Expression of Insulin-Like Growth Factor 1 (IGF-1) and Epidermal Growth Factor (EGF) Receptors and the Effect of IGF-1 and EGF on Androgen and Estrogen Release in the Myometrium of Pigs—In Vitro Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Determination of the Relative Insulin-like Growth Factor 1 Receptor (IGF-1R) and Epidermal Growth Factor Receptor (EGFR) mRNA Transcripts Abundance in the Myometrium
2.2. Sequence Analysis
2.3. Determination of the Relative IGF-1R and EGFR Protein Abundance in the Myometrium
2.4. Incubation of Myometrial Slices
2.5. Determination of A4, T, E1 and E2 Concentration in the Media
2.6. Statistical Analysis
3. Results
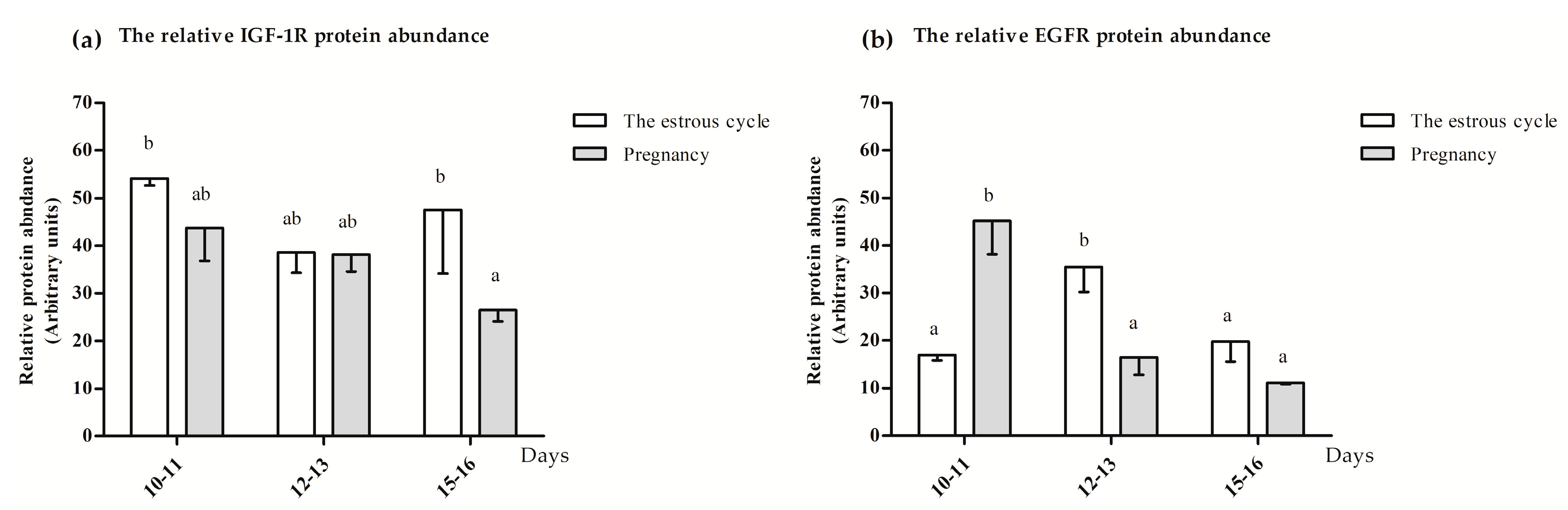
3.1. The Relative Abundance of EGFR and IGF-IR mRNA Transcripts and Proteins in the Myometrium
3.1.1. The Main Effects and Interactions Affecting the Relative Abundance of EGFR and IGF-1R mRNA Transcripts and Proteins
3.1.2. The Relative IGF-1R and EGFR mRNA Transcript Abundances
3.1.3. The IGF-1R and EGFR Protein Location and Abundance
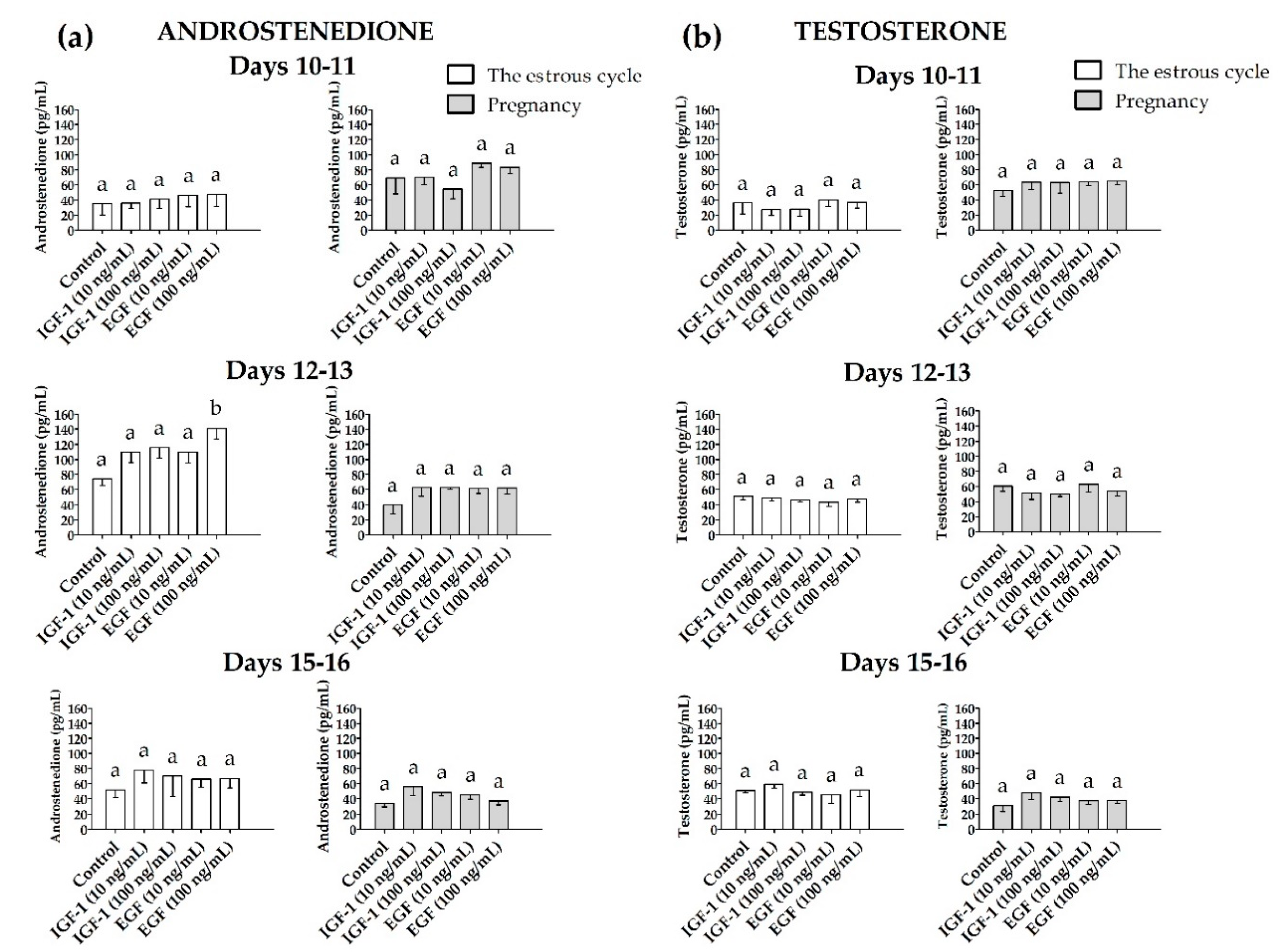
3.2. The Effect of IGF-1 and EGF on the A4, T, E1 and E2 Release from the Myometrium
3.2.1. The Main Effects and Interactions Affecting Myometrial Steroid Hormone Release
3.2.2. Androstenedione (A4) and Testosterone (T) Release
3.2.3. Estrone (E1) and Estradiol-17β (E2) Release
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lemmon, M.A.; Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 2010, 141, 1117–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konopka, B.; Skasko, E.; Kluska, A.; Goluda, M.; Janiec-Jankowska, A.; Paszko, Z.; Ujec, M. Changes in the concentrations of receptors of insulin-like growth factor-I, epithelial growth factor, oestrogens and progestagens in adenomyosis foci, endometrium and myometrium of women during menstrual cycle. Eur. J. Gynaecol. Oncol. 1998, 19, 93–97. [Google Scholar]
- Tang, X.-M.; Rossi, M.J.; Masterson, B.J.; Chegini, N. Insulin-Like Growth Factor I (IGF-I), IGF-I Receptors, and IGF Binding Proteins 1–4 in Human Uterine Tissue: Tissue Localization and IGF-I Action in Endometrial Stromal and Myometrial Smooth Muscle Cells in Vitro. Biol. Reprod. 1994, 50, 1113–1125. [Google Scholar] [CrossRef] [Green Version]
- Hofig, A.; Michel, F.J.; Simmen, F.A.; Simmen, R.C.M. Constitutive Expression of Uterine Receptors for Insulin-Like Growth Factor-I during the Peri-Implantation Period in the Pig1. Biol. Reprod. 1991, 45, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Shynlova, O.; Tsui, P.; Dorogin, A.; Langille, B.L.; Lye, S.J. Insulin-like Growth Factors and Their Binding Proteins Define Specific Phases of Myometrial Differentiation During Pregnancy in the Rat. Biol. Reprod. 2007, 76, 571–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.K.; Tsukamura, H.; Paria, B.C.; Andrews, G.K.; Dey, S.K. Differential expression of epidermal growth factor receptor (EGF-R) gene and regulation of EGF-R bioactivity by progesterone and estrogen in the adult mouse uterus. Endocrinology 1994, 134, 971–981. [Google Scholar] [CrossRef]
- Tamada, H.; Yoh, C.; Inaba, T.; Takano, H.; Kawate, N.; Sawada, T. Epidermal growth factor (EGF) in the goat uterus: Immunohistochemical localization of EGF and EGF receptor and effect of EGF on uterine activity in vivo. Theriogenology 2000, 54, 159–169. [Google Scholar] [CrossRef]
- Hsueh, A.J.W.; Welsh, T.H.; Jones, P.B.C. Inhibition of ovarian and testicular steroiodogenesis by epidermal growth factor. Endocrinology 1981, 108, 2002–2004. [Google Scholar] [CrossRef]
- Caubo, B.; Devinna, R.S.; Tonetta, S.A. Regulation of steroidogenesis in cultured porcine theca cells by growth factors. Endocrinology 1989, 125, 321–326. [Google Scholar] [CrossRef]
- Green, M.L.; Simmen, R.C.M.; Simmen, F.A. Developmental regulation of steroidogenic enzyme gene expression in the periimplantation porcine conceptus: A paracrine role for insulin-like growth factor-I. Endocrinology 1995, 136, 3961–3970. [Google Scholar] [CrossRef]
- Jamnongjit, M.; Gill, A.; Hammes, S.R. Epidermal growth factor receptor signaling is required for normal ovarian steroidogenesis and oocyte maturation. Proc. Natl. Acad. Sci. USA 2005, 102, 16257–16262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaetje, R. IGF-I and EGF influence on steroid secretion and morphology of human granulosa cells of IVF-cycles and natural cycles in vitro. Clin. Exp. Obstet. Gynecol. 1994, 21, 14–23. [Google Scholar] [PubMed]
- Spicer, L.J.; Stewart, R.E. Interactions among basic fibroblast growth factor, epidermal growth factor, insulin, and insulin-like growth factor-I (IGF-I) on cell numbers and steroidogenesis of bovine thecal cells: Role of IGF-I receptors. Biol. Reprod. 1996, 54, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Franczak, A. Endometrial and myometrial secretion of androgens and estrone during early pregnancy and luteolysis in pigs. Reprod. Biol. 2008, 8, 213–228. [Google Scholar] [CrossRef]
- Franczak, A.; Kotwica, G. Secretion of estradiol-17β by porcine endometrium and myometrium during early pregnancy and luteolysis. Theriogenology 2008, 69, 283–289. [Google Scholar] [CrossRef]
- Franczak, A.; Kotwica, G. Androgens and estradiol-17β production by porcine uterine cells: In vitro study. Theriogenology 2010, 73, 232–241. [Google Scholar] [CrossRef]
- Franczak, A.; Zmijewska, A.; Kurowicka, B.; Wojciechowicz, B.; Kotwica, G. Interleukin 1β-induced synthesis and secretion of prostaglandin E2 in the porcine uterus during various periods of pregnancy and the estrous cycle. J. Physiol. Pharmacol. 2010, 61, 733–742. [Google Scholar]
- Wojciechowicz, B.; Kotwica, G.; Kolakowska, J.; Franczak, A. The Activity and Localization of 3β-hydroxysteroid Dehydrogenase/Δ 5-Δ 4 Isomerase and Release of Androstenedione and Progesterone by Uterine Tissues During Early Pregnancy and the Estrous Cycle in Pigs. J. Reprod. Dev. 2013, 59, 49–58. [Google Scholar]
- Franczak, A.; Wojciechowicz, B.; Katwica, G. Novel aspects of cytokine action in porcine uterus—endometrial and myometrial production of estrone (E1) in the presence of interleukin 1β (Il1β), interleukin 6 (Il6) and tumor necrosis factor (TNFα)—in vitro study. Folia Biol. 2013, 61, 253–261. [Google Scholar] [CrossRef] [Green Version]
- Kiezun, M.; Smolinska, N.; Dobrzyn, K.; Szeszko, K.; Rytelewska, E.; Kaminski, T. The effect of orexin A on CYP17A1 and CYP19A3 expression and on oestradiol, oestrone and testosterone secretion in the porcine uterus during early pregnancy and the oestrous cycle. Theriogenology 2017, 90, 129–140. [Google Scholar] [CrossRef]
- Kisielewska, K.; Rytelewska, E.; Gudelska, M.; Kiezun, M.; Dobrzyn, K.; Szeszko, K.; Bors, K.; Wyrebek, J.; Kaminski, T.; Smolinska, N. The effect of orexin B on steroidogenic acute regulatory protein, P450 side-chain cleavage enzyme, and 3β-hydroxysteroid dehydrogenase gene expression, and progesterone and androstenedione secretion by the porcine uterus during early pregnancy and the estrous cycle. J. Anim. Sci. 2019, 97, 851–864. [Google Scholar] [PubMed]
- Kaminski, T.; Smolinska, N.; Kiezun, M.; Dobrzyn, K.; Szeszko, K.; Maleszka, A. Effect of orexin B on CYP17A1 and CYP19A3 expression and oestradiol, oestrone and testosterone secretion in the porcine uterus during early pregnancy and the oestrous cycle. Animal 2018, 12, 1921–1932. [Google Scholar] [CrossRef] [PubMed]
- Franczak, A.; Wojciechowicz, B.; Kolakowska, J.; Kotwica, G. The effect of interleukin-1β, interleukin-6, and tumor necrosis factor-α on estradiol-17β release in the myometrium: The invitro study on the pig model. Theriogenology 2014, 81, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Smolinska, N.; Dobrzyn, K.; Kiezun, M.; Szeszko, K.; Maleszka, A.; Kaminski, T. Effect of adiponectin on the steroidogenic acute regulatory protein, P450 side chain cleavage enzyme and 3β-hydroxysteroid dehydrogenase gene expression, progesterone and androstenedione production by the porcine uterus during early pregnancy. J. Physiol. Pharmacol. 2016, 67, 443–456. [Google Scholar]
- Akins, E.L.; Morrissette, M.C. Gross ovarian changes during estrous cycle of swine. Am. J. Vet. Res. 1968, 29, 1953–1957. [Google Scholar]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Kibbe, W.A. OligoCalc: An online oligonucleotide properties calculator. Nucleic Acids Res. 2007, 35. (webserver issue):. Available online: http://biotools.nubic.northwestern.edu/OligoCalc.html (accessed on 17 October 2019). [CrossRef]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef]
- Staszkiewicz, J.; Skowronski, M.T.; Siawrys, G.; Kaminski, T.; Krazinski, B.E.; Plonka, K.J.; Wylot, B.; Przala, J.; Okrasa, S. Expression of proopiomelanocortin, proenkephalin and prodynorphin genes in porcine luteal cells. Acta Vet. Hung. 2007, 55, 435–449. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kaczmarek, M.M.; Blitek, A.; Schams, D.; Ziecik, A.J. The effect of insulin-like growth factor-I, relaxin and luteinizing hormone on vascular endothelial growth factor secretion by cultured endometrial stromal cells on different days of early pregnancy in pigs. Reprod. Biol. 2008, 8, 163–170. [Google Scholar] [CrossRef]
- Van der Zee, E.; Everts, V.; Hoeben, K.; Beertsen, W. Immunolocalisation of collagenase in rabbit periosteal tissue explants and extraction of the enzyme. The effect of the cytokines IL-1 alpha and EGF. J. Cell Sci. 1994, 107, 1047–1053. [Google Scholar]
- Szafrańska, B.; Ziecik, A.; Okrasa, S. Primary antisera against selected steroids or proteins and secondary antisera against gamma-globulins--an available tool for studies of reproductive processes. Reprod. Biol. 2002, 2, 187–204. [Google Scholar] [PubMed]
- Geisert, R.D.; Thatcher, W.W.; Michael Roberts, R.; Bazer, F.W. Establishment of Pregnancy in the Pig: III. Endometrial Secretory Response to Estradiol Valerate Administered on Day 11 of the Estrous Cycle1,2,3. Biol. Reprod. 1982, 27, 957–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattson, B.A.; Overstrom, E.W.; Albertini, D.F. Transitions in Trophectoderm Cellular Shape and Cytoskeletal Organization in the Elongating Pig Blastocyst. Biol. Reprod. 1990, 42, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Keys, J.L.; King, G.J. Microscopic examination of porcine conceptus-maternal interface between days 10 and 19 of pregnancy. Am. J. Anat. 1990, 188, 221–238. [Google Scholar] [CrossRef]
- Bazer, F.W.; Thatcher, W.W. Theory of maternal recognition of pregnancy in swine based on estrogen controlled endocrine versus exocrine secretion of prostaglandin F2α by the uterine endometrium. Prostaglandins 1977, 14, 397–401. [Google Scholar] [CrossRef]
- McCracken, J.A.; Custer, E.E.; Lamsa, J.C. Luteolysis: A Neuroendocrine-Mediated Event. Physiol. Rev. 1999, 79, 263–323. [Google Scholar] [CrossRef]
- Krzymowski, T.; Stefańczyk-Krzymowska, S. The oestrous cycle and early pregnancy—A new concept of local endocrine regulation. Vet. J. 2004, 168, 285–296. [Google Scholar] [CrossRef]
- Okrasa, S.; Franczak, A.; Zmijewska, A.; Wojciechowicz, B.; Dziekonski, M.; Martyniak, M.; Kolakowska, J.; Zglejc, K.; Kotwica, G. The uterine secretory activity and its physiological changes in the pig. Acta Biol. Cracoviensia. Ser. Zool. 2013, 55–56, 40–57. [Google Scholar]
- Andronowska, A.; Postek, A.; Doboszyńska, T. Epidermal growth factor and epidermal growth factor receptor immunoreactivity in the endothelial cells of the uterine artery and its branches during different stages of the estrous cycle in the pig. Pol. J. Vet. Sci. 2006, 9, 165–170. [Google Scholar]
- Wojciechowicz, B.; Kotwica, G.; Kołakowska, J.; Zglejc, K.; Martyniak, M.; Franczak, A. The alterations in endometrial and myometrial transcriptome at the time of maternal recognition of pregnancy in pigs. Agri Gene 2016, 2, 5–10. [Google Scholar] [CrossRef]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Eukaryotic Transcription and Translation Are Separated in Space and Time. In Biochemistry, 5th ed.; W H Freeman: New York, NY, USA, 2002. [Google Scholar]
- Vogel, C.; Marcotte, E.M. Insights into regulation of protein abundance from proteomics and transcriptomis analyses. Nat. Rev. Genet. 2013, 13, 227–232. [Google Scholar] [CrossRef]
- De Sousa Abreu, R.; Penalva, L.O.; Marcotte, E.M.; Vogel, C. Global signatures of protein and mRNA expression levels. Mol. Biosyst. 2009, 5, 1512–1526. [Google Scholar] [CrossRef] [Green Version]
- Gry, M.; Rimini, R.; Strömberg, S.; Asplund, A.; Pontén, F.; Uhlén, M.; Nilsson, P. Correlations between RNA and protein expression profiles in 23 human cell lines. BMC Genomics 2009, 10, 365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashworth, M.D.; Ross, J.W.; Stein, D.R.; Allen, D.T.; Spicer, L.J.; Geisert, R.D. Endocrine disruption of uterine insulin-like growth factor expression in the pregnant gilt. Reproduction 2005, 130, 545–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Payne, A.H.; Hales, D.B. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 2004, 25, 947–970. [Google Scholar] [CrossRef]
- Makieva, S.; Saunders, P.T.K.; Norman, J.E. Androgens in pregnancy: Roles in parturition. Hum. Reprod. Update 2014, 20, 542–559. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, S.; Mäkelä, S.; Treuter, E.; Tujague, M.; Thomsen, J.; Andersson, G.; Enmark, E.; Pettersson, K.; Warner, M.; Gustafsson, J.Å. Mechanisms of estrogen action. Physiol. Rev. 2001, 81, 1535–1565. [Google Scholar] [CrossRef]
- Franczak, A.; Wojciechowicz, B.; Kolakowska, J.; Zglejc, K.; Kotwica, G. Transcriptomic analysis of the myometrium during peri-implantation period and luteolysis–the study on the pig model. Funct. Integr. Genomics 2014, 14, 673–682. [Google Scholar] [CrossRef] [Green Version]
- Franczak, A.; Wojciechowicz, B.; Kotwica, G. Transcriptomic analysis of the porcine endometrium during early pregnancy and the estrous cycle. Reprod. Biol. 2013, 13, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Kiewisz, J.; Krawczynski, K.; Lisowski, P.; Blitek, A.; Zwierzchowski, L.; Ziecik, A.J.; Kaczmarek, M.M. Global gene expression profiling of porcine endometria on Days 12 and 16 of the estrous cycle and pregnancy. Theriogenology 2014, 82, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.R.; Clyne, C.; Speed, C.; Rubin, G.; Bulun, S. Tissue-specific estrogen biosynthesis and metabolism. Ann. N. Y. Acad. Sci. 2001, 949, 58–67. [Google Scholar] [CrossRef]
- Wojciechowicz, B.; Kotwica, G.; Zglejc, K.; Waszkiewicz, E.; Franczak, A. Expression of 17β-hydroxysteroid dehydrogenase and the effects of LH, FSH and prolactin on oestrone and 17β-oestradiol secretion in the endometrium of pigs during early pregnancy and the oestrous cycle. Reprod. Fertil. Dev. 2017, 29, 975–984. [Google Scholar] [CrossRef]
- Ziecik, A.J.; Przygrodzka, E.; Jalali, B.M.; Kaczmarek, M.M. Regulation of the porcine corpus luteum during pregnancy. Reproduction 2018, 156, 57–67. [Google Scholar] [CrossRef]
- Lee, C.Y.; Green, M.L.; Simmen, R.C.M.; Simmen, F.A. Proteolysis of insulin-like growth factor-binding proteins (IGFBPs) within the pig uterine lumen associated with peri-implantation conceptus development. J. Reprod. Fertil. 1998, 112, 369–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simmen, F.A.; Simmen, R.C.M.; Geisert, R.D.; Botte, F.M.; Bazer, F.W.; Terqui, M. Differential expression, during the estrous cycle and pre- and postimplantation conceptus development, of messenger ribonucleic acids encoding components of the pig uterine insulin-like growth factor system. Endocrinology 1992, 130, 1547–1556. [Google Scholar]





| Gene Symbol | Primer Sequence (5’→3’) | Gene Bank Accession No. | Annealing (°C) | Amplicon Length (bp) |
|---|---|---|---|---|
| IGF-1R | F: TTTGTGCCCAGACCTGAACG R: GTAAAAGGCCGGAGGTTGGA | NM_214172.1 | 60 | 200 |
| EGFR | F: TTCCGTCCGAAACAATCGG R: TTCCCAGTGAGGCACAGAG | NM_214007.1 | 60 | 103 |
| ACTB | F: GGAGATCGTGCGGGACATCAAG R: GGCGTAGAGGTCCTTCCTGATG | U07786.1 | 60 | 268 |
| RNA 18S | F: ACTGAGGCCATGATTAAG R: GCTATCAATCTGTCAATCC | AF102857.1 | 60 | 521 |
| Effect | IGF-1R mRNA | EGFR mRNA | ||
|---|---|---|---|---|
| F | p-Value | F | p-Value | |
| Physiological status 1 | 2.44879 | 0.136039 | 0.09807 | 0.757970 |
| Day of the estrous cycle or pregnancy 2 | 0.45379 | 0.642693 | 3.71779 | 0.045775 |
| Physiological status × Day of the estrous cycle or pregnancy | 0.38678 | 0.685068 | 0.13592 | 0.873849 |
| Effect | IGF-1R Protein | EGFR Protein | ||
| F | p-Value | F | p-Value | |
| Physiological status 1 | 3.8520 | 0.065331 | 0.0022 | 0.962905 |
| Day of the estrous cycle or pregnancy 2 | 1.9167 | 0.175939 | 4.2889 | 0.029976 |
| Physiological status × Day of the estrous cycle or pregnancy | 1.1946 | 0.325719 | 12.9711 | 0.000325 |
| Factors | A4 | T | E1 | E2 | ||||
|---|---|---|---|---|---|---|---|---|
| F | p-Value | F | p-Value | F | p-Value | F | p-Value | |
| Treatment 1 | 2.659 | 0.037423 | 0.36 | 0.836151 | 3.529 | 0.009750 | 1.111 | 0.355716 |
| Physiological status 2 | 1.378 | 0.243377 | 5.85 | 0.017356 | 8.716 | 0.003931 | 1.627 | 0.205110 |
| Days of the estrous cycle or pregnancy 3 | 9.939 | 0.000120 | 2.39 | 0.096971 | 1.312 | 0.273793 | 2.081 | 0.130233 |
| Treatment × Physiological status | 0.169 | 0.953780 | 0.21 | 0.931675 | 0.481 | 0.749270 | 1.470 | 0.216833 |
| Treatment × Days of the estrous cycle or pregnancy | 0.743 | 0.653462 | 0.90 | 0.517208 | 0.723 | 0.670727 | 1.212 | 0.299688 |
| Physiological status × Days of the estrous cycle or pregnancy | 17.850 | 0.000000 | 18.44 | 0.000000 | 2.838 | 0.063284 | 2.402 | 0.095745 |
| Treatment × Physiological status × Days of the estrous cycle or pregnancy | 0.167 | 0.994718 | 0.44 | 0.895146 | 0.845 | 0.565218 | 0.381 | 0.928360 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waszkiewicz, E.M.; Kozlowska, W.; Zmijewska, A.; Franczak, A. Expression of Insulin-Like Growth Factor 1 (IGF-1) and Epidermal Growth Factor (EGF) Receptors and the Effect of IGF-1 and EGF on Androgen and Estrogen Release in the Myometrium of Pigs—In Vitro Study. Animals 2020, 10, 915. https://doi.org/10.3390/ani10050915
Waszkiewicz EM, Kozlowska W, Zmijewska A, Franczak A. Expression of Insulin-Like Growth Factor 1 (IGF-1) and Epidermal Growth Factor (EGF) Receptors and the Effect of IGF-1 and EGF on Androgen and Estrogen Release in the Myometrium of Pigs—In Vitro Study. Animals. 2020; 10(5):915. https://doi.org/10.3390/ani10050915
Chicago/Turabian StyleWaszkiewicz, Ewa Monika, Wiktoria Kozlowska, Agata Zmijewska, and Anita Franczak. 2020. "Expression of Insulin-Like Growth Factor 1 (IGF-1) and Epidermal Growth Factor (EGF) Receptors and the Effect of IGF-1 and EGF on Androgen and Estrogen Release in the Myometrium of Pigs—In Vitro Study" Animals 10, no. 5: 915. https://doi.org/10.3390/ani10050915






