Muscle Cortisol Levels, Expression of Glucocorticoid Receptor and Oxidative Stress Markers in the Teleost Fish Argyrosomus regius Exposed to Transport Stress
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Travel Condition from Fish Hatchery and Sampling
2.2. Cortisol Measurement
2.3. RNA Extraction and Real-Time PCR
2.4. Immunohistochemistry (IHC)
2.5. Statistical Analysis
3. Results
3.1. Cortisol Measurement
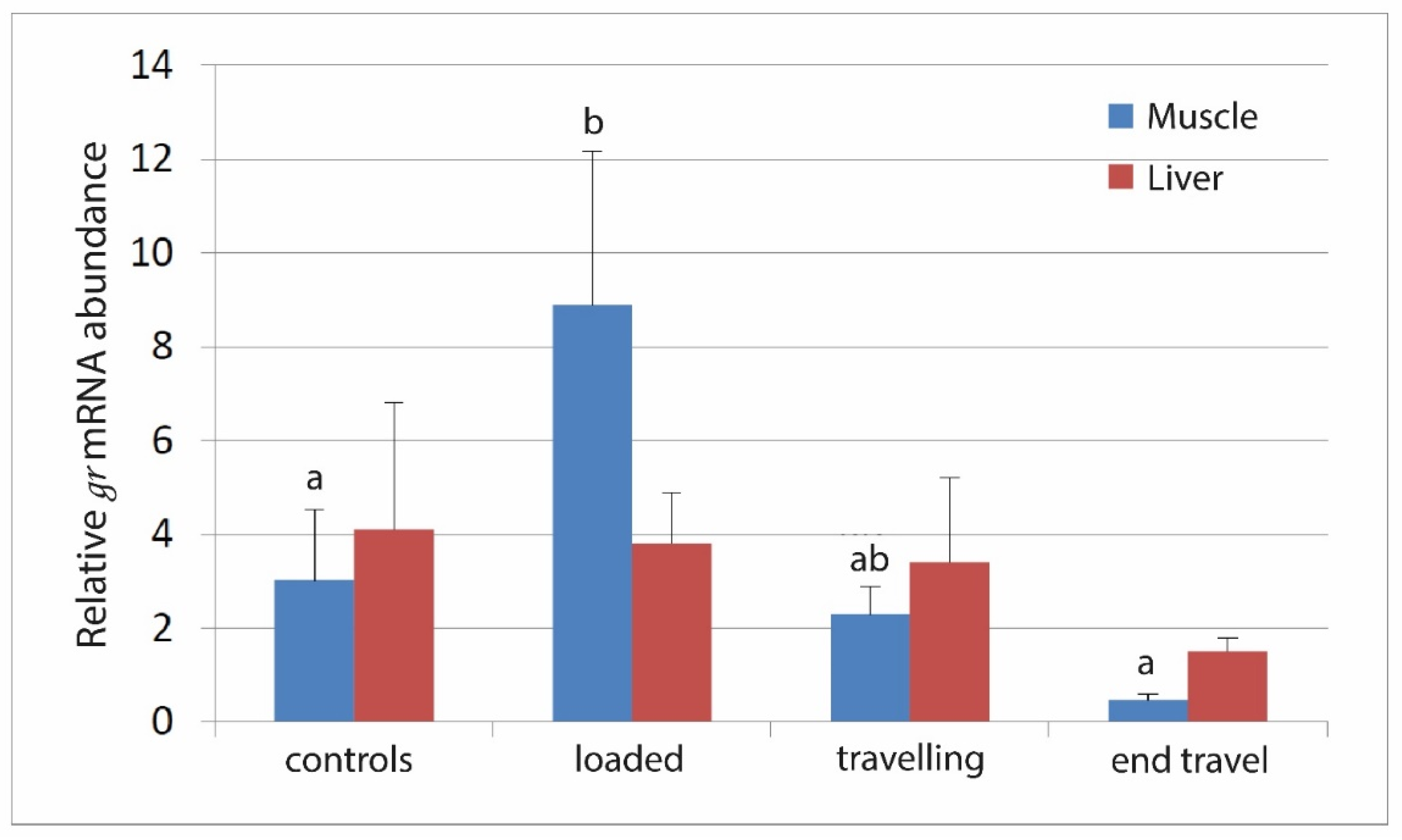
3.2. RNA Expression
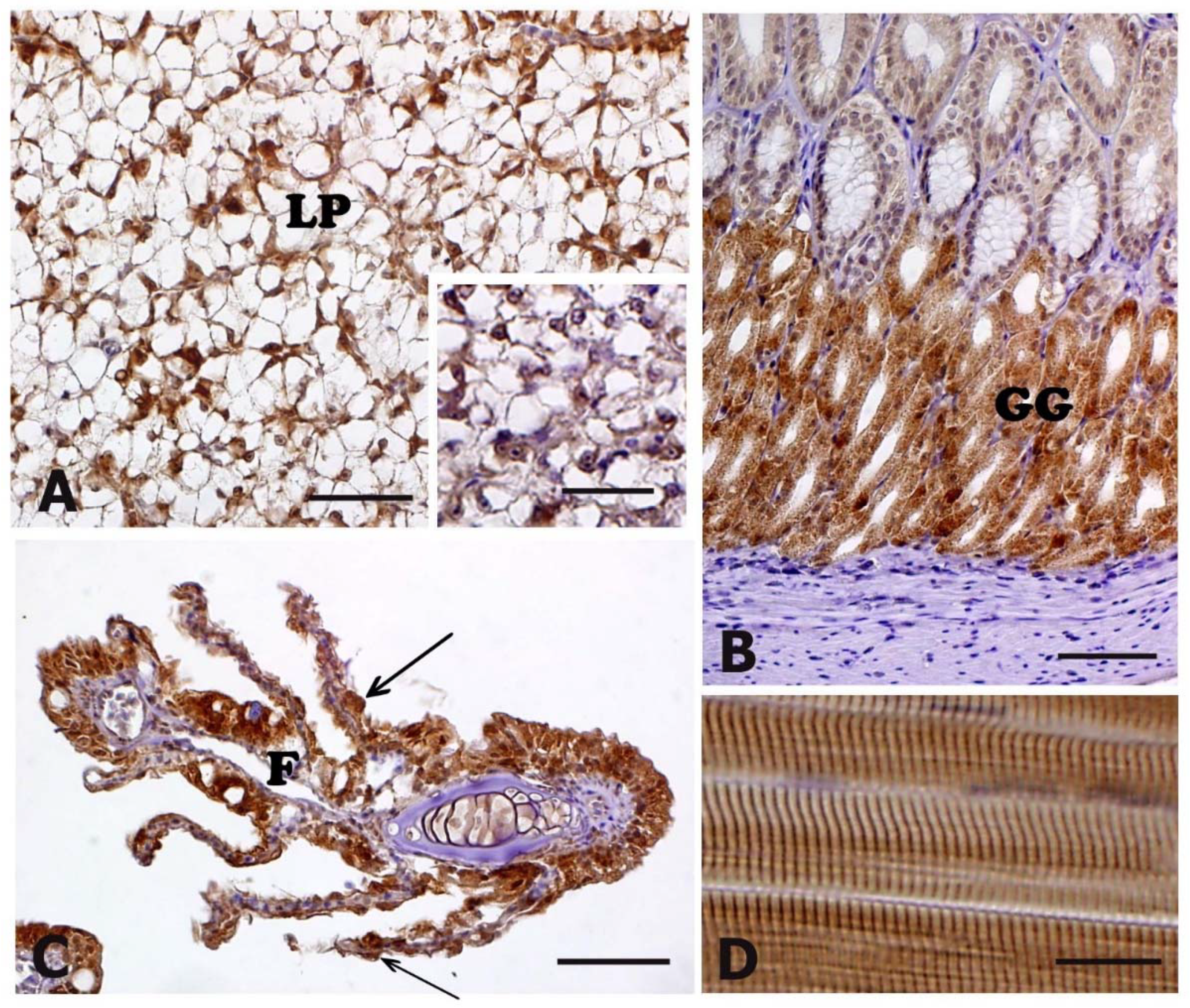
3.3. HSP70 Immunohistochemistry
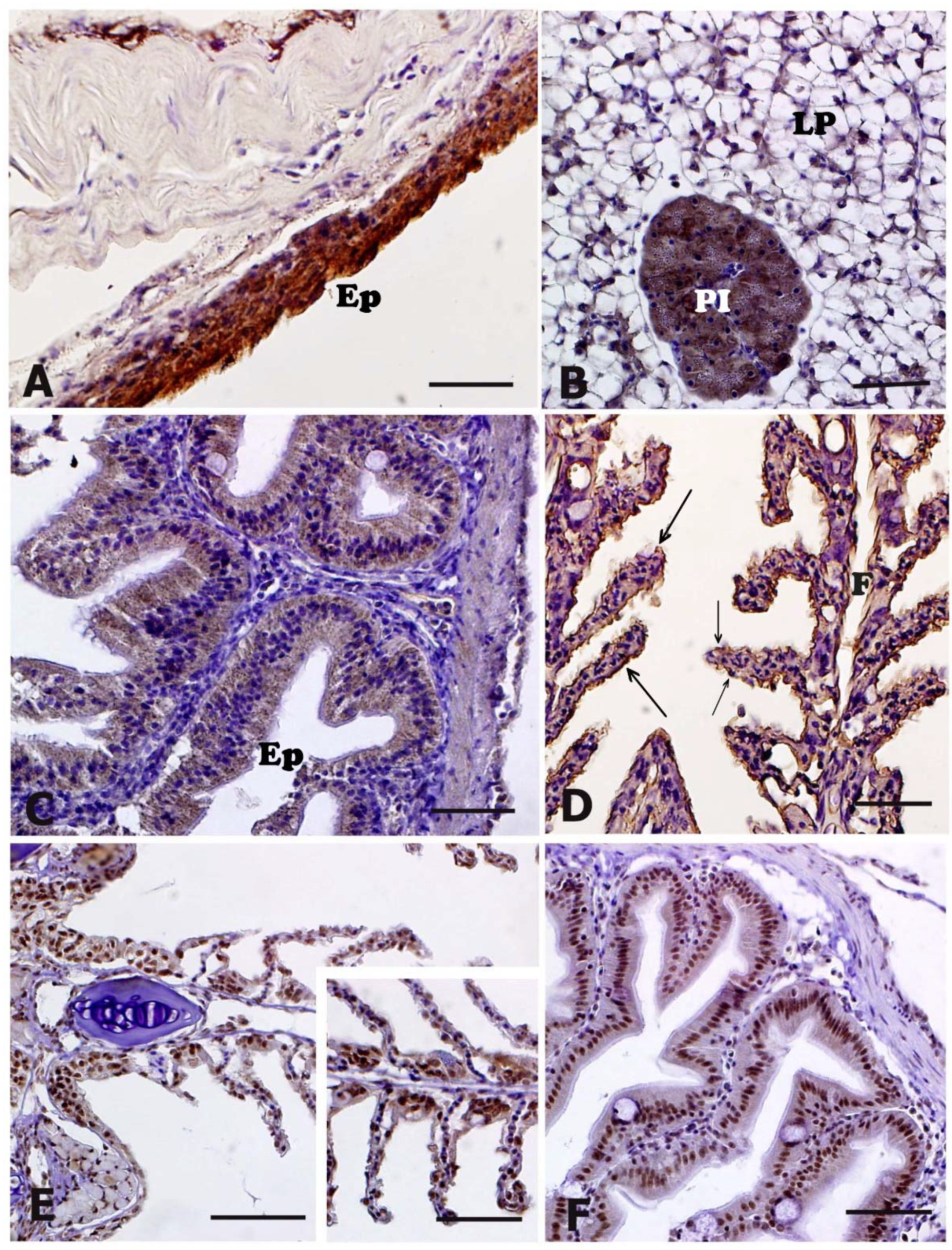
3.4. HNE Immunohistochemistry
3.5. NT and 8-OHdG Immunohistochemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spezzani, C.; Ruffo, G.; Fabris, A.; Mordenti, O.; Manfrin, A.; Salati, F.; Giorietto, F.; Salogni, C. Manuale per la Gestione del Controllo del Benessere dei Pesci Durante il Trasporto su Strada; Italian Ministry of Health: Rome, Italy, 2018; pp. 1–59.
- EFSA (European Food Safety Authority). Opinion of the scientific panel for animal health and welfare on a request from the commission related to the welfare of animals during transport. EFSA J. 2004, 2, 1–36. [Google Scholar] [CrossRef] [Green Version]
- OIE (World Health Animal Organization). Welfare of Farmed Fish during Transport. OIE Aquatic Animal Health Code, 2nd ed.; World organization for animal health: Paris, France, 2015; pp. 1–4. [Google Scholar]
- Kino, T.; Chrousos, G.P. Glucocorticoid and mineralocorticoid receptors and associated diseases. Essays Biochem. 2004, 40, 137–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palstra, A.P.; Mendez, S.; Dirks, R.P.; Schaaf, M.J.M. Cortisol Acting Through the Glucocorticoid Receptor Is Not Involved in Exercise-Enhanced Growth, But Does Affect the White Skeletal Muscle Transcriptome in Zebrafish (Danio rerio). Front. Physiol. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadoel, B.; Vijayan, M.M. Stress and growth. In Biology of Stress in Fish, 2nd ed.; Schreck, C.B., Tort, L., Farrell, A.P., Brauner, C.J., Eds.; Elsevier: London, UK, 2016; Volume 35, pp. 167–205. [Google Scholar] [CrossRef]
- Kuo, T.; Harris, C.A.; Wang, J.C. Metabolic functions of glucocorticoid receptor in skeletal muscle. Mol. Cell. Endocrinol. 2013, 380, 79–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benítez-Dorta, V.; Caballero, M.J.; Izquierdo, M.; Manchado, M.; Infante, C.; Zamorano, M.J.; Montero, D. Total substitution of fish oil by vegetable oils in Senegalese sole (Solea senegalensis) diets: Effects on fish performance, biochemical composition, and expression of some glucocorticoid receptor-related genes. Fish. Physiol. Biochem. 2013, 39, 335–349. [Google Scholar] [CrossRef]
- Mommsen, T.P.; Vijayan, M.M.; Moon, T.W. Cortisol in teleosts: Dynamics, mechanisms of action, and metabolic regulation. Rev. Fish Biol. Fish. 1999, 9, 211–268. [Google Scholar] [CrossRef]
- Schreck, C.B. Stress and compensation in teleostean fishes: Response to social and physical factors. In Stress and Fish; Pickering, A.D., Ed.; Academic Press: London, UK, 1981; pp. 295–321. [Google Scholar]
- Wendelaar Bonga, S.E. The stress response in fish. Physiol. Rev. 1997, 77, 591–625. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 3rd ed.; Oxford University Press: Oxford, UK, 1999; pp. 1–905. [Google Scholar] [CrossRef]
- Davies, K.J.A. Oxidative stress, the paradox of aerobic life. In Free Radical and Oxidative Stress: Environment, Drugs and Food Additives, 2nd ed.; Rice Evans, C., Halliwell, B., Land, G.G., Eds.; Portland Press: London, UK, 1995; Volume 40, pp. 1–31. [Google Scholar]
- Winston, G.W.; Di Giulio, R.T. Prooxidant and antioxidant mechanisms in aquatic organisms. Aquat. Toxicol. 1991, 19, 137–161. [Google Scholar] [CrossRef]
- Ischiropoulos, H.; Zhu, L.; Chen, J.; Tsai, M.; Martin, J.C.; Smith, C.D.; Beckman, J.S. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch. Biochem. Biophys. 2002, 298, 431–437. [Google Scholar] [CrossRef]
- Aldini, G.; Dalle-donne, I.; Facino, R.M.; Milzani, A.; Carini, M. Intervention Strategies to Inhibit Protein Carbonylation by Reactive Carbonyls. Med. Res. Rev. 2006, 27, 817–868. [Google Scholar] [CrossRef]
- Ploch, S.A.; Lee, Y.; MacLean, E.; Di Giulio, R.T. Oxidative stress in liver of brown bullhead and channel catfish following exposure to tert-butyl hydroperoxide. Aquat. Toxicol. 1999, 46, 231–240. [Google Scholar] [CrossRef]
- Pilger, A.; Rüdiger, H.W. 8-Hydroxy-2’-deoxyguanosine as a marker of oxidative DNA damage related to occupational and environmental exposure. Int. Arch. Occup. Environ. Health 2006, 80, 1–15. [Google Scholar] [CrossRef]
- Poltronieri, C.; Maccatrozzo, L.; Simontacchi, C.; Bertotto, D.; Funkenstein, B.; Patruno, M.; Radaelli, G. Quantitative RT-PCR analysis and immunohistochemical localization of HSP70 in sea bass Dicentrarchus labrax exposed to transport stress. Eur. J. Histochem. 2007, 51, 125–136. [Google Scholar] [CrossRef]
- Poltronieri, C.; Negrato, E.; Bertotto, D.; Majolini, D.; Simontacchi, C.; Radaelli, G. Immunohistochemical localization of constitutive and inducible Heat Shock Protein 70 in carp (Cyprinus carpio) and trout (Oncorhynchus mykiss) exposed to transport stress. Eur. J. Histochem. 2008, 52, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Bertotto, D.; Poltronieri, C.; Negrato, E.; Majolini, D.; Radaelli, G.; Simontacchi, C. Alternative matrices for cortisol measurement in fish. Aquac. Res. 2010, 41, 1261–1267. [Google Scholar] [CrossRef]
- Bertotto, D.; Poltronieri, C.; Negrato, E.; Richard, J.; Pascoli, F.; Simontacchi, C.; Radaelli, G. Whole body cortisol and expression of HSP70, IGF-I and MSTN in early development of sea bass subjected to heat shock. Gen Comp Endocrinol. 2011, 174, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Pascoli, F.; Negrato, E.; Di Giancamillo, A.; Bertotto, D.; Domeneghini, C.; Simontacchi, C.; Mutinelli, F.; Radaelli, G. Evaluation of oxidative stress biomarkers in Zosterisessor ophiocephalus from the Venice Lagoon, Italy. Aquat. Toxicol. 2011, 101, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Sandodden, R.; Findstad, B.; Iversen, M. Transport stress in Atlantic salmon (Salmo salar L.): Anaesthesia and recovery. Aquac. Res. 2001, 32, 87–90. [Google Scholar] [CrossRef]
- Chandroo, K.P.; Cooke, S.J.; Mckinley, R.S.; Moccia, R.D. Use of electromyogram telemetry to assess the behavioural and energetic responses of rainbow trout, Oncorhynchus mykiss (Walbaum) to transportation stress. Aquac. Res. 2005, 36, 1226–1238. [Google Scholar] [CrossRef]
- Refaey, M.M.; Tian, X.; Tang, R.; Li, D. Changes in physiological responses, muscular composition and flesh quality of channel catfish Ictalurus punctatus suffering from transport stress. Aquaculture 2017, 478, 9–15. [Google Scholar] [CrossRef]
- Refaey, M.M.; Li, D. Transport stress changes blood biochemistry, antioxidant defense system, and hepatic HSPs mRNA expressions of channel catfish Ictalurus punctatus. Front. Physiol. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Paterson, B.D.; Rimmer, M.A.; Keike, G.M.; Semmens, G.I. Physiological responses of the Asian sea bass, Lates calcarifer to water quality deterioration during simulated live transport: Acidosis, red-cell swelling, and levels of ions and ammonia in the plasma. Aquaculture 2003, 218, 717–728. [Google Scholar] [CrossRef]
- Barton, B.A.; Iwama, G.K. Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annu. Rev. Fish Dis. 1991, 1, 3–26. [Google Scholar] [CrossRef]
- Honryo, T.; Oakada, T.; Kawahara, M.; Kurata, M.; Agawa, Y.; Sawada, Y.; Miyashita, S.; Takii, K.; Ishibashi, Y. Estimated time for recovery from transportation stress and starvation in juvenile Pacific bluefin tuna Thunnus orientalis. Aquaculture 2018, 484, 175–183. [Google Scholar] [CrossRef]
- Wu, B.; Wang, Q.; Cao, J.; Mei, J.; Xie, J. Effects of ascorbic acid and β-1,3-glucan on survival, physiological response and flesh quality of cultured tiger grouper (Epinephelus fuscoguttatus) during simulated transport in water. Biology 2020, 9, 37. [Google Scholar] [CrossRef] [Green Version]
- Aluru, N.; Vijayan, M.M. Hepatic transcriptome response to glucocorticoid receptor activation in rainbow trout. Physiol. Genom. 2007, 31, 483–491. [Google Scholar] [CrossRef]
- Faught, E.; Vijayan, M.M. Mechanisms of cortisol action in fish hepatocytes. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2016, 199, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Iwama, G.K.; Thomas, P.T.; Forsyth, R.B.; Vijayan, M.M. Heat shock protein expression in fish. Rev. Fish Biol. Fish. 1998, 8, 35–56. [Google Scholar] [CrossRef]
- Sanders, B.M.; Nguyen, J.; Martin, L.S.; Howe, S.R.; Coventry, S. Induction and subcellular localization of two major stress proteins in response to copper in the fathead minnow Pimephales promelas. Comp. Biochem. Physiol. C. 1995, 112, 335–343. [Google Scholar] [CrossRef]
- Williams, J.H.; Farag, A.M.; Stansbury, M.A.; Young, P.A.; Bergman, H.L.; Petersen, N.S. Accumulation of HSP70 in juvenile and adult rainbow trout gill exposed to metal-contaminated water and/or diet. Environ. Toxicol. Chem. 1996, 15, 1324–1328. [Google Scholar] [CrossRef]
- Vijayan, M.M.; Pereira, C.; Forsyth, R.B.; Kennedy, C.J.; Ywama, G.K. Handling stress does not affect the expression of hepatic heat shock protein 70 and conjugation heat-shock-cognate HSC71 gene from rainbow trout. Eur. J Biochem. 1997, 204, 893–900. [Google Scholar]
- Vijayan, M.M.; Pereira, C.; Kruzynski, G.; Ywama, G.K. Sublethal concentrations of contaminant induce the expression of hepatic heat shock protein 70 in two salmonids. Aquat. Toxicol. 1998, 40, 101–108. [Google Scholar] [CrossRef]
- Schmidt, H.; Posthaus, H.; Busato, A.; Wahli, T.; Meier, W.; Burkhardt-Holm, P. Transient increase in chloride cell number and heat shock protein expression (hsp70) in brown trout (Salmo trutta fario) exposed to sudden temperature elevation. Biol. Chem. 1998, 379, 1227–1233. [Google Scholar] [CrossRef]
- Duffy, L.K.; Scofield, E.; Rodgers, T.; Patton, M.; Bowyer, R.T. Comparative baseline levels of mercury, HSP70 and HSP60 in subsistence fish from the Yukon-Kuskokwim delta region of Alaska. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1999, 124, 181–186. [Google Scholar] [CrossRef]
- Hassanein, H.M.A.; Banhawy, M.A.; Soliman, F.M.; Abdel-Rehim, S.A.; Muller, W.E.G.; Schroder, H.C. Induction of HSP70 by the herbicide oxyfluoren (goal) in the Egyptian Nile fish, Oreochromis niloticus. Arch. Env. Contain. Toxicol. 1999, 37, 78–84. [Google Scholar] [CrossRef]
- Currie, S.; Moyes, C.D.; Tufts, B.L. The effects of heat shock and acclimatation temperature on hsp70 and hsp30 mRNA expression in rainbow trout: In vivo and in vitro comparisons. J. Fish Biol. 2000, 56, 398–408. [Google Scholar] [CrossRef]
- Ali, K.S.; Dorgai, L.; Ábrahám, M.; Hermesz, E. Tissue- and stressor-specific differential expression of two hsc70 genes in carp. Biochem. Biophys. Res. Commun. 2003, 307, 503–509. [Google Scholar] [CrossRef]
- Ali, K.S.; Dorgai, L.; Gazdag, A.; Ábrahám, M.; Hermesz, E. Identification and induction of hsp70 gene by heat shock and cadmium exposure in carp. Acta Biol. Hung. 2003, 54, 323–334. [Google Scholar] [CrossRef]
- Zarate, J.; Bradley, T.M. Heat shock proteins are not sensitive indicators of hatchery stress in salmon. Aquaculture 2003, 223, 175–187. [Google Scholar] [CrossRef]
- Gornati, R.; Papis, E.; Rimoldi, S.; Terova, G.; Saroglia, M.; Bernardini, G. Rearing density influences the expression of stress-related genes in sea bass (Dicentrarchus labrax L). Gene 2004, 341, 111–118. [Google Scholar] [CrossRef]
- Deane, E.E.; Woo, N.Y. Cloning and characterization of the hsp70 multigene family from silver sea bream: Modulated gene expression between warm and cold temperature acclimatation. Biochem. Biophys. Res Commun. 2005, 330, 776–783. [Google Scholar] [CrossRef]
- Ojima, N.; Yamashita, M.; Watabe, S. Comparative expression analysis of two paralogous Hsp70s in rainbow trout cells exposed to heat stress. Biochim. Biophys. Acta 2005, 1681, 99–106. [Google Scholar] [CrossRef]
- Zarantoniello, M.; Bortoletti, M.; Olivotto, I.; Ratti, S.; Poltronieri, C.; Negrato, E.; Caberlotto, S.; Radaelli, G.; Bertotto, D. Salinity, Temperature and Ammonia Acute Stress Response in Seabream (Sparus aurata) Juveniles: A Multidisciplinary Study. Animals 2021, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, E.; Civettini, M.; Carbonara, P.; Zupa, W.; Lembo, G.; Manfrin, A. Development of molecular and histological methods to evaluate stress oxidative biomarkers in sea bass (Dicentrarchus labrax). Fish Physiol. Biochem. 2020, 46, 1577–1588. [Google Scholar] [CrossRef] [PubMed]
- Eissa, N.; Wang, H.P. Transcriptional stress responses to environmental and husbandry stressors in aquaculture species. Rev. Aquac. 2016, 8, 61–88. [Google Scholar] [CrossRef]
- Negrato, E.; Vascellari, M.; Capolongo, F.; Binato, G.; Da Dalt, L.; Papo, M.B.; Gioacchini, G.; Carnevali, O.; Bertotto, D.; Radaelli, G. Expression of 8-OHdG in Zosterisessor ophiocephalus from the Venetian lagoon, Italy. Eur. J. Histochem. 2013, 57, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Topal, A.; Gergit, A.; Özkaraca, M. Assessment of oxidative DNA damage, oxidative stress responses and histopathological alterations in gill and liver tissues of Oncorhynchus mykiss treated with linuron. Hum. Exp. Toxicol. 2020, 20, 1–10. [Google Scholar] [CrossRef]


| Target Protein | Antibody | Species | Dilution | Characterization | Immunizing Antigen/Source |
|---|---|---|---|---|---|
| Heat Shock Protein 70 (HSP70) | Anti-HSP70 | mouse monoclonal | 1:600 (IHC) | Immunohistochemistry | Recombinant fragment from human Hsp70 (Abcam, UK) |
| 4-hydroxy-2-nonenal (HNE) | Anti-HNE | mouse monoclonal | 1:50 (IHC) | Immunohistochemistry | 4-hydroxy-2-nonenal modified KLH (Abcam, UK) |
| Nitrotyrosine (NT) | Anti-NT | mouse monoclonal | 1:1000 (IHC) | Immunohistochemistry | 3-(4-hydroxy-3-nitrophenylacetamido) propionic acid conjugated to bovine serum albumin (BSA) (GeneTex, Inc., USA) |
| 8 Hydroxy-2’-guanosine (8-OHdG) | Anti-8-OHdG | mouse monoclonal | 1:3000 (IHC) | Immunohistochemistry | 8-Hydroxy-2’-deoxyguanosine conjugated Keyhole Limpet Hemocyanin (Abcam, UK) |
| Tissue | CTRL | Transport Stress (End Travel) | ||||||
|---|---|---|---|---|---|---|---|---|
| HSP70 | HNE | NT | 8-OHdG | HSP70 | HNE | NT | 8-OHdG | |
| Skin | + | + | + | + | + | + | ++ | + |
| Oropharyngeal cavity | +/− | ++ | +/− | +/− | +/− | ++ | +/− | +/− |
| Stomach | ++ | + | +/− | ++ | ++ | + | + | ++ |
| Intestine | + | ++ | + | + | + | ++ | + | + |
| Liver and pancreas | ++ | + | + | + | ++ | + | + | + |
| Gills | ++ | ++ | + | ++ | ++ | ++ | ++ | ++ |
| Muscle | ++ | + | +/− | − | ++ | + | + | +/− |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bortoletti, M.; Maccatrozzo, L.; Radaelli, G.; Caberlotto, S.; Bertotto, D. Muscle Cortisol Levels, Expression of Glucocorticoid Receptor and Oxidative Stress Markers in the Teleost Fish Argyrosomus regius Exposed to Transport Stress. Animals 2021, 11, 1160. https://doi.org/10.3390/ani11041160
Bortoletti M, Maccatrozzo L, Radaelli G, Caberlotto S, Bertotto D. Muscle Cortisol Levels, Expression of Glucocorticoid Receptor and Oxidative Stress Markers in the Teleost Fish Argyrosomus regius Exposed to Transport Stress. Animals. 2021; 11(4):1160. https://doi.org/10.3390/ani11041160
Chicago/Turabian StyleBortoletti, Martina, Lisa Maccatrozzo, Giuseppe Radaelli, Stefano Caberlotto, and Daniela Bertotto. 2021. "Muscle Cortisol Levels, Expression of Glucocorticoid Receptor and Oxidative Stress Markers in the Teleost Fish Argyrosomus regius Exposed to Transport Stress" Animals 11, no. 4: 1160. https://doi.org/10.3390/ani11041160







