Effects of Intermittent Mild Cold Stimulation on mRNA Expression of Immunoglobulins, Cytokines, and Toll-Like Receptors in the Small Intestine of Broilers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
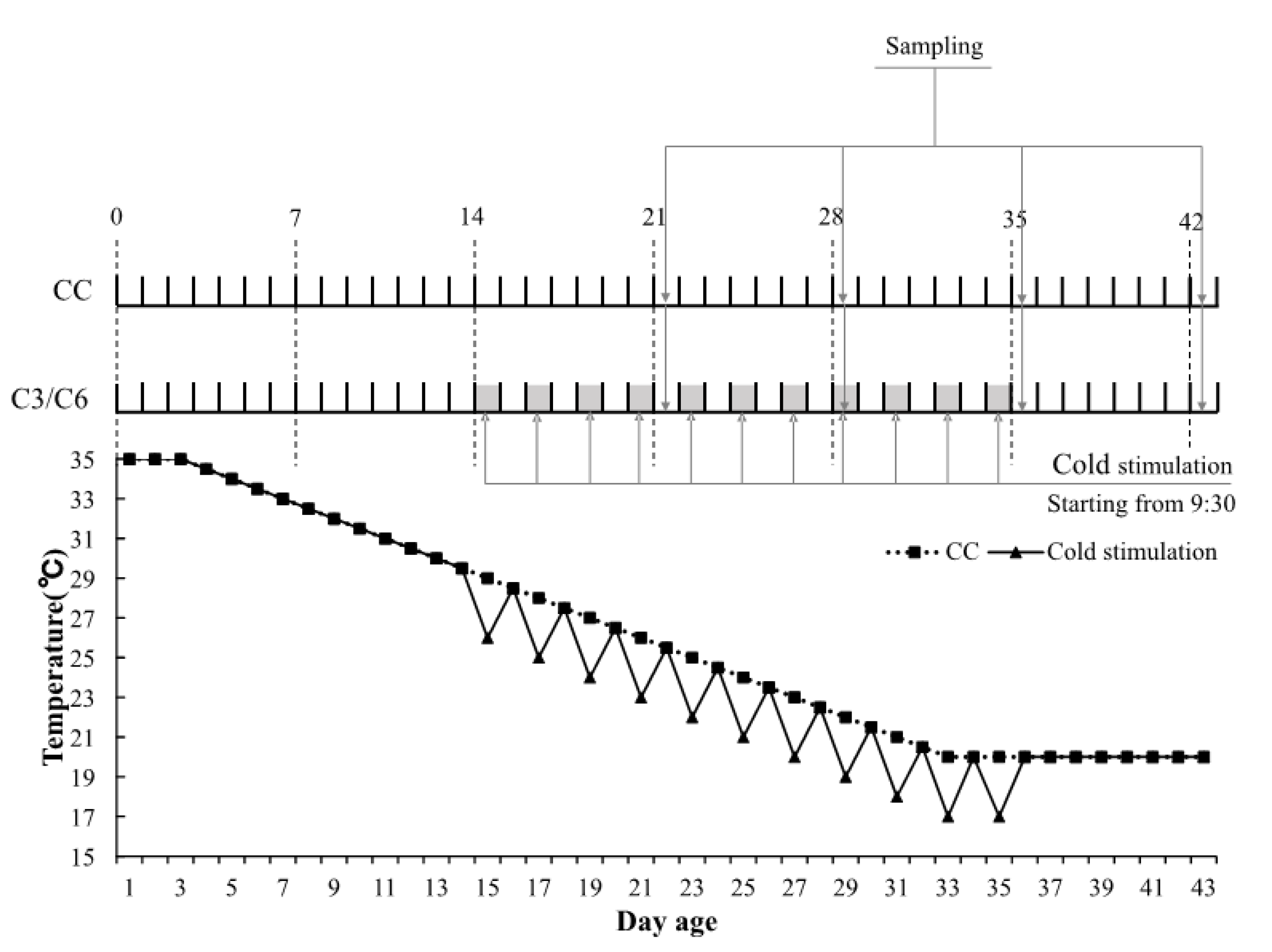
2.1. Animals and Experimental Design
2.2. Total RNA Extraction and Reverse Transcription
2.3. Quantitative Real-Time PCR (qRT-PCR)
2.4. Statistical Analysis
3. Results
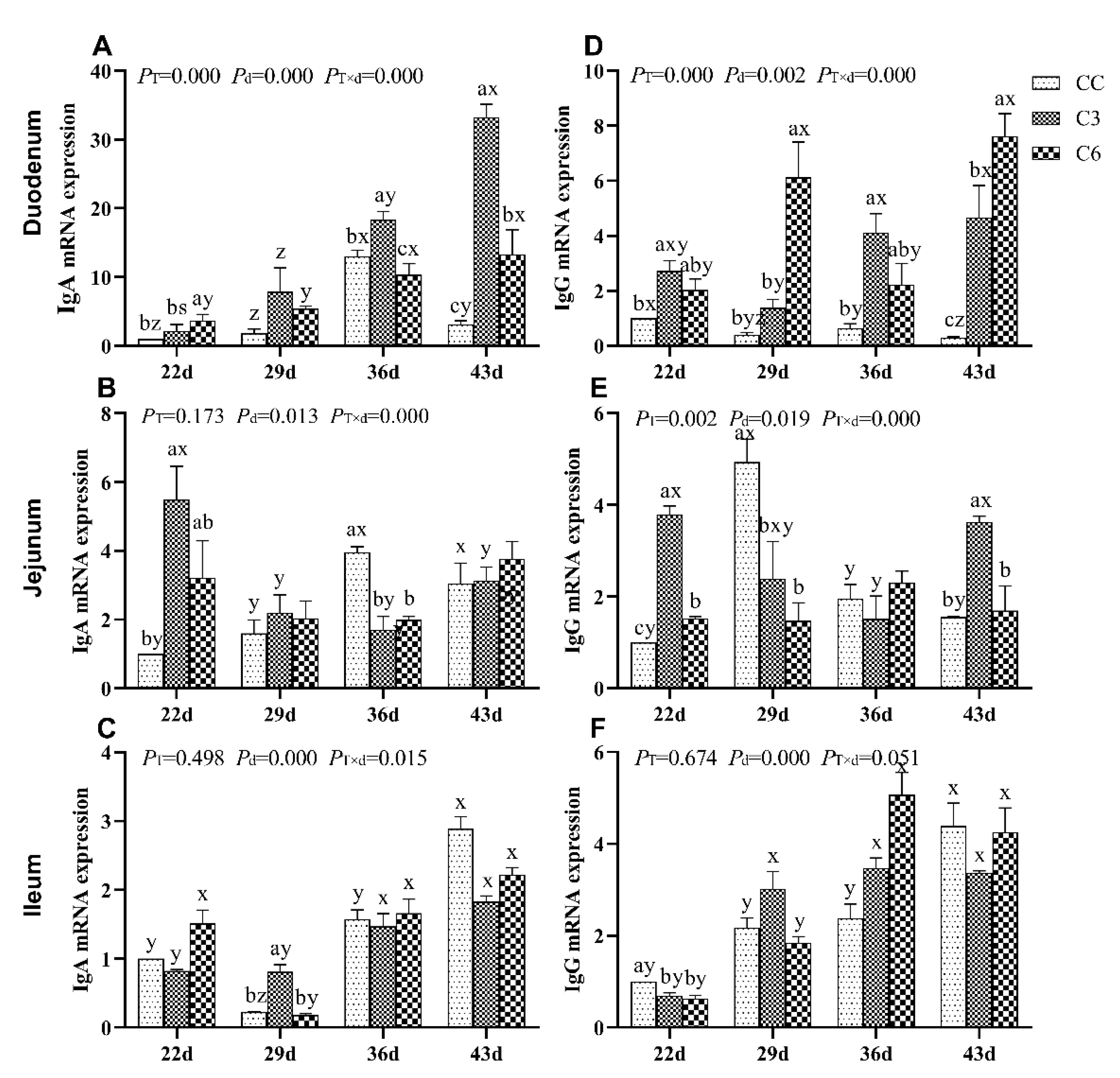
3.1. Changes in mRNA Expression Levels of Immunoglobulins
3.2. Changes in mRNA Expression Levels of Cytokines in Duodenum
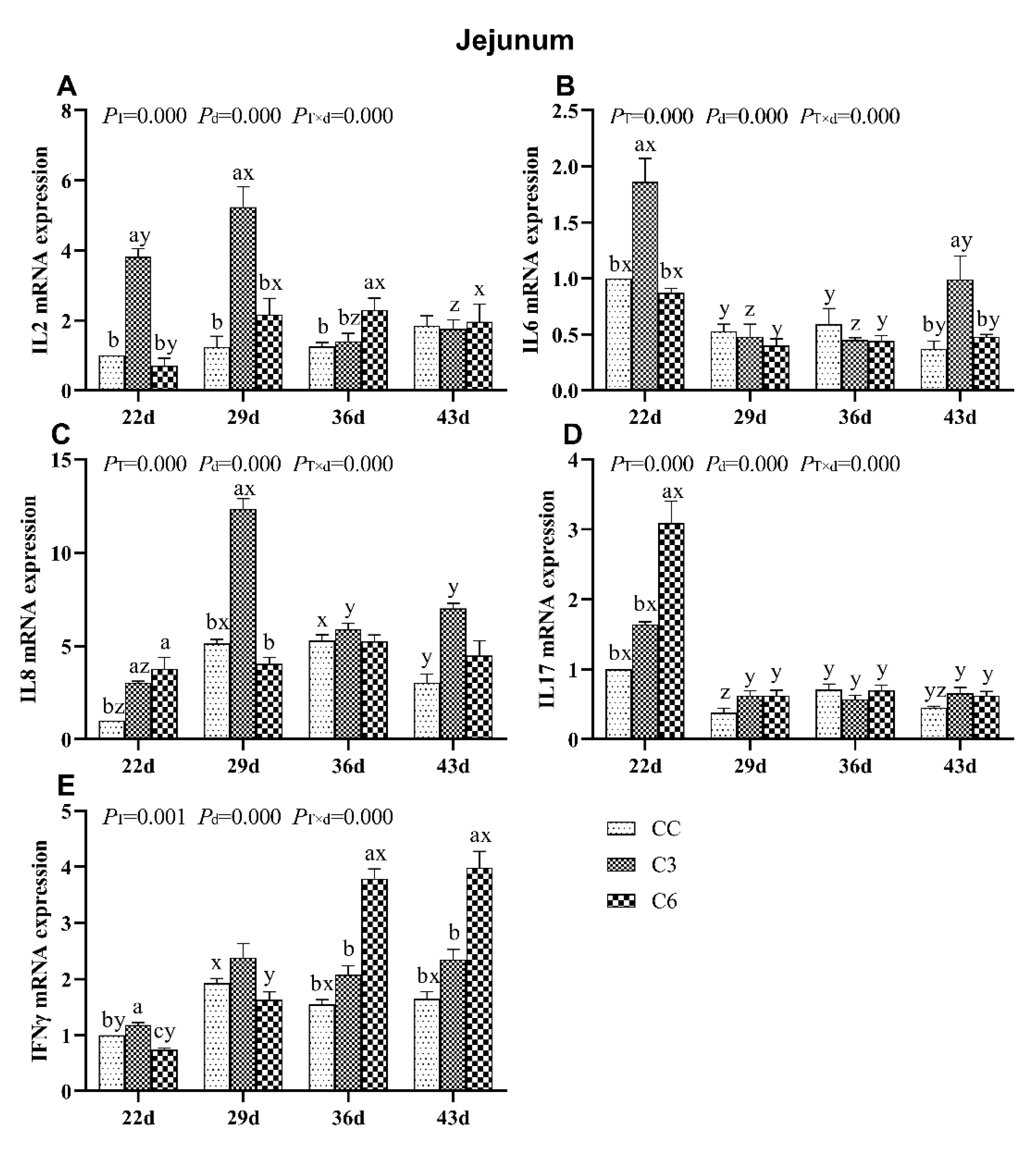
3.3. Changes in mRNA Expression Levels of Cytokines in Jejunum
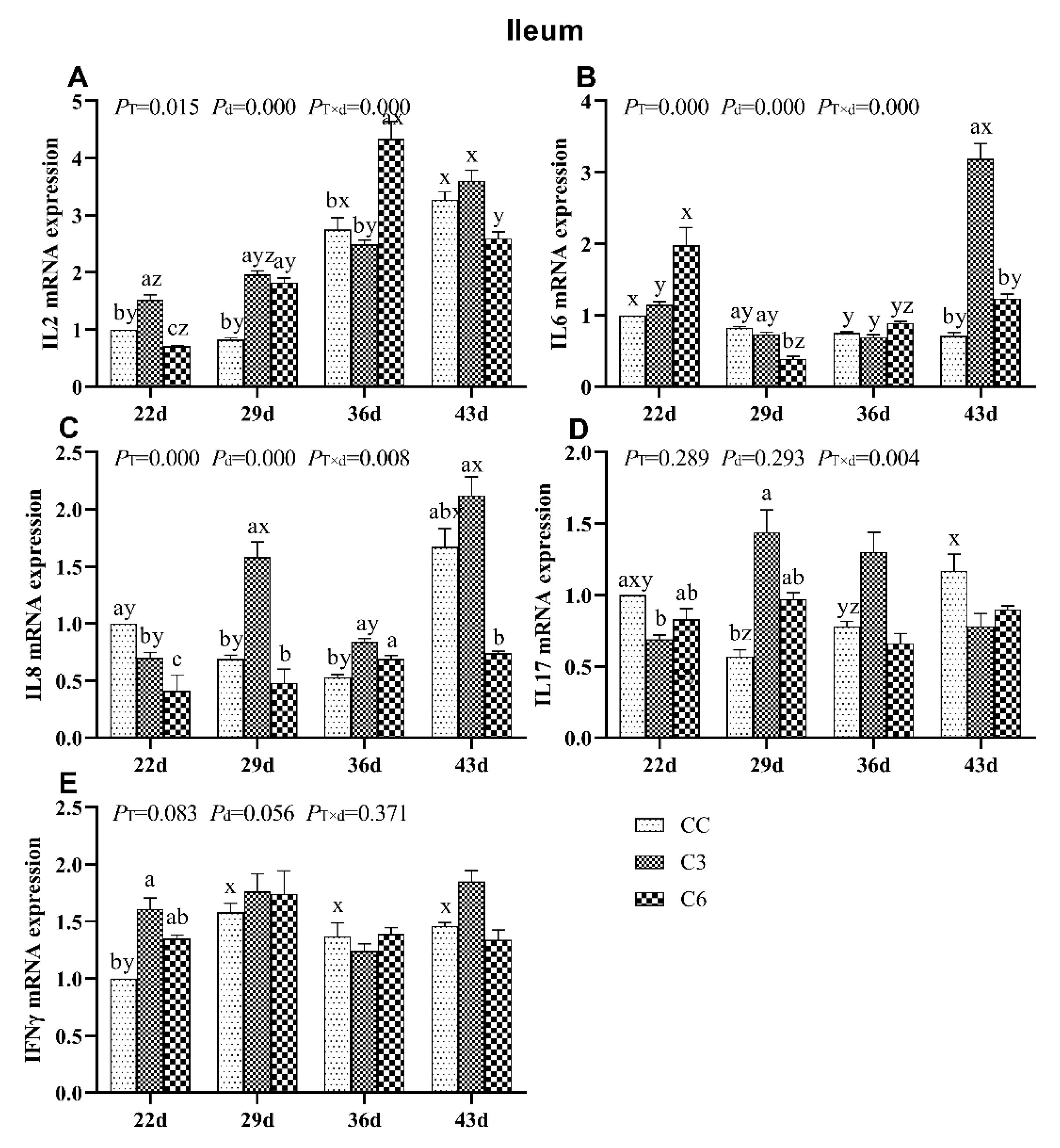
3.4. Changes in mRNA Expression Levels of Cytokines in Ileum
3.5. Changes in mRNA Expression Levels of TLRs in Duodenum
3.6. Changes in mRNA Expression Levels of TLRs in Jejunum
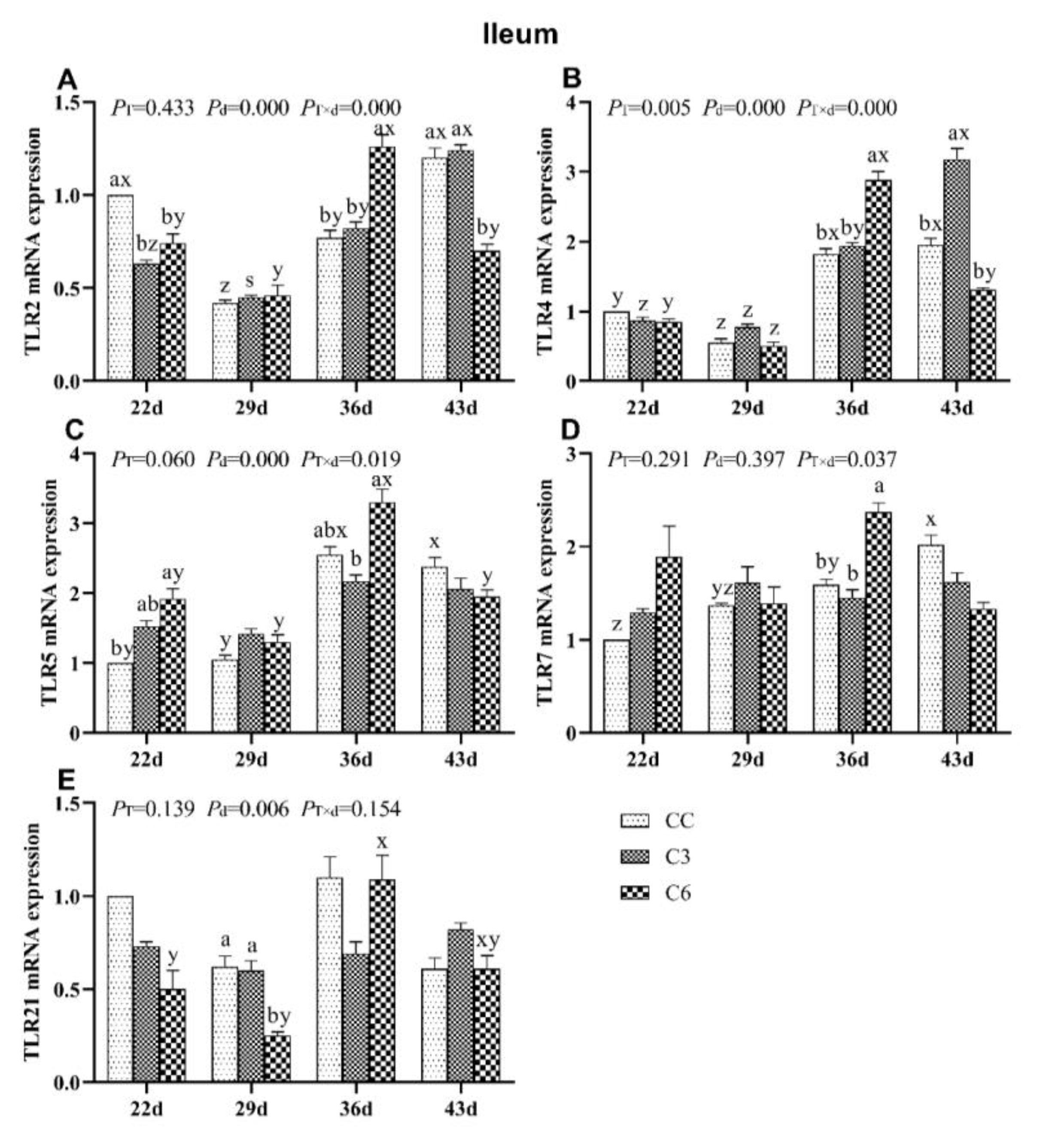
3.7. Changes in mRNA Expression Levels of TLRs in Ileum
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hangalapura, B.N. Cold Stress and Immunity: Do Chickens Adapt to Cold by Trading-Off Immunity for Thermoregulation? Wageningen University and Research Centre: Wageningen, NLD, The Netherlands, 2006. [Google Scholar]
- Jia, Z.; Chen, A.; Wang, C.; He, M.; Xu, J.; Fu, H.; Zhang, X.; Lv, W.; Guo, Z. Amelioration effects of Kaempferol on immune response following chronic intermittent cold-stress. Res. Vet. Sci. 2019, 125, 390–396. [Google Scholar] [CrossRef]
- Liu, Y.; Xue, G.; Li, S.; Fu, Y.; Yin, J.; Zhang, R.; Li, J. Effect of Intermittent and Mild Cold Stimulation on the Immune Function of Bursa in Broilers. Animals 2020, 10, 1275. [Google Scholar] [CrossRef] [PubMed]
- Rintamäki, H. Predisposing factors and prevention of frostbite. Int. J. Circumpolar Health 2000, 59, 114–121. [Google Scholar] [PubMed]
- Yang, Z.; Liu, C.; Zheng, W.; Teng, X.; Li, S. The functions of antioxidants and heat shock proteins are altered in the immune organs of selenium-deficient broiler chickens. Biol. Trace Elem. Res. 2016, 169, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Shinder, D.; Luger, D.; Rusal, M.; Rzepakovsky, V.; Bresler, V.; Yahav, S. Early age cold conditioning in broiler chickens (Gallus domesticus): Thermotolerance and growth responses. J. Therm. Biol. 2002, 27, 517–523. [Google Scholar] [CrossRef]
- Furness, J.B.; Rivera, L.R.; Cho, H.J.; Bravo, D.M.; Callaghan, B. The gut as a sensory organ. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 729–740. [Google Scholar] [CrossRef]
- Fu, J.; Liu, C.P.; Zhang, Z.W.; Xing, M.W.; Xu, S.W. Influence of inflammatory pathway markers on oxidative stress induced by cold stress in intestine of quails. Res. Vet. Sci. 2013, 95, 495–501. [Google Scholar] [CrossRef]
- Kaushik, S.; Kaur, J. Effect of chronic cold stress on intestinal epithelial cell proliferation and inflammation in rats. Stress Int. J. Biol. Stress 2005, 8, 191–197. [Google Scholar] [CrossRef]
- Zhao, F.Q.; Zhang, Z.W.; Yao, H.D.; Wang, L.L.; Liu, T.; Yu, X.Y.; Li, S.; Xu, S.W. Effects of cold stress on mRNA expression of immunoglobulin and cytokine in the small intestine of broilers. Res. Vet. Sci. 2013, 95, 146–155. [Google Scholar] [CrossRef]
- Shira, E.B.; Friedman, A. Innate immune functions of avian intestinal epithelial cells: Response to bacterial stimuli and localization of responding cells in the developing avian digestive tract. PLoS ONE 2018, 13, e0200393. [Google Scholar]
- Perozo, F.; Finol, G.; Mavarez, Y. Levels of immunoglobulin-a in trachea, gut and bile samples of chickens vaccinated against newcastle disease. Rev. Científica 2007, 17, 226–230. [Google Scholar]
- Zhang, X.; Zhang, X.; Yang, Q. Effect of compound mucosal immune adjuvant on mucosal and systemic immune responses in chicken orally vaccinated with attenuated Newcastle-disease vaccine. Vaccine 2007, 25, 3254–3262. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Li, S.; Xin, H.; Li, J.; Li, X.; Zhang, R.; Li, J.; Bao, J. Proper cold stimulation starting at an earlier age can enhance immunity and improve adaptability to cold stress in broilers. Poult. Sci. 2019, 99, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Adachi, M.; Oda, N.; Kokubu, F.; Huang, S.-K. IL-17 cytokine family. J. Allergy Clin. Immunol. 2004, 114, 1265–1273. [Google Scholar] [CrossRef]
- Dugué, B.; Leppanen, E. Adaptation related to cytokines in man: Effects of regular swimming in ice-cold water. Clin. Physiol. 2000, 20, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Rhind, S.G.; Castellani, J.W.; Brenner, I.K.; Shephard, R.J.; Zamecnik, J.; Montain, S.J.; Young, A.J.; Shek, P.N. Intracellular monocyte and serum cytokine expression is modulated by exhausting exercise and cold exposure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 281, R66–R75. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Zhang, X.; Xin, H.; Li, S.; Li, J.; Zhang, R.; Li, X.; Li, J.; Bao, J. Effects of prior cold stimulation on inflammatory and immune regulation in ileum of cold-stressed broilers. Poult. Sci. 2018, 97, 4228–4237. [Google Scholar] [CrossRef]
- Takeda, K.; Akira, S. Toll-like receptors in innate immunity. Int. Immunol. 2005, 17, 1–14. [Google Scholar] [CrossRef]
- Cario, E. Bacterial interactions with cells of the intestinal mucosa: Toll-like receptors and NOD2. Gut 2005, 54, 1182–1193. [Google Scholar] [CrossRef]
- Cosseau, C.; Devine, D.A.; Dullaghan, E.; Gardy, J.L.; Chikatamarla, A.; Gellatly, S.; Lorraine, L.Y.; Pistolic, J.; Falsafi, R.; Tagg, J. The commensal Streptococcus salivarius K12 downregulates the innate immune responses of human epithelial cells and promotes host-microbe homeostasis. Infect. Immun. 2008, 76, 4163–4175. [Google Scholar] [CrossRef] [Green Version]
- Paul, A.; Dangi, S.S.; Gupta, M.; Singh, J.; Thakur, N.; Naskar, S.; Nanda, P.K.; Mohanty, N.; Das, A.K.; Bandopadhayay, S. Expression of TLR genes in Black Bengal goat (Capra hircus) during different seasons. Small Rumin. Res. 2015, 124, 17–23. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, R.; Su, Y.; Bi, Y.; Li, X.; Zhang, X.; Li, J.; Bao, J. Effects of Acute Cold Stress After Long-Term Cold Stimulation on Antioxidant Status, Heat Shock Proteins, Inflammation and Immune Cytokines in Broiler Heart. Front. Physiol. 2018, 9, 1589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Svensson, E.; Råberg, L.; Koch, C.; Hasselquist, D. Energetic stress, immunosuppression and the costs of an antibody response. Funct. Ecol. 1998, 12, 912–919. [Google Scholar] [CrossRef]
- Rybakina, E.G.; Shanin, S.N.; Kozinets, I.A.; Fomicheva, E.E.; Korneva, E.A. Cellular mechanisms of cold stress-related immunosuppression and the action of interleukin 1. Int. J. Tissue React. 1997, 19, 135–140. [Google Scholar]
- Banerjee, S.K.; Aviles, H.; Fox, M.T.; Monroy, F.P. Cold Stress-Induced Modulation of Cell Immunity during Acute Toxoplasma gondii Infection in Mice. J. Parasitol. 1999, 85, 442–447. [Google Scholar] [CrossRef]
- Hangalapura, B.; Nieuwland, M.; de Vries Reilingh, G.; Heetkamp, M.; van den Brand, H.; Kemp, B.; Parmentier, H. Effects of cold stress on immune responses and body weight of chicken lines divergently selected for antibody responses to sheep red blood cells. Poult. Sci. 2003, 82, 1692–1700. [Google Scholar] [CrossRef]
- Hangalapura, B.N.; Nieuwland, M.G.B.; Buyse, J.; Kemp, B.; Parmentier, H.K. Effect of Duration of Cold Stress on Plasma Adrenal and Thyroid Hormone Levels and Immune Responses in Chicken Lines Divergently Selected for Antibody Responses. Poult. Sci. 2006, 83, 1644–1649. [Google Scholar] [CrossRef]
- Hangalapura, B.N.; Nieuwland, M.G.B.; de Vries Reilingh, G.; van den Brand, H.; Kemp, B.; Parmentier, H.K. Durations of cold stress modulates overall immunity of chicken lines divergently selected for antibody responses. Poult. Sci. 2004, 83, 765–775. [Google Scholar] [CrossRef]
- Su, Y.; Wei, H.; Bi, Y.; Wang, Y.; Zhao, P.; Zhang, R.; Li, X.; Li, J.; Bao, J. Pre-cold acclimation improves the immune function of trachea and resistance to cold stress in broilers. J. Cell. Physiol. 2019, 234, 7198–7212. [Google Scholar] [CrossRef]
- Woof, J.M.; Kerr, M.A. The function of immunoglobulin A in immunity. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2006, 208, 270–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyna-Garfias, H.; Miliar, A.; Jarillo-Luna, A.; Rivera-Aguilar, V.; Pacheco-Yepez, J.; Baeza, I.; Campos-Rodríguez, R. Repeated restraint stress increases IgA concentration in rat small intestine. Brain Behav. Immun. 2010, 24, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Carr, D.J.J.; Woolley, T.W.; Blalock, J.E. Phentolamine but not propranolol blocks the immunopotentiating effect of cold stress on antigen-specific IgM production in mice orally immunized with sheep red blood cells. Brain Behav. Immun. 1992, 6, 50–63. [Google Scholar] [CrossRef]
- Diehl, S.; Rincón, M. The two faces of IL-6 on Th1/Th2 differentiation. Mol. Immunol. 2002, 39, 531–536. [Google Scholar] [CrossRef]
- McAdam, A.J.; Pulaski, B.A.; Harkins, S.S.; Hutter, E.K.; Lord, E.M.; Frelinger, J.G. Synergistic effects of co-expression of the Th1 cytokines il-2 and IFNγ on generation of murine tumor-reactive cytotoxic cells. Int. J. Cancer 1995, 61, 628–634. [Google Scholar] [CrossRef]
- Ito, K.; Hanazawa, T.; Tomita, K.; Barnes, P.J.; Adcock, I.M. Oxidative stress reduces histone deacetylase 2 activity and enhances IL-8 gene expression: Role of tyrosine nitration. Biochem. Biophys. Res. Commun. 2004, 315, 240–245. [Google Scholar] [CrossRef]
- Hangalapura, B.N.; Kaiser, M.G.; van der Poel, J.J.; Parmentier, H.K.; Lamont, S.J. Cold stress equally enhances in vivo pro-inflammatory cytokine gene expression in chicken lines divergently selected for antibody responses. Dev. Comp. Immunol. 2006, 30, 503–511. [Google Scholar] [CrossRef]
- Brenner, I.; Castellani, J.; Gabaree, C.; Young, A.; Zamecnik, J.; Shephard, R.; Shek, P. Immune changes in humans during cold exposure: Effects of prior heating and exercise. J. Appl. Physiol. 1999, 87, 699–710. [Google Scholar] [CrossRef]
- Castellani, J.W.; Brenner, I.K.; Rhind, S.G. Cold exposure: Human immune responses and intracellular cytokine expression. Med. Sci. Sports Exerc. 2002, 34, 2013–2020. [Google Scholar] [CrossRef]
- Luo, B.; Shi, H.; Wang, L.; Shi, Y.; Wang, C.; Yang, J.; Wan, Y.; Niu, J. Rat Lung Response to PM2.5 Exposure under Different Cold Stresses. Int. J. Environ. Res. Public Health 2014, 11, 12915–12926. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Genovese, K.J.; Swaggerty, C.L.; MacKinnon, K.M.; Kogut, M.H. Co-stimulation with TLR3 and TLR21 ligands synergistically up-regulates Th1-cytokine IFN-γ and regulatory cytokine IL-10 expression in chicken monocytes. Dev. Comp. Immunol. 2012, 36, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Arslan, F.; de Kleijn, D.P.; Pasterkamp, G. Innate immune signaling in cardiac ischemia. Nat. Rev. Cardiol. 2015, 8, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Napper, S.; Dadgar, S.; Arsenault, R.J.; Trost, B.; Scruten, E.; Kusalik, A.; Shand, P. Induction of tissue- and stressor-specific kinomic responses in chickens exposed to hot and cold stresses. Poult. Sci. 2015, 94, 1333–1345. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.B.; Mark, M.R.; Gray, A.; Huang, A.; Godowski, P.J. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature 1998, 395, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Okla, M.; Wang, W.; Kang, I.; Pashaj, A.; Carr, T.; Chung, S. Activation of Toll-like Receptor 4 (TLR4) Attenuates Adaptive Thermogenesis via Endoplasmic Reticulum Stress. J. Biol. Chem. 2015, 290, 26476–26490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gewirtz, A.T.; Navas, T.A.; Lyons, S.; Godowski, P.J.; Madara, J.L. Cutting Edge: Bacterial Flagellin Activates Basolaterally Expressed TLR5 to Induce Epithelial Proinflammatory Gene Expression. J. Immunol. 2001, 167, 1882–1885. [Google Scholar] [CrossRef] [Green Version]
- Blasius, A.L.; Beutler, B. Intracellular Toll-like Receptors. Immunity 2010, 32, 305–315. [Google Scholar] [CrossRef] [Green Version]
- Keestra, A.M.; de Zoete, M.R.; Bouwman, L.I.; van Putten, J.P.M. Chicken TLR21 Is an Innate CpG DNA Receptor Distinct from Mammalian TLR9. J. Immunol. 2010, 185, 460–467. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Li, W.; Zheng, X.-H.; Rong, X.; Huang, L.-G. Glutamine downregulates TLR-2 and TLR-4 expression and protects intestinal tract in preterm neonatal rats with necrotizing enterocolitis. J. Pediatr. Surg. 2014, 49, 1057–1063. [Google Scholar] [CrossRef]
- Basu, M.; Paichha, M.; Swain, B.; Lenka, S.S.; Singh, S.; Chakrabarti, R.; Samanta, M. Modulation of TLR2, TLR4, TLR5, NOD1 and NOD2 receptor gene expressions and their downstream signaling molecules following thermal stress in the Indian major carp catla (Catla catla). 3 Biotech 2015, 5, 1021–1030. [Google Scholar] [CrossRef] [Green Version]



| Gene | Reference Sequence | Primer Sequences (5′–3′) |
|---|---|---|
| β-actin | NM_205518.1 | F: CACCACAGCCGAGAGAGAAAT |
| R: TGACCATCAGGGAGTTCATAGC | ||
| IgA | NM_205287.1 | F: TGCTAGTGGTTGTGGTGCTTGTG |
| R: CGGAGGCGGAGGAGACGATG | ||
| IgG | XM_025146241.1 | F: CGATTCCAGCCTCAGCGTCAC |
| R: TAGGTGCCGTTGAAGTGTTCTTGG | ||
| IFNγ | NM_205149.1 | F: GAACTGGACAGGGAGAAATGAGA |
| R: ACGCCATCAGGAAGGTTGTT | ||
| IL2 | NM_204153.1 | F: CTGTATTTCGGTAGCAATG |
| R: ACTCCTGGGTCTCAGTTG | ||
| IL6 | NM_204628.1 | F: AAATCCCTCCTCGCCAATCT |
| R: CCCTCACGGTCTTCTCCATAAA | ||
| IL8 | NM_205018.1 | F: GGCTTGCTAGGGGAAATGA |
| R: AGCTGACTCTGACTAGGAAACTGT | ||
| IL17 | NM_204460.1 | F: GCCATTCCAGGTGCGTGAACTC |
| R: CGGCGGAGGACGAGGATCTC | ||
| TLR2 | XM_001232192294a | F: GATTGTGGACAACATCATTGACTC |
| R: AGAGCTGCTTTCAAGTTTTCCC | ||
| TLR4 | NM_001030693.1190a | F: AGTCTGAAATTGCTGAGCTCAAAT |
| R: GCGACGTTAAGCCATGGAAG | ||
| TLR5 | NM_001024586124a | F: CCTTGTGCTTTGAGGAACGAGA |
| R: CACCCATCTTTGAGAAACTGCC | ||
| TLR7 | NM_001011688219b | F: TTCTGGCCACAGATGTGACC |
| R: CCTTCAACTTGGCAGTGCAG | ||
| TLR21 | NM_001030558112a | F: TGCCCCTCCCACTGCTGTCCACT |
| R: AAAGGTGCCTTGACATCCT |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Li, J.; Liu, Y.; Li, C.; Zhang, R.; Bao, J. Effects of Intermittent Mild Cold Stimulation on mRNA Expression of Immunoglobulins, Cytokines, and Toll-Like Receptors in the Small Intestine of Broilers. Animals 2020, 10, 1492. https://doi.org/10.3390/ani10091492
Li S, Li J, Liu Y, Li C, Zhang R, Bao J. Effects of Intermittent Mild Cold Stimulation on mRNA Expression of Immunoglobulins, Cytokines, and Toll-Like Receptors in the Small Intestine of Broilers. Animals. 2020; 10(9):1492. https://doi.org/10.3390/ani10091492
Chicago/Turabian StyleLi, Shuang, Jianhong Li, Yanhong Liu, Chun Li, Runxiang Zhang, and Jun Bao. 2020. "Effects of Intermittent Mild Cold Stimulation on mRNA Expression of Immunoglobulins, Cytokines, and Toll-Like Receptors in the Small Intestine of Broilers" Animals 10, no. 9: 1492. https://doi.org/10.3390/ani10091492





