purification of cynarases from artichoke (Cynara scolymus L.): Enzymatic properties of cynarase A. Phytochemistry 2005, 66, 41–49. [Google Scholar] [CrossRef]
- Agboola, S.; Chen, S.; Zhao, J. Formation of bitter peptides during ripening of ovine milk cheese made with different coagulants. Dairy Sci. Technol. 2004, 84, 567–578. [Google Scholar] [CrossRef]
- Tejada, L.; Abellán, A.; Cayuela, J.M.; Martínez-Cacha, A.; Fernández-Salguero, J. Proteolysis in goats’ milk cheese made with calf rennet and plant coagulant. Int. Dairy J. 2008, 18, 139–146. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; Recio, I.; Amigo, L. β-Lactoglobulin as source of bioactive peptides. Amino Acids 2008, 35, 257–265. [Google Scholar] [CrossRef]
- Alvarado, C.; Guerra, M. Lactosuero como fuente de péptidos bioactivos. An Venez Nutr 2010, 23, 42–49. [Google Scholar]
- Heimgartner, U.; Pietrzak, M.; Geertsen, R.; Brodelius, P.; da Silva Figueiredo, A.C.; Pais, M.S.S. Purification and partial characterization of milk clotting proteases from flowers of Cynara cardunculus. Phytochemistry 1990, 29, 1405–1410. [Google Scholar] [CrossRef]
- Faro, C.; Ramalho-Santos, M.; Vieira, M.; Mendes, A.; Simões, I.; Andrade, R.; Veríssimo, P.; Lin, X.; Tang, J.; Pires, E. Cloning and characterization of cDNA encoding cardosin A, an RGD-containing plant aspartic proteinase. J. Biol. Chem. 1999, 274, 28724–28729. [Google Scholar] [CrossRef]
- Llorente, B.E.; Obregón, W.D.; Avilés, F.X.; Caffini, N.O.; Vairo-Cavalli, S. Use of artichoke (Cynara scolymus) flower extract as a substitute for bovine rennet in the manufacture of Gouda-type cheese: Characterization of aspartic proteases. Food Chem. 2014, 159, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Malcata, F.X. Studies pertaining to coagulant and proteolytic activities of plant proteases from Cynara cardunculus. Food Chem. 2005, 89, 19–26. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Cushman, D.W.; Cheung, H.S. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem. Pharmacol. 1971, 20, 1637–1648. [Google Scholar] [CrossRef]
- Miguel, M.; Recio, I.; Gómez-Ruiz, J.A.; Ramos, M.; López-Fandiño, R. Angiotensin I-converting enzyme inhibitory activity of peptides derived from egg white proteins by enzymatic hydrolysis. J. Food Prot. 2004, 67, 1914–1920. [Google Scholar] [CrossRef]
- Bersuder, P.; Hole, M.; Smith, G. Antioxidants from heated histidine-glucose model system. I: Investigation of the antioxidant role of histidine and isolation of antioxidants by high-performance liquid chromatography. J. Am. Oil Chem. Soc. 1998, 75. [Google Scholar] [CrossRef]
- De Gobba, C.; Espejo-Carpio, F.J.; Skibsted, L.H.; Otte, J. Antioxidant peptides from goat milk protein fractions hydrolysed by two commercial proteases. Int. Dairy J. 2014, 39, 28–40. [Google Scholar] [CrossRef]
- R Core Team. R: A language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 25 September 2019).
- Sousa, M.J.; Malcata, F.X. Proteolysis of ovine and caprine caseins in solution by enzymatic extracts from flowers of Cynara cardunculus. Enzyme Microb. Technol. 1998, 22, 305–314. [Google Scholar] [CrossRef]
- Verissimo, P.; Esteves, C.; Faro, C.; Pires, E. The vegetable rennet of Cynara cardunculus L. contains two proteinases with chymosin and pepsin-like specificities. Biotechnol. Lett. 1995, 17, 621–626. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, J.; Agboola, S. Isolation and partial characterization of rennet-like proteases from Australian cardoon (Cynara cardunculus L.). J. Agric. Food Chem. 2003, 51, 3127–3134. [Google Scholar] [CrossRef]
- Ramalho-Santos, M.; Veríssimo, P.; Faro, C.; Pires, E. Action on bovine αs1-casein of cardosins A and B, aspartic proteinases from the flowers of the cardoon Cynara cardunculus L. Biochim. Biophys. Acta BBA Protein Struct. Mol. Enzymol. 1996, 1297, 83–89. [Google Scholar] [CrossRef]
- Mao, X.-Y.; Ni, J.-R.; Sun, W.-L.; Hao, P.-P.; Fan, L. Value-added utilization of yak milk casein for the production of angiotensin-I-converting enzyme inhibitory peptides. Food Chem. 2007, 103, 1282–1287. [Google Scholar] [CrossRef]
- Wu, S.; Qi, W.; Li, T.; Lu, D.; Su, R.; He, Z. Simultaneous production of multi-functional peptides by pancreatic hydrolysis of bovine casein in an enzymatic membrane reactor via combinational chromatography. Food Chem. 2013, 141, 2944–2951. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Chatli, M.K.; Singh, R.; Mehta, N.; Kumar, P. Antioxidant and antimicrobial activity of camel milk casein hydrolysates and its fractions. Small Rumin. Res. 2016, 139, 20–25. [Google Scholar] [CrossRef]
- Petrat-Melin, B.; Kristiansen, G.H.; Le, T.T.; Poulsen, N.A.; Larsen, L.B.; Young, J.F. In vitro gastrointestinal digestion of purified bovine kappa-casein variants A, B, and E: Effects on antioxidant and angiotensin 1-converting enzyme inhibitory capacity. Int. Dairy J. 2016, 57, 44–51. [Google Scholar] [CrossRef]
- Galán, E.; Prados, F.; Pino, A.; Tejada, L.; Fernández-Salguero, J. Influence of different amounts of vegetable coagulant from cardoon Cynara cardunculus and calf rennet on the proteolysis and sensory characteristics of cheeses made with sheep milk. Int. Dairy J. 2008, 18, 93–98. [Google Scholar] [CrossRef]
- Carrera, E.; Gaya, P.; Medina, M.; Nunez, M. Effect of milk coagulant on the formation of hydrophobic and hydrophilic peptides during the manufacture of cows’ milk Hispanico cheese. Milchwissenschaft 1999, 54, 146–149. [Google Scholar]
- Cheung, H.S.; Wang, F.L.; Ondetti, M.A.; Sabo, E.F.; Cushman, D.W. Binding of peptide substrates and inhibitors of angiotensin-converting enzyme. Importance of the COOH-terminal dipeptide sequence. J. Biol. Chem. 1980, 255, 401–407. [Google Scholar]
- Ren, J.; Zhao, M.; Shi, J.; Wang, J.; Jiang, Y.; Cui, C.; Kakuda, Y.; Xue, S.J. Purification and identification of antioxidant peptides from grass carp muscle hydrolysates by consecutive chromatography and electrospray ionization-mass spectrometry. Food Chem. 2008, 108, 727–736. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, X.; Zhuang, Y. Purification and characterization of novel antioxidant peptides from enzymatic hydrolysates of tilapia (Oreochromis niloticus) skin gelatin. Peptides 2012, 38, 13–21. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bueno-Gavilá, E.; Abellán, A.; Bermejo, M.S.; Salazar, E.; Cayuela, J.M.; Prieto-Merino, D.; Tejada, L. Characterization of Proteolytic Activity of Artichoke (Cynara scolymus L.) Flower Extracts on Bovine Casein to Obtain Bioactive Peptides. Animals 2020, 10, 914. https://doi.org/10.3390/ani10050914
Bueno-Gavilá E, Abellán A, Bermejo MS, Salazar E, Cayuela JM, Prieto-Merino D, Tejada L. Characterization of Proteolytic Activity of Artichoke (Cynara scolymus L.) Flower Extracts on Bovine Casein to Obtain Bioactive Peptides. Animals. 2020; 10(5):914. https://doi.org/10.3390/ani10050914
Chicago/Turabian StyleBueno-Gavilá, Estefanía, Adela Abellán, María Soledad Bermejo, Eva Salazar, José María Cayuela, David Prieto-Merino, and Luis Tejada. 2020. "Characterization of Proteolytic Activity of Artichoke (Cynara scolymus L.) Flower Extracts on Bovine Casein to Obtain Bioactive Peptides" Animals 10, no. 5: 914. https://doi.org/10.3390/ani10050914
APA StyleBueno-Gavilá, E., Abellán, A., Bermejo, M. S., Salazar, E., Cayuela, J. M., Prieto-Merino, D., & Tejada, L. (2020). Characterization of Proteolytic Activity of Artichoke (Cynara scolymus L.) Flower Extracts on Bovine Casein to Obtain Bioactive Peptides. Animals, 10(5), 914. https://doi.org/10.3390/ani10050914




