Distribution Change of Invasive American Bullfrogs (Lithobates catesbeianus) by Future Climate Threaten Endangered Suweon Treefrog (Hyla suweonensis) in South Korea
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collecting Location Data
2.2. Predicting Present and Future Distribution
3. Results
3.1. Potential Distribution of Lithobates catesbeianus
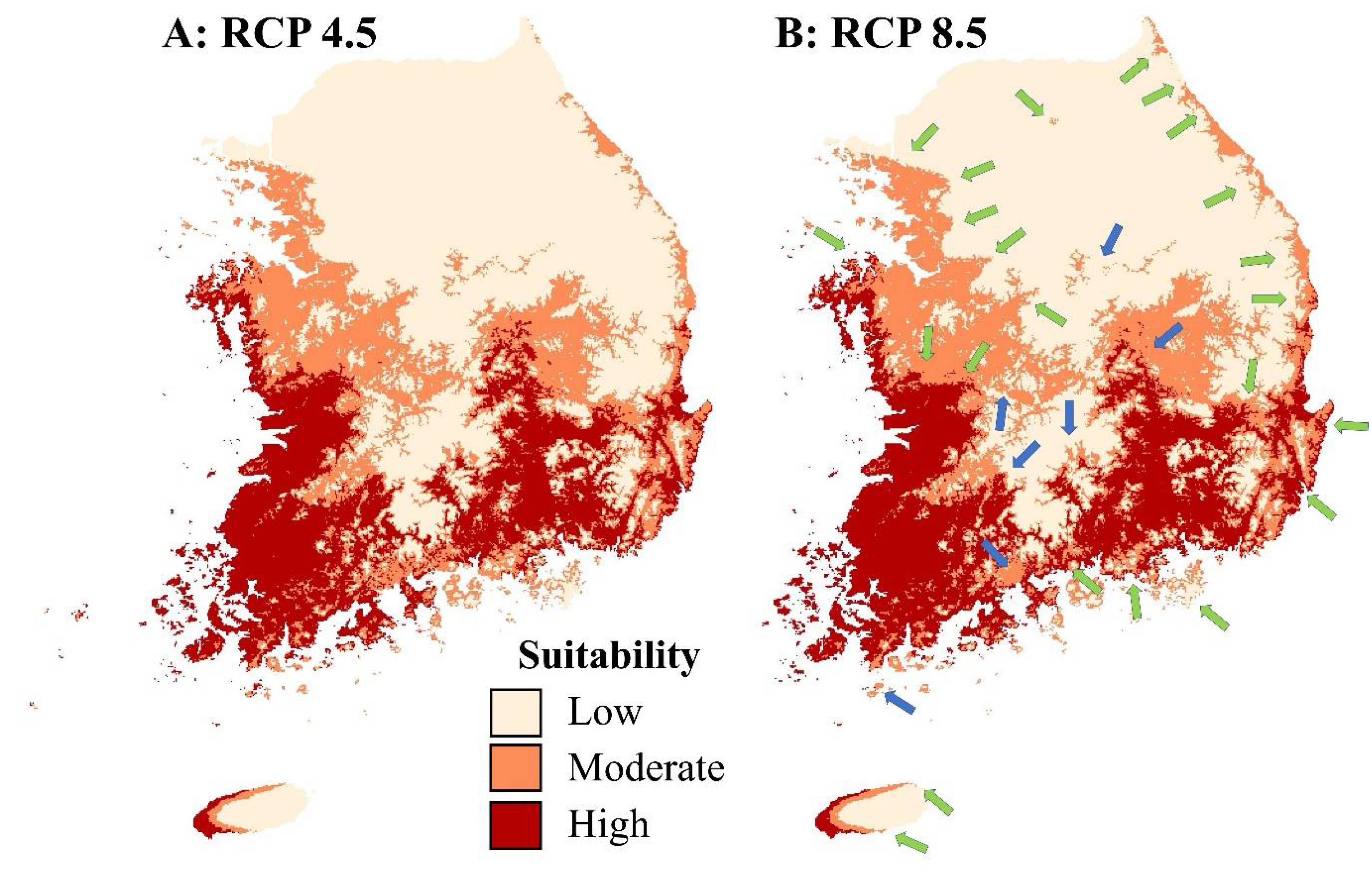
3.2. Future Distribution of Lithobates catesbeianus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shanmuganathan, T.; Pallister, J.; Doody, S.; McCallum, H.; Robinson, T.; Sheppard, A.; Hardy, C.; Halliday, D.; Venables, D.; Voysey, R.; et al. Biological Control of the Cane Toad in Australia: A review. Anim. Conserv. 2010, 13, 16–23. [Google Scholar] [CrossRef]
- Kim, H.K. The Bullfrog (Rana catesbeiana) and its Culture. J. Korean Res. Inst. Bett. Liv. Ewha 1973, 10, 173–200. [Google Scholar]
- Cadi, A.; Delmas, V.; Prévot-Julliard, A.C.; Joly, P.; Pieau, C.; Girondot, M. Successful Reproduction of the Introduced Slider Turtle (Trachemys scripta elegans) in the South of France. Aquat. Conserv. 2004, 14, 237–246. [Google Scholar] [CrossRef]
- Koo, K.S.; Park, H.R.; Choi, J.H.; Sung, H.C. Present Status of Non-Native Amphibians and Reptiles Traded in Korean Online Pet Shop. Korean Jour. Environ. Ecol. 2020, 34, 106–114. [Google Scholar] [CrossRef]
- Oh, H.S.; Hong, C.E. Current Conditions of Habitat for Rana catesbeiana and Trachemys scripta elegans Imported to Jeju-do, Including Proposed Management Plans. Korean J. Environ. Ecol. 2007, 21, 311–317. [Google Scholar]
- Pujol-Buxó, E.; Riaño, G.M.; Llorente, G.A. Mild Segregation in the Breeding Preferences of an Invasive Anuran (Discoglossus pictus) and its Main Native Competitor (Epidalea calamita) in Ephemeral Ponds. Amphib. Reptil. 2019, 1, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Groffen, J.; Kong, S.; Jang, Y.; Borzée, A. The Invasive American Bullfrog (Lithobates catesbeianus) in the Republic of Korea: History and Recommendations for population control. Manag. Biol. Invasion. 2019, 10, 517–535. [Google Scholar] [CrossRef] [Green Version]
- Schlaepfer, M.A.; Sax, D.F.; Olden, J.D. The Potential Conservation Value of Non-Native Species. Conserv. Biol. 2011, 25, 428–437. [Google Scholar] [CrossRef]
- Beard, K.H.; O’Neill, E.M. Infection of an Invasive Frog Eleutherodactylus coqui by the Chytrid Fungus Batrachochytrium dendrobatidis in Hawaii. Biol. Conserv. 2005, 126, 591–595. [Google Scholar] [CrossRef] [Green Version]
- Borzée, A.; Kosch, T.A.; Kim, M.; Jang, Y. Introduced Bullfrogs are Associated with Increased Batrachochytrium dendrobatidis Prevalence and Reduced Occurrence of Korean Treefrogs. PLoS ONE 2017, 12, e0190551. [Google Scholar] [CrossRef]
- Hoffmann, B.D.; Broadhurst, L.M. The Economic Cost of Managing Invasive Species in Australia. NeoBiota 2016, 31, 1. [Google Scholar] [CrossRef] [Green Version]
- Gozlan, R.E. Interference of Non-Native Species with Fisheries and Aquaculture. Impact Biol. Invasions Ecosy. Serv. 2017, 12, 119–137. [Google Scholar] [CrossRef]
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species: A Selection From The Global Invasive Species Database; Invasive Species Specialist Group: Auckland, New Zealand, 2000. [Google Scholar]
- IUCN. Lithobates catesbeianus. In The IUCN Red List of Threatened Species. 2015. Available online: http://dx.doi.org/10.2305/IUCN.UK.2015-4.RLTS.T58565A53969770.en (accessed on 23 September 2021).
- Schroeder, E.E.; Baskett, T.S. Age estimation, growth rates, and population structure in Missouri bullfrogs. Copeia 1968, 583–592. [Google Scholar] [CrossRef]
- Xuan, L.; Yiming, L.; McGarrity, M. Geographical Variation in Body Size and Sexual Size Dimorphism of Introduced American Bullfrogs in Southwestern China. Biol. Invasions. 2010, 12, 2037–2047. [Google Scholar] [CrossRef]
- Kang, H.J.; Koo, K.S.; Sung, H.C. Current Distribution of American Bullfrog Rana catesbeiana Shaw, 1802 in the Republic of Korea. BioInvasions. Rec. 2019, 8, 942–946. [Google Scholar] [CrossRef]
- Schwalbe, C.R.; Rosen, P.C. Preliminary Report on Effect of Bullfrogs in Wetland Herpetofaunas in Southeastern Arizona. General Technical Report-US Department of Agriculture, Forest Service, (RM-166). 1988, pp. 166–173. Available online: https://arizona.pure.elsevier.com/en/publications/preliminary-report-on-effect-of-bullfrogs-in-wetland-herpetofauna (accessed on 23 September 2021).
- Lee, H.J. Distribution and Characteristics of Reeve’s Turtle (Chinemys reevesii) Populations in Jeolla-do and Gyeongsangnam-do. Master’s Thesis, Kangwon National University, Chuncheon-si, South Korea, 2010; p. 48.
- Park, C.D.; Lee, C.W.; Lim, J.C.; Yang, B.G.; Lee, J.H. A Study on the Diet Items of American Bullfrog (Lithobates catesbeianus) in Gahang Wetland, Korea. Korean Jour. Environ. Ecol. 2018, 32, 55–65. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Thuiller, W.; Miaud, C. Prediction and Validation of the Potential Global Distribution of a Problematic Alien Invasive Species—The American Bullfrog. Divers. Distrib. 2007, 13, 476–485. [Google Scholar] [CrossRef]
- Giovanelli, J.G.; Haddad, C.F.; Alexandrino, J. Predicting the Potential Distribution of the Alien Invasive American Bullfrog (Lithobates catesbeianus) in Brazil. Biol. Invasions. 2008, 10, 585–590. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Maiorano, L.; Falcucci, A.; Dendoncker, N.; Boitani, L.; Padoa-Schioppa, E.; Miaud, C.; Thuiller, W. Knowing the Past to Predict the Future: Land-Use Change and the Distribution of Invasive Bullfrogs. Glob. Chang. Biol. 2010, 16, 528–537. [Google Scholar] [CrossRef]
- Iñiguez, C.A.; Morejón, F.J. Potential Distribution of the American Bullfrog (Lithobates catesbeianus) in Ecuador. South. Am. J. Herpetol. 2012, 7, 85–90. [Google Scholar] [CrossRef]
- Yap, T.A.; Koo, M.S.; Ambrose, R.F.; Vredenburg, V.T. Introduced Bullfrog Facilitates Pathogen Invasion in the Western United States. PLoS ONE 2018, 13, e0188384. [Google Scholar] [CrossRef]
- Swei, A.; Rowley, J.J.; Rödder, D.; Diesmos, M.L.; Diesmos, A.C.; Briggs, C.J.; Brown, R.; Cao, T.T.; Cheon, T.L.; Chong, R.A.; et al. Is Chytridiomycosis an Emerging Infectious Disease in Asia? PLoS ONE 2011, 6, e23179. [Google Scholar] [CrossRef] [Green Version]
- Kuramoto, M. Mating Calls of Treefrogs (genus Hyla) in the Far East, with Description of a New Species from Korea. Copeia 1980, 100–108. [Google Scholar] [CrossRef]
- Hua, X.; Fu, C.; Li, J.; DeOca, A.N.M.; Wiens, J.J. A Revised Phylogeny of Holarctic Treefrogs (genus Hyla) based on Nuclear and Mitochondrial DNA Sequences. Herpetologica 2009, 65, 246–259. [Google Scholar] [CrossRef]
- Borzée, A.; Jang, Y. Description of a Seminatural Habitat of the Endangered Suweon Treefrog, Hyla suweonensis. Anim. Cells. Syst. 2015, 19, 1–5. [Google Scholar] [CrossRef]
- Borzée, A.; Choi, Y.; Kim, Y.E.; Jablonski, P.; Jang, Y. Interspecific Variation in Seasonal Migration and Brumation Behaviour in Two Closely Related Species of Treefrogs. Front. Ecol. Evol. 2019, 7, 55. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.D.; Miller-Rushing, A.J. Degradation, Urbanization, and Restoration: A Review of the Challenges and Future of Conservation on the Korean Peninsula. Biol Cons. 2014, 176, 262–276. [Google Scholar] [CrossRef]
- Borzée, A. Why are Anurans Threatened? The case of Dryophytes suweonensis. Ph.D. Thesis, Seoul National University, Seoul, Korea, December 2018; p. 489. [Google Scholar]
- NIE. Guidelines for the 4th National Natural Environment Survey. In Ministry of Environment; Northeast Asian Plant Research Institute: Seocheon, Korea, 2012; p. 486. [Google Scholar]
- QGIS. QGIS Geographic Information System ver 3.12.2. Open Source Geospatial Foundation Project. 2020. Available online: http://qgis.osgeo.org (accessed on 24 September 2020).
- Borzée, A.; Kim, K.; Heo, K.; Jablonski, P.G.; Jang, Y. Impact of Land Reclamation and Agricultural Water Regime on the Distribution and Conservation Status of the Endangered Dryophytes suweonensis. PeerJ 2017, 5. [Google Scholar] [CrossRef] [Green Version]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L. WorldClim; Version 1.3.; University of California: Berkeley, CA, USA, 2005. [Google Scholar]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very High Resolution Interpolated Climate Surfaces for Global Land Areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Salas, E.A.L.; Seamster, V.A.; Harings, N.M.; Boykin, K.G.; Alvarez, G.; Dixon, K.W. Projected Future Bioclimate-envelope Suitability for Reptile and Amphibian Species of Concern in South Central USA. Herpetol. Conserv. Biol. 2017, 12, 522–547. [Google Scholar]
- Koo, K.S.; Park, D.; Oh, H.S. Analyzing Habitat Characteristics and Predicting Present and Future Suitable Habitats of Sibynophis chinensis based on a Climate Change Scenario. J. Asia Pac. Biodivers. 2019, 12, 1–6. [Google Scholar] [CrossRef]
- Yi, Y.J.; Cheng, X.; Yang, Z.F.; Zhang, S.H. Maxent Modeling for Predicting the Potential Distribution of Endangered Medicinal Plant (H. riparia Lour) in Yunnan, China. Ecol. Eng. 2016, 92, 260–269. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; p. 1535. [Google Scholar]
- Philips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Hanley, J.A.; McNeil, B.J. The Meaning and Use of the Area Under a Receiver Operating Characteristic (ROC) Curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nori, J.; Urbina-Cardona, J.N.; Loyola, R.D.; Lescano, J.N.; Leynaud, G.C. Climate Change and American Bullfrog Invasion: What Could We Expect in South America? PLoS ONE 2011, 6, e025718. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Bioclimate Variables | Mean | Min | Max | |
|---|---|---|---|---|
| Bio03 | Isothermality (BIO2/BIO7) (×100) | 27.8 | 22.0 | 33.0 |
| Bio05 | Max Temperature of Warmest Month (°C) | 30.0 | 27.0 | 31.0 |
| Bio06 | Min Temperature of Coldest Month (°C) | −4.8 | −11.1 | 2.5 |
| Bio12 | Annual Precipitation (mm) | 1263.6 | 1003.0 | 1637.0 |
| Bio13 | Precipitation of Wettest Month (mm) | 256.5 | 172.0 | 416.0 |
| Bioclimate Variables | Contribution for Modeling (%) | ||
|---|---|---|---|
| Present | Future | ||
| RCP 4.5 | RCP 8.5 | ||
| Bio03 | 1.5 | 2.7 | 1.3 |
| Bio05 | 33.4 | 31.1 | 27.6 |
| Bio06 | 57.4 | 56.0 | 63.3 |
| Bio12 | 3.4 | 3.2 | 2.2 |
| Bio13 | 4.3 | 7.0 | 5.7 |
| Potential Distribution | Number of Overlap Points | |
|---|---|---|
| RCP 4.5 | RCP 8.5 | |
| Present | 78/122 (63.9%) | |
| Future | 79/122 (64.8%) | 88/122 (72.1%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koo, K.S.; Choe, M. Distribution Change of Invasive American Bullfrogs (Lithobates catesbeianus) by Future Climate Threaten Endangered Suweon Treefrog (Hyla suweonensis) in South Korea. Animals 2021, 11, 2865. https://doi.org/10.3390/ani11102865
Koo KS, Choe M. Distribution Change of Invasive American Bullfrogs (Lithobates catesbeianus) by Future Climate Threaten Endangered Suweon Treefrog (Hyla suweonensis) in South Korea. Animals. 2021; 11(10):2865. https://doi.org/10.3390/ani11102865
Chicago/Turabian StyleKoo, Kyo Soung, and Minjee Choe. 2021. "Distribution Change of Invasive American Bullfrogs (Lithobates catesbeianus) by Future Climate Threaten Endangered Suweon Treefrog (Hyla suweonensis) in South Korea" Animals 11, no. 10: 2865. https://doi.org/10.3390/ani11102865






