Phenotyping of the Visceral Adipose Tissue Using Dual Energy X-ray Absorptiometry (DXA) and Magnetic Resonance Imaging (MRI) in Pigs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Test Procedure
2.2.1. Magnetic Resonance Imaging
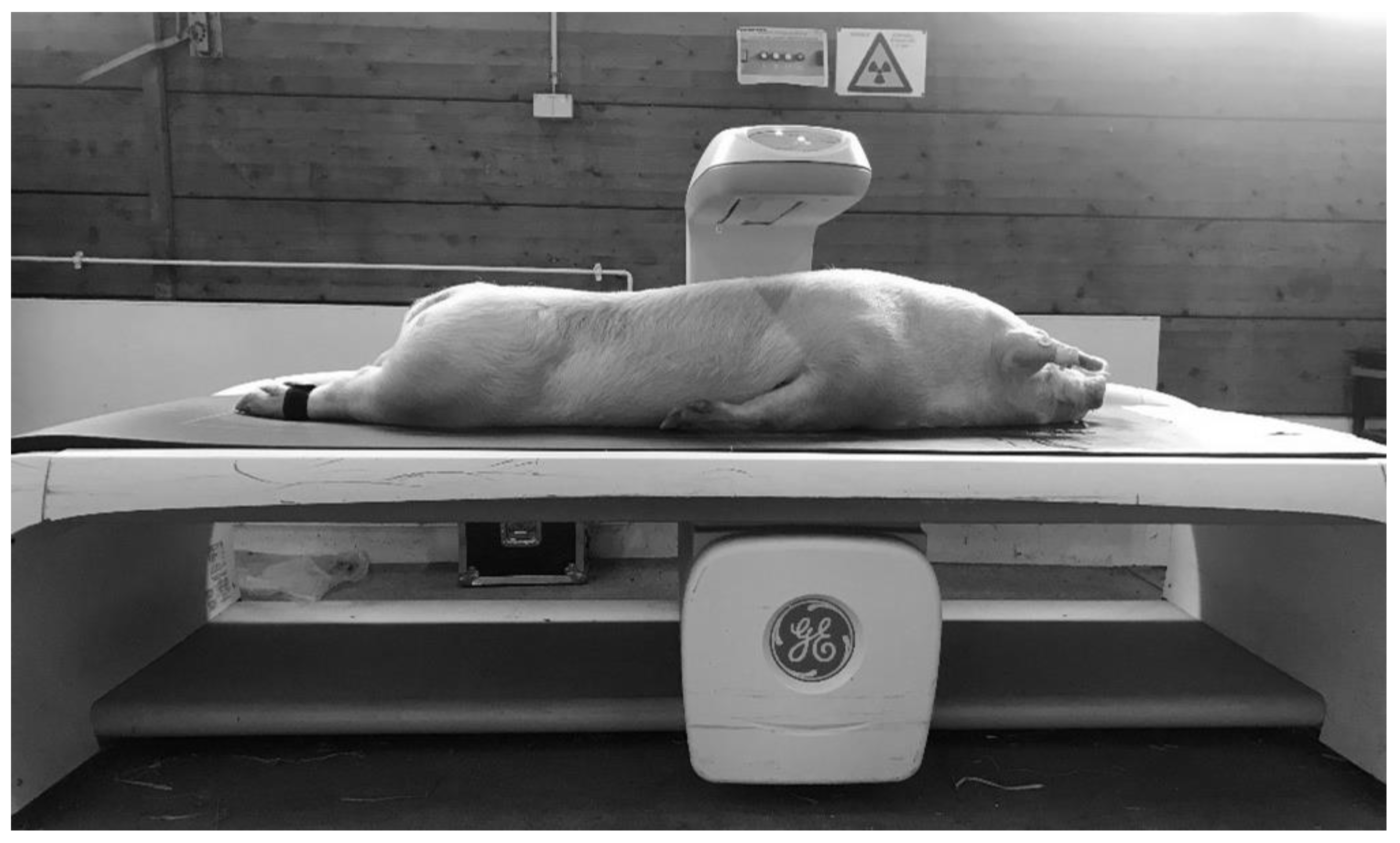
2.2.2. Dual Energy X-ray Absorptiometry
2.3. Data Analysis
2.3.1. MRI Evaluation
2.3.2. DXA Evaluation
2.3.3. Statistical Analysis
3. Results
3.1. Internal DXA Measurement Results
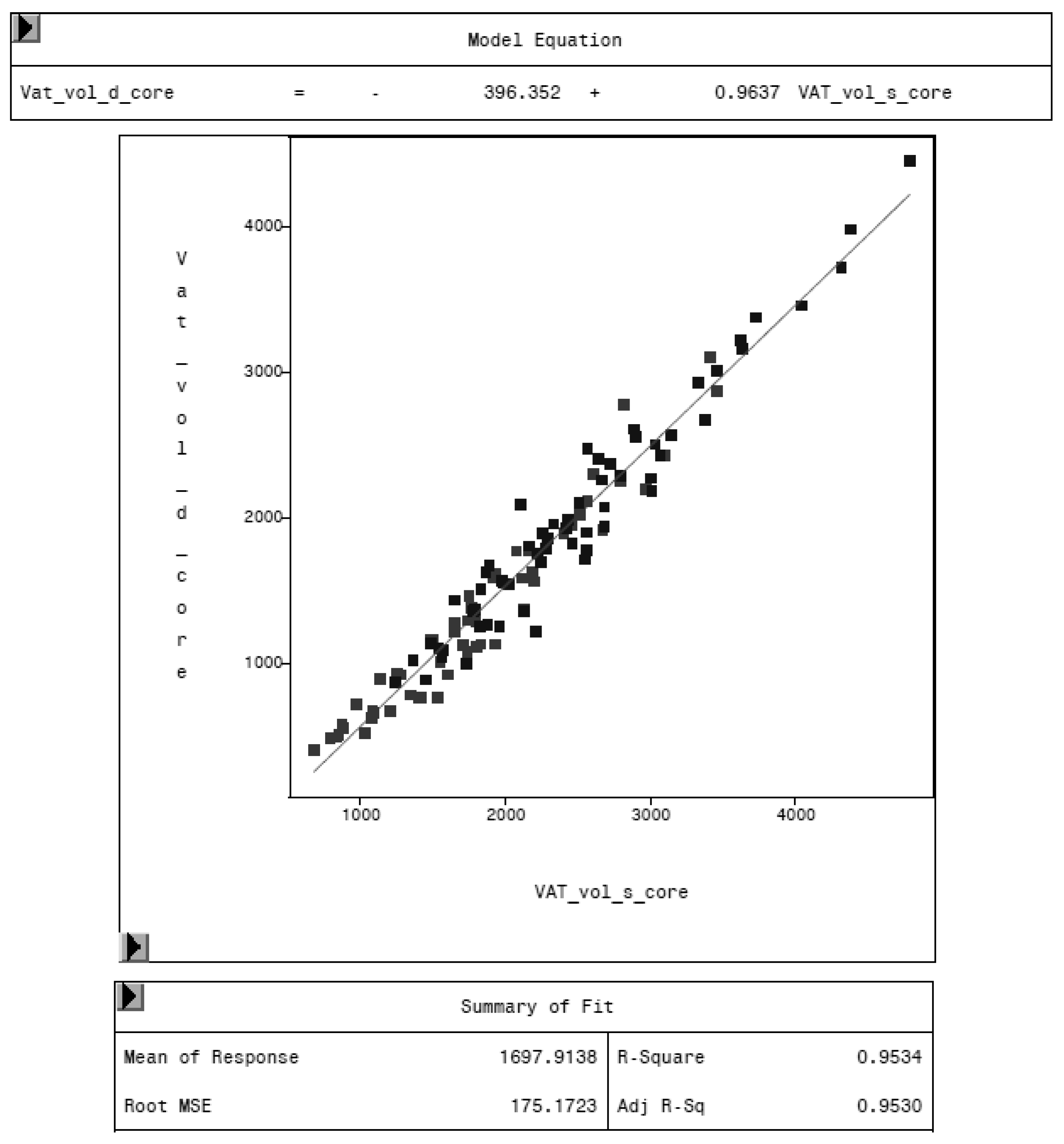
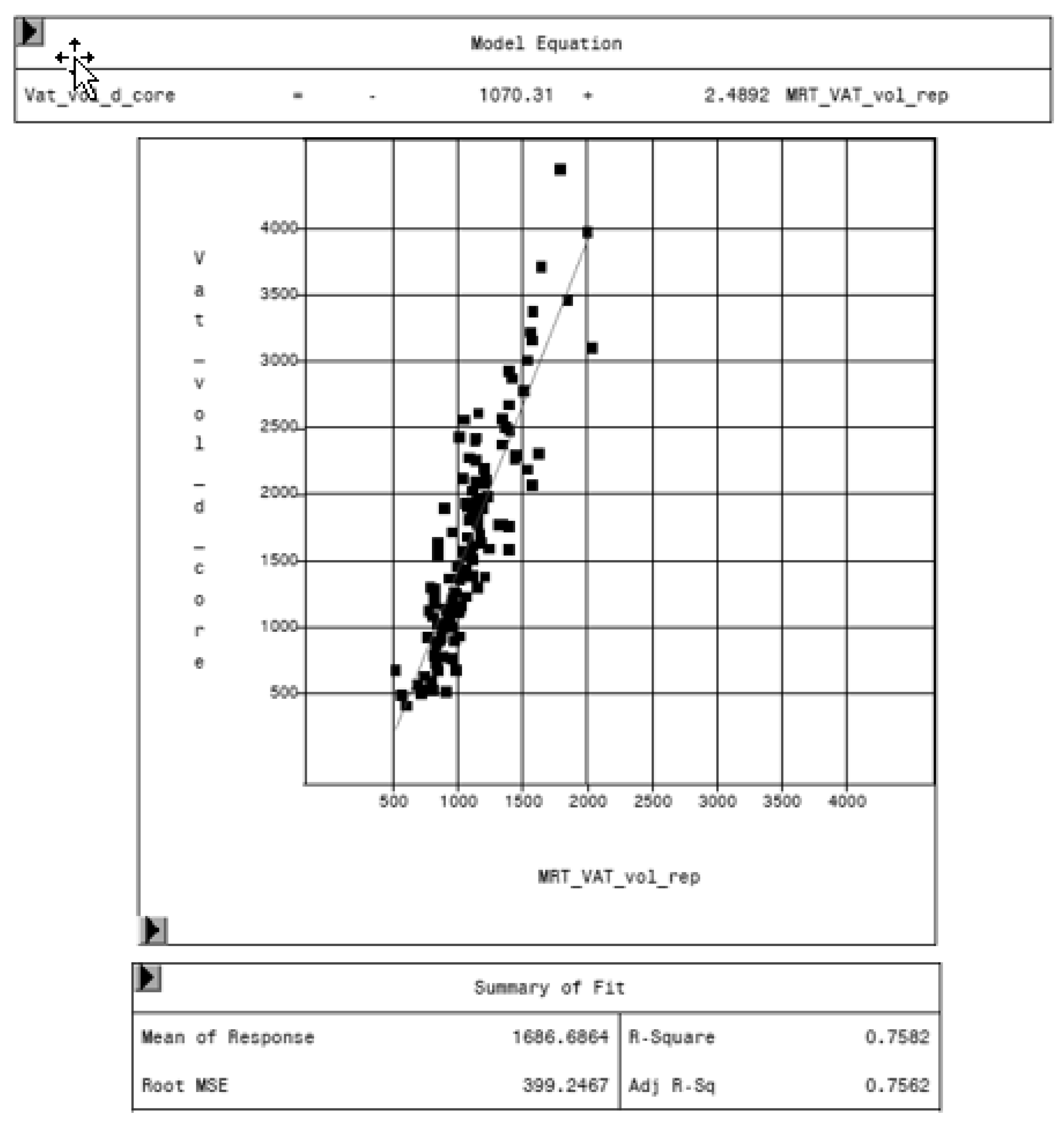
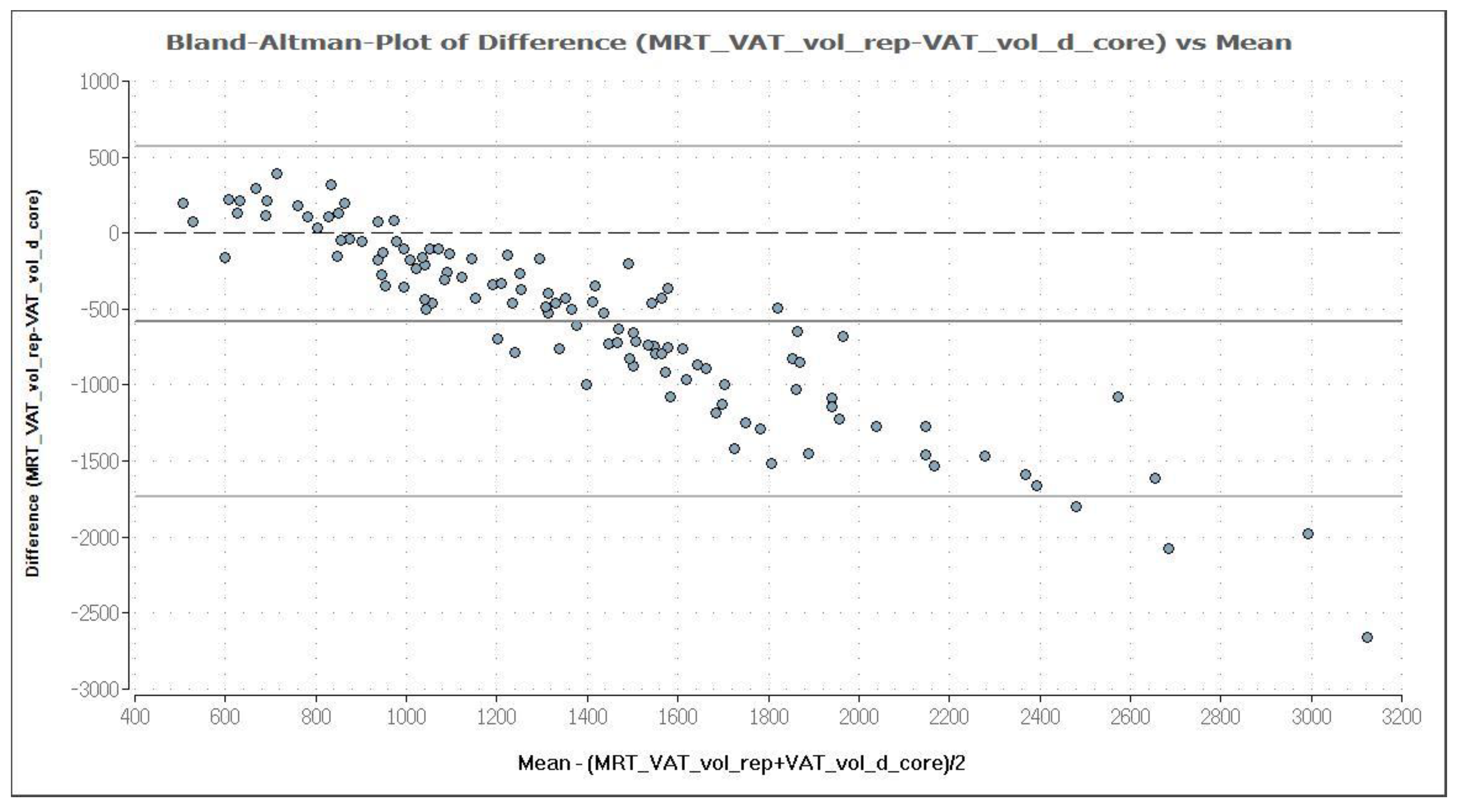
3.2. Comparison of MRI and DXA Results
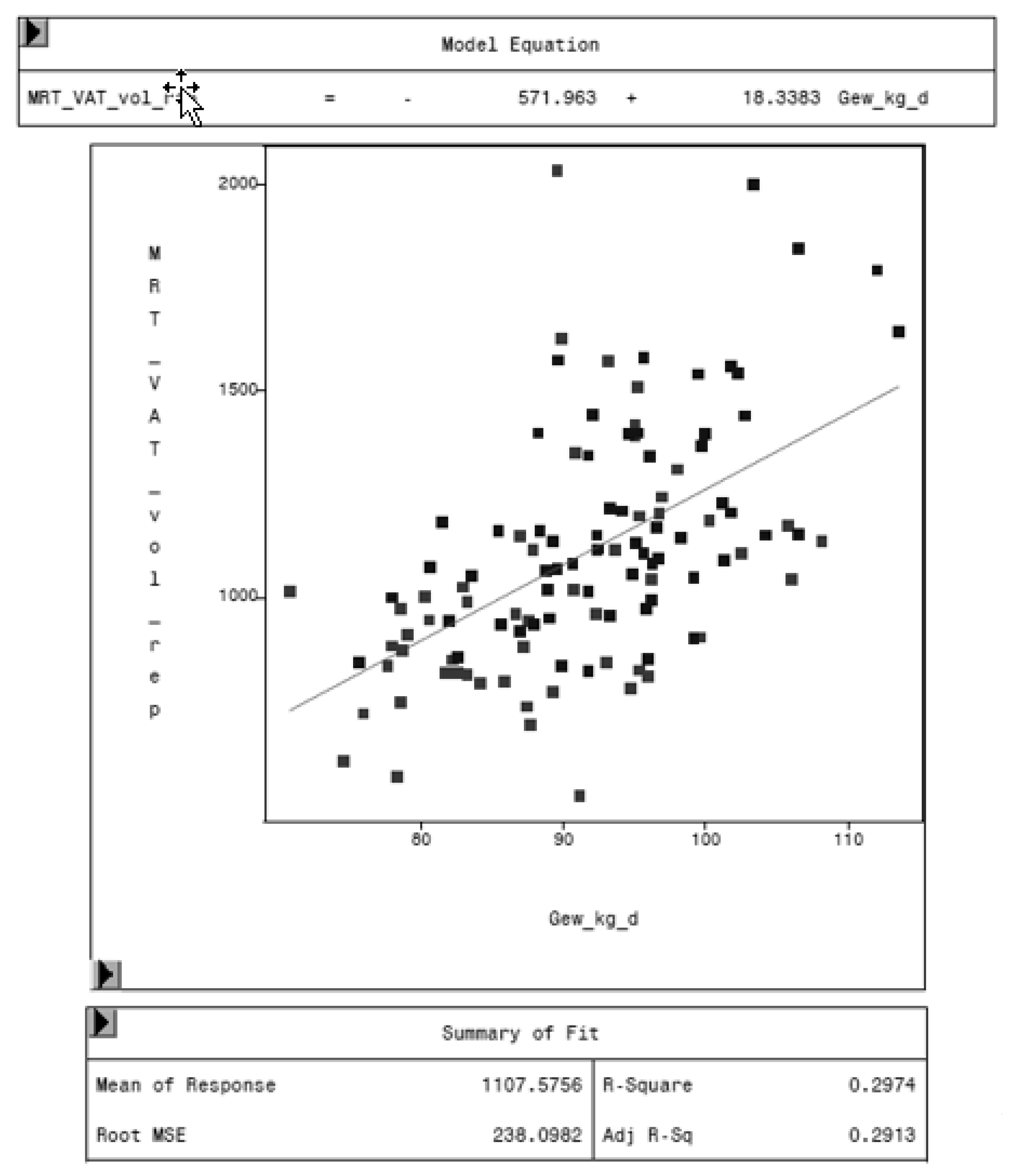
3.3. Relationship of VAT, Weight, and Age
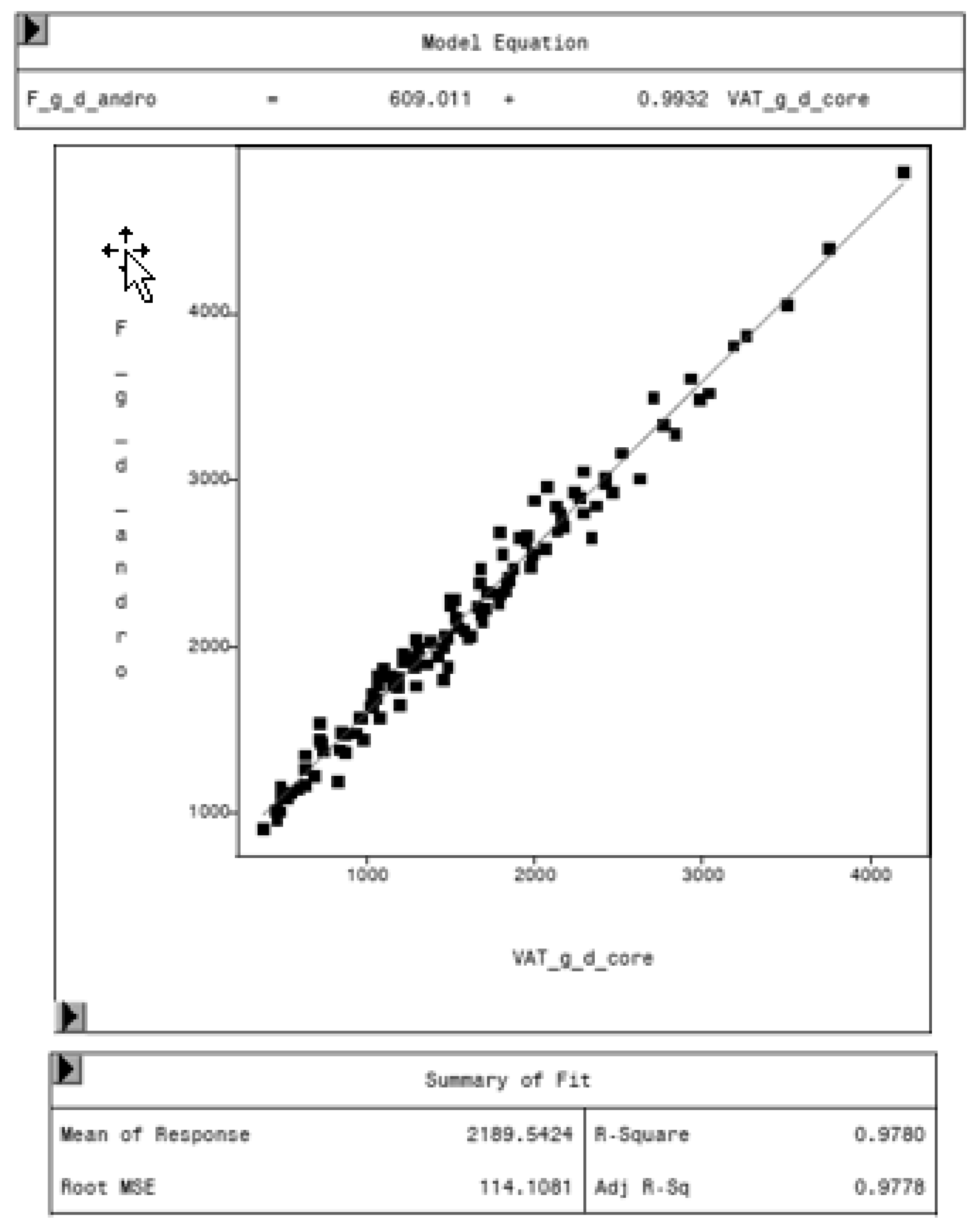
3.4. Variation in Fat Mass and VAT Volume by Sex and Genetic Origin
3.5. Variation in Weight by Sex and Genetic Origin
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 27 March 2020).
- Statistisches Bundesamt. Zahl der Todesfälle im Jahr 2017 um 2,3 % Gestiegen. Available online: https://www.destatis.de/DE/Themen/Gesellschaft-Umwelt/Gesundheit/Todesursachen/todesfaelle.html;jsessionid=942A0C43C62E6604E5EB4BC9B457D203.internet721 (accessed on 27 March 2020).
- Dornquast, C.; Kroll, L.E.; Neuhauser, H.K.; Willich, S.N.; Reinhold, T.; Busch, M.A. Regional Differences in the Prevalence of Cardiovascular Disease. Dtsch. Arztebl. Int. 2016, 113, 704–711. [Google Scholar] [CrossRef]
- World Health Organization. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 27 March 2020).
- Robert Koch-Institut. Gesundheit in Deutschland: Gesundheitsberichterstattung des Bundes; Gemeinsam Getragen von RKI und Destatis; RKI: Berlin, Germany, 2015. [Google Scholar]
- Nationale VersorgungsLeitlinie Chronische Herzinsuffizienz—Langfassung. Available online: https://www.leitlinien.de/mdb/downloads/nvl/herzinsuffizienz/archiv/hi-lang-1.3.pdf (accessed on 27 March 2020).
- Fox, C.S.; Massaro, J.M.; Hoffmann, U.; Pou, K.M.; Maurovich-Horvat, P.; Liu, C.-Y.; Vasan, R.S.; Murabito, J.M.; Meigs, J.B.; Cupples, L.A.; et al. Abdominal visceral and subcutaneous adipose tissue compartments: Association with metabolic risk factors in the Framingham Heart Study. Circulation 2007, 116, 39–48. [Google Scholar] [CrossRef]
- Liu, J.; Fox, C.S.; Hickson, D.A.; May, W.D.; Hairston, K.G.; Carr, J.J.; Taylor, H.A. Impact of Abdominal Visceral and Subcutaneous Adipose Tissue on Cardiometabolic Risk Factors: The Jackson Heart Study. J. Clin. Endocrinol. Metab. 2010, 95, 5419–5426. [Google Scholar] [CrossRef]
- Rodríguez, R.R.; González-Bulnes, A.; Garcia-Contreras, C.; Elena Rodriguez-Rodriguez, A.; Astiz, S.; Vazquez-Gomez, M.; Luis Pesantez, J.; Isabel, B.; Salido-Ruiz, E.; González, J.; et al. The Iberian pig fed with high-fat diet: A model of renal disease in obesity and metabolic syndrome. Int. J. Obes. (Lond.) 2020, 44, 457–465. [Google Scholar] [CrossRef]
- Lanzas, C.; Ayscue, P.; Ivanek, R.; Gröhn, Y.T. Model or meal? Farm animal populations as models for infectious diseases of humans. Nat. Rev. Microbiol. 2010, 8, 139–148. [Google Scholar] [CrossRef]
- Renner, S.; Blutke, A.; Clauss, S.; Deeg, C.A.; Kemter, E.; Merkus, D.; Wanke, R.; Wolf, E. Porcine models for studying complications and organ crosstalk in diabetes mellitus. Cell Tissue Res. 2020, 380, 341–378. [Google Scholar] [CrossRef]
- Rohrer, G.; Beever, J.E.; Rothschild, M.F.; Schook, L.; Gibbs, R.; Weinstock, G. Porcine Genomic Sequencing Initiative; Porcine Sequencing White Paper; Department of Animal Science, Iowa State University: Ames, IA, USA, 2002; pp. 1–10. [Google Scholar]
- Hishikawa, D.; Hong, Y.-H.; Roh, S.-g.; Miyahara, H.; Nishimura, Y.; Tomimatsu, A.; Tsuzuki, H.; Gotoh, C.; Kuno, M.; Choi, K.-C.; et al. Identification of genes expressed differentially in subcutaneous and visceral fat of cattle, pig, and mouse. Physiol. Genom. 2005, 21, 343–350. [Google Scholar] [CrossRef]
- Rothammer, S.; Kremer, P.V.; Bernau, M.; Fernandez-Figares, I.; Pfister-Schär, J.; Medugorac, I.; Scholz, A.M. Genome-wide QTL mapping of nine body composition and bone mineral density traits in pigs. Genet. Sel. Evol. 2014, 46, 1–11. [Google Scholar] [CrossRef]
- Scholz, A.M.; Bünger, L.; Kongsro, J.; Baulain, U.; Mitchell, A.D. Non-invasive methods for the determination of body and carcass composition in livestock: Dual-energy X-ray absorptiometry, computed tomography, magnetic resonance imaging and ultrasound: Invited review. Animal 2015, 9, 1250–1264. [Google Scholar] [CrossRef]
- Siemens, A.G. Technical Documents. MAGNETOM C! syngo MR, M4-030.815.01.03.02; Siemens AG: Munich, Germany, 2004. [Google Scholar]
- Pietrobelli, A.; Gallagher, D.; Baumgartner, R.; Ross, R.; Heymsfield, S.B. Lean R value for DXA two-component soft-tissue model: Influence of age and tissue or organ type. Appl. Radiat. Isot. 1998, 49, 743–744. [Google Scholar] [CrossRef]
- GE Healthcare. Lunar CoreScan Application from GE Healthcare. Available online: https://www.gehealthcare.com/-/jssmedia/1cd6ce71f5cd4ac1b782fa1815ddb23b.pdf (accessed on 21 May 2020).
- GE Healthcare. Advancements in DXA Body Composition Analysis: Metabolic Phenotyping with CoreScan. Available online: https://www3.gehealthcare.com/~/media/downloads/us/product/product-categories/metabolic-health/gated-pdfs/Whitepaper-Metabolic-Phenotyping-with-CoreScan-JB47862XX.pdf (accessed on 27 March 2020).
- GE Healthcare. Lunar. enCORE-Based X-ray Bone Densitometer. User Manual, 5th ed.; GE Healthcare: Madison, WI, USA, 2010. [Google Scholar]
- Altman, D.G.; Bland, J.M. Measurement in Medicine: The Analysis of Method Comparison Studies. J. R. Stat. Soc. Ser. D Stat. 1983, 32, 307–317. [Google Scholar] [CrossRef]
- Staiano, A.E.; Katzmarzyk, P.T. Ethnic and sex differences in body fat and visceral and subcutaneous adiposity in children and adolescents. Int. J. Obes. (Lond.) 2012, 36, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Maislin, G.; Ahmed, M.M.; Gooneratne, N.; Thorne-Fitzgerald, M.; Kim, C.; Teff, K.; Arnardottir, E.S.; Benediktsdottir, B.; Einarsdottir, H.; Juliusson, S.; et al. Single slice vs. volumetric MR assessment of visceral adipose tissue: Reliability and validity among the overweight and obese. Obesity 2012, 20, 2124–2132. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Gallagher, D. Assessment methods in human body composition. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 566–572. [Google Scholar] [CrossRef]
- Shuster, A.; Patlas, M.; Pinthus, J.H.; Mourtzakis, M. The clinical importance of visceral adiposity: A critical review of methods for visceral adipose tissue analysis. Br. J. Radiol. 2012, 85, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.L.; Bell, J.D. Influence of undersampling on magnetic resonance imaging measurements of intra-abdominal adipose tissue. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 211–218. [Google Scholar] [CrossRef]
- Snijder, M.B.; Visser, M.; Dekker, J.M.; Seidell, J.C.; Fuerst, T.; Tylavsky, F.; Cauley, J.; Lang, T.; Nevitt, M.; Harris, T.B. The prediction of visceral fat by dual-energy X-ray absorptiometry in the elderly: A comparison with computed tomography and anthropometry. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Micklesfield, L.K.; Goedecke, J.H.; Punyanitya, M.; Wilson, K.E.; Kelly, T.L. Dual-energy X-ray performs as well as clinical computed tomography for the measurement of visceral fat. Obesity 2012, 20, 1109–1114. [Google Scholar] [CrossRef]
- Fourman, L.T.; Kileel, E.M.; Hubbard, J.; Holmes, T.; Anderson, E.J.; Looby, S.E.; Fitch, K.V.; Feldpausch, M.N.; Torriani, M.; Lo, J.; et al. Comparison of visceral fat measurement by dual-energy X-ray absorptiometry to computed tomography in HIV and non-HIV. Nutr. Diabetes 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Schlegel, P.; Gutzwiller, A. Dietary Calcium to Digestible Phosphorus Ratio for Optimal Growth Performance and Bone Mineralization in Growing and Finishing Pigs. Animals (Basel) 2020, 10, 178. [Google Scholar] [CrossRef]
- Hartnett, P.; Boyle, L.; Younge, B.; O’Driscoll, K. The Effect of Group Composition and Mineral Supplementation during Rearing on Measures of Cartilage Condition and Bone Mineral Density in Replacement Gilts. Animals (Basel) 2019, 9, 637. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.D.; Scholz, A.M.; Wang, P.C.; Song, H. Body composition analysis of the pig by magnetic resonance imaging. J. Anim. Sci. 2001, 79, 1800–1813. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.D.; Conway, J.M.; Potts, W.J. Body composition analysis of pigs by dual-energy x-ray absorptiometry. J. Anim. Sci. 1996, 74, 2663–2671. [Google Scholar] [CrossRef]
- Fowler, P.A.; Fuller, M.F.; Glasbey, C.A.; Cameron, G.G.; Foster, M.A. Validation of the in vivo measurement of adipose tissue by magnetic resonance imaging of lean and obese pigs. Am. J. Clin. Nutr. 1992, 56, 7–13. [Google Scholar] [CrossRef]
- Scholz, A.M.; Förster, M. Genauigkeit der Dualenergie-Röntgenabsorptiometrie (DXA) zur Ermittlung der Körperzusammensetzung von Schweinen in vivo. Arch. Anim. Breed. 2006, 49, 462–476. [Google Scholar] [CrossRef]
- Baulain, U.; Friedrichs, M.; Höreth, R.; Henning, M.; Tholen, E. Use of MRI to Assess Carcass and Primal Cut Composition in Different Pig Breeds. 2010. Available online: www.kongressband.de/wcgalp2010/assets/pdf/0357.pdf (accessed on 27 March 2020).
- Mohammad, A.; de Lucia Rolfe, E.; Sleigh, A.; Kivisild, T.; Behbehani, K.; Wareham, N.J.; Brage, S.; Mohammad, T. Validity of visceral adiposity estimates from DXA against MRI in Kuwaiti men and women. Nutr. Diabetes 2017, 7, e238. [Google Scholar] [CrossRef]
- Neeland, I.J.; Grundy, S.M.; Li, X.; Adams-Huet, B.; Vega, G.L. Comparison of visceral fat mass measurement by dual-X-ray absorptiometry and magnetic resonance imaging in a multiethnic cohort: The Dallas Heart Study. Nutr. Diabetes 2016, 6, e221. [Google Scholar] [CrossRef]
- Reinhardt, M.; Piaggi, P.; DeMers, B.; Trinidad, C.; Krakoff, J. Cross calibration of two dual-energy X-ray densitometers and comparison of visceral adipose tissue measurements by iDXA and MRI. Obesity (Silver Spring) 2017, 25, 332–337. [Google Scholar] [CrossRef]
- Taylor, A.E.; Kuper, H.; Varma, R.D.; Wells, J.C.; Bell, J.D.; Radhakrishna, K.V.; Kulkarni, B.; Kinra, S.; Timpson, N.J.; Ebrahim, S.; et al. Validation of dual energy X-ray absorptiometry measures of abdominal fat by comparison with magnetic resonance imaging in an Indian population. PLoS ONE 2012, 7, e51042. [Google Scholar] [CrossRef]
- GE Healthcare. Lunar Technology Advantages. Available online: https://www3.gehealthcare.com/~/media/downloads/us/product/product-categories/metabolic-health/gated-pdfs/BMD-Global-Lunar-Technology-Advantages-JB45946XX.pdf (accessed on 28 April 2020).
- Lukaski, H.C.; Marchello, M.J.; Hall, C.B.; Schafer, D.M.; Siders, W.A. Soft tissue composition of pigs measured with dual X-ray absorptiometry: Comparison with chemical analyses and effects of carcass thicknesses. Nutrition 1999, 15, 697–703. [Google Scholar] [CrossRef]
- Laskey, M.A.; Lyttle, K.D.; Flaxman, M.E.; Barber, R.W. The influence of tissue depth and composition on the performance of the Lunar dual-energy X-ray absorptiometer whole-body scanning mode. Eur. J. Clin. Nutr. 1992, 46, 39–45. [Google Scholar] [PubMed]
- Jebb, S.A.; Goldberg, G.R.; Jennings, G.; Elia, M. Dual-energy X-ray absorptiometry measurements of body composition: Effects of depth and tissue thickness, including comparisons with direct analysis. Clin. Sci. 1995, 88, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.; Rothney, M.P.; Peters, D.M.; Wacker, W.K.; Davis, C.E.; Shapiro, M.D.; Ergun, D.L. Dual-energy X-ray absorptiometry for quantification of visceral fat. Obesity (Silver Spring) 2012, 20, 1313–1318. [Google Scholar] [CrossRef]
- Björntorp, P. The regulation of adipose tissue distribution in humans. Int. J. Obes. Relat. Metab. Disord. 1996, 20, 291–302. [Google Scholar] [PubMed]
- Pedersen, S.B.; Bruun, J.M.; Hube, F.; Kristensen, K.; Hauner, H.; Richelsen, B. Demonstration of estrogen receptor subtypes α and β in human adipose tissue: Influences of adipose cell differentiation and fat depot localization. Mol. Cell. Endocrinol. 2001, 182, 27–37. [Google Scholar] [CrossRef]
- Dieudonne, M.N.; Pecquery, R.; Boumediene, A.; Leneveu, M.C.; Giudicelli, Y. Androgen receptors in human preadipocytes and adipocytes: Regional specificities and regulation by sex steroids. Am. J. Physiol. 1998, 274, C1645–C1652. [Google Scholar] [CrossRef]
- O’Brien, S.N.; Welter, B.H.; Mantzke, K.A.; Price, T.M. Identification of progesterone receptor in human subcutaneous adipose tissue. J. Clin. Endocrinol. Metab. 1998, 83, 509–513. [Google Scholar] [CrossRef]
- Wells, J.C.K. Sexual dimorphism of body composition. Best Pract. Res. Clin. Endocrinol. Metab. 2007, 21, 415–430. [Google Scholar] [CrossRef]
- Pedersen, S.B.; Hansen, P.S.; Lund, S.; Andersen, P.H.; Odgaard, A.; Richelsen, B. Identification of oestrogen receptors and oestrogen receptor mRNA in human adipose tissue. Eur. J. Clin. Investig. 1996, 26, 262–269. [Google Scholar] [CrossRef]
- Christoffersen, B.; Golozoubova, V.; Pacini, G.; Svendsen, O.; Raun, K. The Young Göttingen Minipig as a Model of Childhood and Adolescent Obesity: Influence of Diet and Gender. Obesity 2012, 1, 11. [Google Scholar] [CrossRef]
- Christoffersen, B.O.; Grand, N.; Golozoubova, V.; Svendsen, O.; Raun, K. Gender-associated differences in metabolic syndrome-related parameters in Göttingen minipigs. Comp. Med. 2007, 57, 493–504. [Google Scholar] [PubMed]
- Clapper, J.A.; Clark, T.M.; Rempel, L.A. Serum concentrations of IGF-I, estradiol-17beta, testosterone, and relative amounts of IGF binding proteins (IGFBP) in growing boars, barrows, and gilts. J. Anim. Sci. 2000, 78, 2581–2588. [Google Scholar] [CrossRef] [PubMed]
- Christoffersen, B.O.; Gade, L.P.; Golozoubova, V.; Svendsen, O.; Raun, K. Influence of castration-induced testosterone and estradiol deficiency on obesity and glucose metabolism in male Göttingen minipigs. Steroids 2010, 75, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Farnworth, E.R.; Kramer, J.K.G. Fat metabolism in growing swine: A review. Can. J. Anim. Sci. 1987, 67, 301–318. [Google Scholar] [CrossRef]
- Irshad, A.; Kandeepan, G.; Kumar, S.; Ashish, K.A.; Vishnuraj, M.R.; Shukla, V. Factors influencing carcass composition of livestock: A review. J. Anim. Prod. Adv. 2013, 3, 177–186. [Google Scholar]
- Mohrmann, M.; Roehe, R.; Susenbeth, A.; Baulain, U.; Knap, P.W.; Looft, H.; Plastow, G.S.; Kalm, E. Association between body composition of growing pigs determined by magnetic resonance imaging, deuterium dilution technique, and chemical analysis. Meat Sci. 2006, 72, 518–531. [Google Scholar] [CrossRef]
- Giles, L.R.; Eamens, G.J.; Arthur, P.F.; Barchia, I.M.; James, K.J.; Taylor, R.D. Differential growth and development of pigs as assessed by X-ray computed tomography. J. Anim. Sci. 2009, 87, 1648–1658. [Google Scholar] [CrossRef]
- Kolstad, K. Fat deposition and distribution in three genetic lines of pigs from 10 to 105 kilograms liveweight. Quality of meat and fat in pigs as affected by genetics and nutrition. In Proceedings of the Joint Session of the Eaap Commissions on Pig Production, Animal Genetics and Animal Nutrition, Zurich, Switzerland, 25 August 1999; pp. 199–202. [Google Scholar]
- Koopmans, S.J.; Schuurman, T. Considerations on pig models for appetite, metabolic syndrome and obese type 2 diabetes: From food intake to metabolic disease. Eur. J. Pharmacol. 2015, 759, 231–239. [Google Scholar] [CrossRef]
- Bauer, A.; Judas, M. Schlachtkörperqualität von Mastebern in Vergleich zu Sauen und Börgen. Züchtungskunde 2014, 5, 374–389. [Google Scholar]
- Scholz, A.M. In-vivo-Methoden zur Analyse von Muskelstoffwechsel und Körperzusammensetzung beim Schwein unter Besonderer Berücksichtigung Genetischer Einflüsse. Ph.D. Thesis, Tierärztliche Fakultät der Ludwig-Maximilians-Universität, München, Germany, 2002. [Google Scholar]
- Ernst, C.W.; Steibel, J.P. Molecular advances in QTL discovery and application in pig breeding. Trends Genet. 2013, 29, 215–224. [Google Scholar] [CrossRef]
- Pig QTL Database. Available online: https://www.animalgenome.org/cgi-bin/QTLdb/SS/index (accessed on 27 March 2020).
- Hu, Z.-l.; Dracheva, S.; Jang, W.; Maglott, D.; Bastiaansen, J.; Rothschild, M.F.; Reecy, J.M. A QTL resource and comparison tool for pigs: PigQTLDB. Mamm. Genome 2005, 16, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Ntalla, I.; Panoutsopoulou, K.; Vlachou, P.; Southam, L.; William Rayner, N.; Zeggini, E.; Dedoussis, G.V. Replication of established common genetic variants for adult BMI and childhood obesity in Greek adolescents: The TEENAGE study. Ann. Hum. Genet. 2013, 77, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Speliotes, E.K.; Willer, C.J.; Berndt, S.I.; Monda, K.L.; Thorleifsson, G.; Jackson, A.U.; Allen, H.L.; Lindgren, C.M.; Luan, J.; Mägi, R.; et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat. Genet. 2010, 42, 937–948. [Google Scholar] [CrossRef]
- Rothammer, S.; Bernau, M.; Kremer-Rücker, P.V.; Medugorac, I.; Scholz, A.M. Genome-wide QTL mapping results for regional DXA body composition and bone mineral density traits in pigs. Arch. Anim. Breed. 2017, 60, 51–59. [Google Scholar] [CrossRef]
- International Mouse Phenotyping Consortium. Available online: https://www.mousephenotype.org/ (accessed on 21 April 2020).
- Thomas, G.; Betters, J.L.; Lord, C.C.; Brown, A.L.; Marshall, S.; Ferguson, D.; Sawyer, J.; Davis, M.A.; Melchior, J.T.; Blume, L.C.; et al. The serine hydrolase ABHD6 Is a critical regulator of the metabolic syndrome. Cell Rep. 2013, 5, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Nagai, K.; Nagata, Y.; Doronbekov, K.; Nishimura, S.; Yoshioka, S.; Fujita, T.; Shiga, K.; Miyake, T.; Taniguchi, Y.; et al. Exploration of genes showing intramuscular fat deposition-associated expression changes in musculus longissimus muscle. Anim. Genet. 2006, 37, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Serão, N.V.L.; Veroneze, R.; Ribeiro, A.M.F.; Verardo, L.L.; Braccini Neto, J.; Gasparino, E.; Campos, C.F.; Lopes, P.S.; Guimarães, S.E.F. Candidate gene expression and intramuscular fat content in pigs. J. Anim. Breed. Genet. 2011, 128, 28–34. [Google Scholar] [CrossRef]
- Kogelman, L.J.A.; Kadarmideen, H.N.; Mark, T.; Karlskov-Mortensen, P.; Bruun, C.S.; Cirera, S.; Jacobsen, M.J.; Jørgensen, C.B.; Fredholm, M. An f2 pig resource population as a model for genetic studies of obesity and obesity-related diseases in humans: Design and genetic parameters. Front. Genet. 2013, 4, 29. [Google Scholar] [CrossRef]
- Wakchaure, R.; Ganguly, S.; Praveen, K.P.; Sharma, S.; Kumar, A.; Mahajan, T.; Qadri, K. Importance of Heterosis in Animals: A Review. Int. J. Adv. Eng. Technol. Innov. Sci. 2015, 1, 1–5. [Google Scholar]
- Christians, C.J.; Johnson, R.K. Crossbreeding Programs for Commercial Pork Production; EM-Cooperative Extension Service, College of Agriculture, Washington State University (USA): Pullman, WA, USA, 1978. [Google Scholar]
- Bennnett, G.L.; Tess, M.W.; Dickerson, G.E.; Johnson, R.K. Simulation of Heterosis Effects on Costs of Pork Production. J. Anim. Sci. 1983, 56, 792–800. [Google Scholar] [CrossRef]
- Müller, E.; Moser, G.; Bartenschilager, H.; Geldermann, H. Trait values of growth, carcass and meat quality in Wild Boar, Meishan and Pietrain pigs as well as their crossbred generations. J. Anim. Breed. Genet. 2000, 117, 189–202. [Google Scholar] [CrossRef]





| Number | Age (days) | Weight (kg) | |||
|---|---|---|---|---|---|
| Mean | Standard Deviation | Mean | Standard Deviation | ||
| Castrated males | 63 | 147.4 | 3.5 | 93.9 | 7.6 |
| Females | 57 | 146.9 | 3.1 | 89.0 | 8.4 |
| MHF1 | 58 | 147.8 | 3.5 | 93.0 | 8.1 |
| MHF2 | 62 | 146.5 | 3.1 | 90.3 | 8.5 |
| ViscFat Sequence | Ham Sequence | |
|---|---|---|
| pixel size (mm x mm) | 1.9 × 1.6 | 1.9 × 1.6 |
| examination time (min) | 12.22 | 10.27 |
| signal-to-noise ratio | 1.00 | 1.00 |
| repetition time (TR) (ms) | 441 | 370 |
| echo time (TE) (ms) | 18 | 18 |
| slice number | 30 | 20 |
| slice thickness (mm) | 6 | 6 |
| Acquisition | axial | axial |
| distance factor (%) | 20 | 20 |
| matrix size | 211 × 250 | 211 × 250 |
| field of view (FoV) (mm) | 400 | 400 |
| VAT Volume (cm3) | |||
|---|---|---|---|
| DXA “Thick” | DXA “Standard” | MRI | |
| Arithmetic mean | 1686.69 | 2173.08 | 1108.33 |
| Standard deviation | 805.08 | 815.17 | 283.76 |
| DXA VAT Volume “Thick” (cm3) | DXA VAT Volume “Standard” (cm3) | |
|---|---|---|
| Model equation | −1070.31 + 2.4892 MRT_VAT_vol_rep | −537.384 + 2.4368 MRT_VAT_vol_rep |
| Root MSE | 399.2 | 443.4 |
| Adj. R-Sq | 0.756 | 0.707 |
| Pr > t | <0.0001 | <0.0001 |
| MRI VAT Volume (cm3) | DXA VAT Volume “Thick” (cm3) | |
|---|---|---|
| Model equation | −3908.40 + 34.0868 age (days) | −14,353.7 + 109.005 age (days) |
| Root MSE | 261.9 | 724.9 |
| Adj. R-Sq | 0.16 | 0.20 |
| Pr > t | <0.0001 | < 0.0001 |
| Sex | Genetic Origin | ||||||
|---|---|---|---|---|---|---|---|
| Castrated Males | Females | MHF1 | MHF2 | ||||
| DXA | Mode “Thick” | Gew_kg_d [kg] | LSM | 93.59 | 89.69 | 93.19 | 90.09 |
| SEE | 1.33 | 1.39 | 1.57 | 1.50 | |||
| Pr > t | 0.0079 | 0.1268 | |||||
| F_g_d_ges [g] | LSM | 15,092 | 12,554 | 14,532 | 13,114 | ||
| SEE | 466 | 492 | 533 | 507 | |||
| Pr > t | <0.0001 | 0.0504 | |||||
| F_proz_d_ges [%] | LSM | 16.35 | 14.14 | 15.86 | 14.63 | ||
| SEE | 0.40 | 0.43 | 0.46 | 0.44 | |||
| Pr > t | <0.0001 | 0.0498 | |||||
| F_g_d_andro [g] | LSM | 2409 | 1957 | 2343 | 2023 | ||
| SEE | 91 | 96 | 104 | 99 | |||
| Pr > t | 0.0002 | 0.0243 | |||||
| VAT_g_d_core [g] | LSM | 1868 | 1290 | 1741 | 1416 | ||
| SEE | 89 | 94 | 103 | 98 | |||
| Pr > t | <0.0001 | 0.0200 | |||||
| VAT_vol_d_core [cm3] | LSM | 1979.53 | 1367.33 | 1845.77 | 1501.88 | ||
| SEE | 94.82 | 100.01 | 108.99 | 103.75 | |||
| Pr > t | <0.0001 | 0.0200 | |||||
| Mode “Standard” | F_g_s_ges [g] | LSM | 16,164 | 13,677 | 15,537 | 14,304 | |
| SEE | 454 | 481 | 511 | 486 | |||
| Pr > t | <0.0001 | 0.0787 | |||||
| F_proz_s_ges [%] | LSM | 17.56 | 15.46 | 17.01 | 16.02 | ||
| SEE | 0.37 | 0.39 | 0.41 | 0.39 | |||
| Pr > t | <0.0001 | 0.0830 | |||||
| F_g_s_andro [g] | LSM | 2604 | 2164 | 2531 | 2237 | ||
| SEE | 86 | 91 | 96 | 91 | |||
| Pr > t | 0.0003 | 0.0272 | |||||
| VAT_g_s_core [g] | LSM | 2335 | 1744 | 2182 | 1897 | ||
| SEE | 84 | 89 | 92 | 88 | |||
| Pr > t | <0.0001 | 0.0269 | |||||
| VAT_vol_s_core [cm3] | LSM | 2475.38 | 1848.46 | 2312.55 | 2011.30 | ||
| SEE | 88.62 | 93.93 | 97.66 | 92.74 | |||
| Pr > t | <0.0001 | 0.0270 | |||||
| MRI | MRT_VAT_vol_rep [cm3] | LSM | 1188.83 | 1016.65 | 1196.40 | 1009.08 | |
| SEE | 47.48 | 49.08 | 56.44 | 53.86 | |||
| Pr > t | 0.0001 | 0.0074 | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
C. Weigand, A.; Schweizer, H.; Aline Knob, D.; Scholz, A.M. Phenotyping of the Visceral Adipose Tissue Using Dual Energy X-ray Absorptiometry (DXA) and Magnetic Resonance Imaging (MRI) in Pigs. Animals 2020, 10, 1165. https://doi.org/10.3390/ani10071165
C. Weigand A, Schweizer H, Aline Knob D, Scholz AM. Phenotyping of the Visceral Adipose Tissue Using Dual Energy X-ray Absorptiometry (DXA) and Magnetic Resonance Imaging (MRI) in Pigs. Animals. 2020; 10(7):1165. https://doi.org/10.3390/ani10071165
Chicago/Turabian StyleC. Weigand, Anna, Helen Schweizer, Deise Aline Knob, and Armin M. Scholz. 2020. "Phenotyping of the Visceral Adipose Tissue Using Dual Energy X-ray Absorptiometry (DXA) and Magnetic Resonance Imaging (MRI) in Pigs" Animals 10, no. 7: 1165. https://doi.org/10.3390/ani10071165
APA StyleC. Weigand, A., Schweizer, H., Aline Knob, D., & Scholz, A. M. (2020). Phenotyping of the Visceral Adipose Tissue Using Dual Energy X-ray Absorptiometry (DXA) and Magnetic Resonance Imaging (MRI) in Pigs. Animals, 10(7), 1165. https://doi.org/10.3390/ani10071165







