Characteristics of High-Level Aminoglycoside-Resistant Enterococcus faecalis Isolated from Bulk Tank Milk in Korea
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Isolation
2.2. Antimicrobial Susceptibility Testing
2.3. Detection of HLAR Enterococci
2.4. Detection of Antimicrobial Resistance and Virulence Genes
2.5. Statistical Analysis
3. Results
3.1. Prevalence of MDR and HLAR E. faecalis
3.2. Antimicrobial Resistance of HLAR E. faecalis
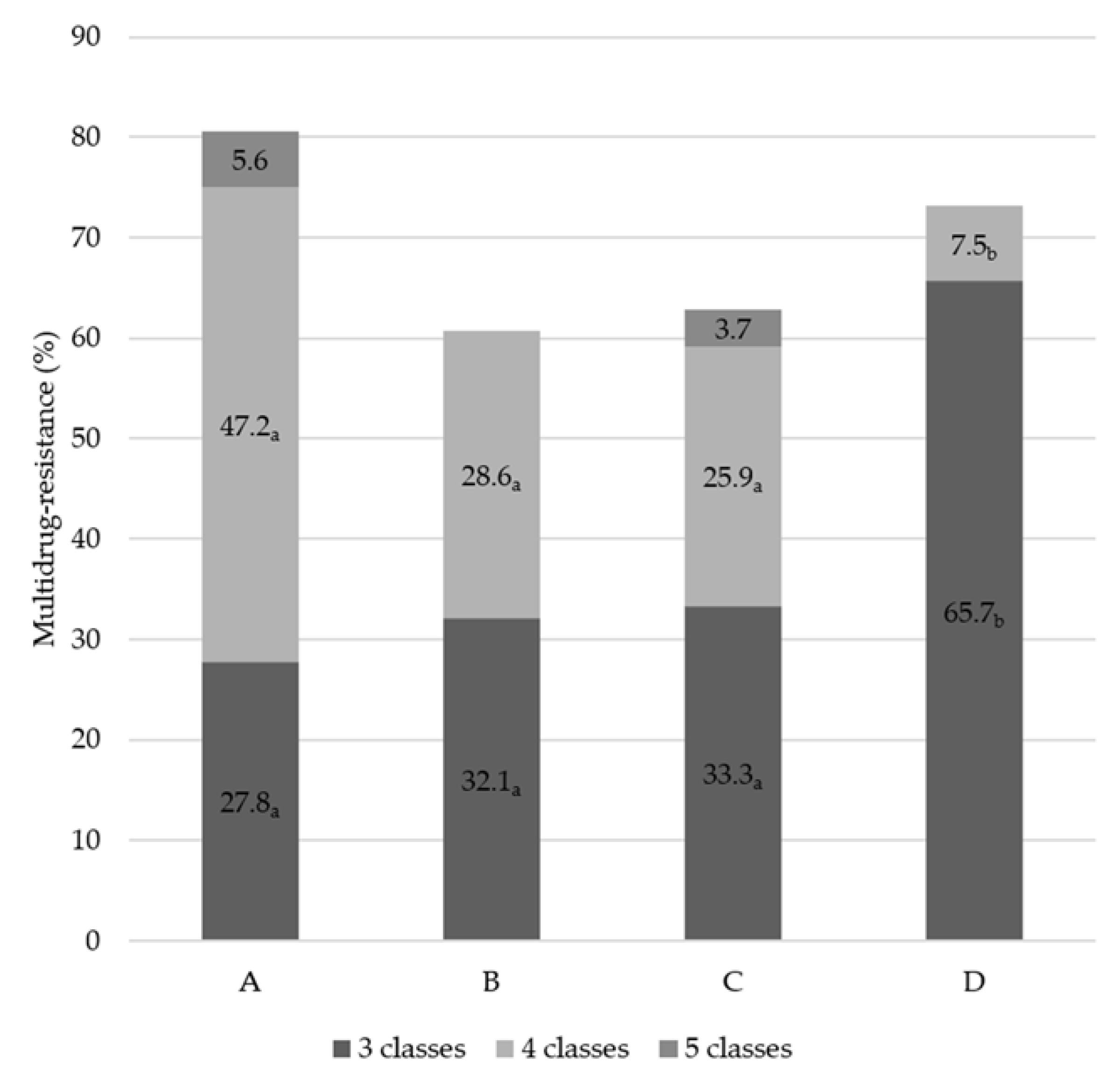
3.3. Distribution of MDR Patterns
3.4. Distribution of Antimicrobial Resistance Genes
3.5. Distribution of Virulence Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mingeot-Leclercq, M.P.; Glupczynski, Y.; Tulkens, P.M. Aminoglycosides: Activity and resistance. Antimicrob. Agents Chemother. 1999, 43, 727–737. [Google Scholar] [CrossRef]
- Krause, K.M.; Serio, A.W.; Kane, T.R.; Connolly, L.E. Aminoglycosides: An Overview. Cold Spring Harbor Perspect. Med. 2016, 1–18. [Google Scholar] [CrossRef]
- Verraes, C.; Van Boxstael, S.; Van Meervenne, E.; Van Coillie, E.; Butaye, P.; Catry, B.; de Schaetzen, M.A.; Van Huffel, X.; Imberechts, H.; Dierick, K.; et al. Antimicrobial resistance in the food chain: A review. Int. J. Environ. Res. Public Health 2013, 10, 2643–2669. [Google Scholar] [CrossRef]
- Economou, V.; Gousia, P. Agriculture and food animals as a source of antimicrobial-resistant bacteria. Infect. Drug Resist. 2015, 8, 49–61. [Google Scholar] [CrossRef]
- Giraffa, G. Enterococci from foods. FEMS Microbiol. Rev. 2002, 26, 163–171. [Google Scholar] [CrossRef]
- Rózańska, H.; Lewtak-Piłat, A.; Kubajka, M.; Weiner, M. Occurrence of enterococci in mastitic cow’s milk and their antimicrobial resistance. J. Vet. Res. 2019, 63, 93–97. [Google Scholar] [CrossRef]
- Chow, J.W. Special Section: Antimicrobial Resistance Aminoglycoside Resistance in Enterococci. Clin. Infect. Dis. 2000, 31, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Khodabandeh, M.; Mohammadi, M.; Abdolsalehi, M.R.; Hasannejad-Bibalan, M.; Gholami, M.; Alvandimanesh, A.; Pournajaf, A.; Rajabnia, R. High-Level Aminoglycoside Resistance in Enterococcus Faecalis and Enterococcus Faecium; as a Serious Threat in Hospitals. Infect. Disord. Drug Targets 2018, 20, 223–228. [Google Scholar] [CrossRef]
- Murray, B.E. The life and times of the enterococcus. Clin. Microbiol. Rev. 1990, 3, 46–65. [Google Scholar] [CrossRef]
- Chajęcka-Wierzchowska, W.; Zadernowska, A.; García-Solache, M. Ready-to-eat dairy products as a source of multidrug-resistant Enterococcus strains: Phenotypic and genotypic characteristics. J. Dairy Sci. 2020, 103, 4068–4077. [Google Scholar] [CrossRef]
- Palmeri, M.; Mancuso, I.; Gaglio, R.; Arcuri, L.; Barreca, S.; Barbaccia, P.; Scatassa, M.L. Identification and evaluation of antimicrobial resistance of enterococci isolated from raw ewes’ and cows’ milk collected in western Sicily: A preliminary investigation. Ital. J. Food Saf. 2021, 9. [Google Scholar] [CrossRef]
- Dutka-Malen, S.; Evers, S.; Courvalin, P. Erratum: Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J. Clin. Microbiol. 1995, 33, 1434. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). M100 Performance Standards for Antimicrobial Susceptibility Testing; CLSI: Wayne, PA, USA, 2019; Volume 29. [Google Scholar]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Clark, N.C.; Olsvik, Ø.; Swenson, J.M.; Spiegel, C.A.; Tenover, F.C. Detection of a streptomycin/spectinomycin adenylyltransferase gene (aadA) in Enterococcus faecalis. Antimicrob. Agents Chemother. 1999, 43, 157–160. [Google Scholar] [CrossRef]
- Aarestrup, F.M.; Agerso, Y.; Gerner-Smidt, P.; Madsen, M.; Jensen, L.B. Comparison of antimicrobial resistance phenotypes and resistance genes in Enterococcus faecalis and Enterococcus faecium from humans in the community, broilers, and pigs in Denmark. Diagn. Microbiol. Infect. Dis. 2000, 37, 127–137. [Google Scholar] [CrossRef]
- Vakulenko, S.B.; Susan, M.; Donabedian, A.M.V.; Zervos, M.J.; Lerner, S.A.; Chow, J.W. Multiplex PCR for detection of aminoglycoside resistance genes in enterococci. Antimicrob. Agents Chemother. 2003, 47, 1423–1426. [Google Scholar] [CrossRef]
- Kehrenberg, C.; Schwarz, S. Distribution of florfenicol resistance genes fexA and cfr among chloramphenicol-resistant Staphylococcus isolates. Antimicrob. Agents Chemother. 2006, 50, 1156–1163. [Google Scholar] [CrossRef]
- Di Cesare, A.; Luna, G.M.; Vignaroli, C.; Pasquaroli, S.; Tota, S.; Paroncini, P.; Biavasco, F. Aquaculture Can Promote the Presence and Spread of Antibiotic-Resistant Enterococci in Marine Sediments. PLoS ONE 2013, 8, e62838. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, Y.; Cai, J.; Schwarz, S.; Cui, L.; Hu, Z.; Zhang, R.; Li, J.; Zhao, Q.; He, T.; et al. A novel gene, optrA, that confers transferable resistance to oxazolidinones and phenicols and its presence in Enterococcus faecalis and Enterococcus faecium of human and animal origin. J. Antimicrob. Chemother. 2015, 70, 2182–2190. [Google Scholar] [CrossRef]
- Osman, K.M.; Badr, J.; Orabi, A.; Elbehiry, A.; Saad, A.; Ibrahim, M.D.S.; Hanafy, M.H. Poultry as a vector for emerging multidrug resistant Enterococcus spp.: First report of vancomycin (van) and the chloramphenicol–florfenicol (cat-fex-cfr) resistance genes from pigeon and duck faeces. Microb. Pathog. 2019, 128, 195–205. [Google Scholar] [CrossRef]
- Huang, J.; Wang, M.; Gao, Y.; Chen, L.; Wang, L. Emergence of plasmid-mediated oxazolidinone resistance gene poxtA from CC17 Enterococcus faecium of pig origin. J. Antimicrob. Chemother. 2019, 74, 2524–2530. [Google Scholar] [CrossRef]
- Billström, H.; Lund, B.; Sullivan, Å.; Nord, C.E. Virulence and antimicrobial resistance in clinical Enterococcus faecium. Int. J. Antimicrob. Agents 2008, 32, 374–377. [Google Scholar] [CrossRef]
- Song, H.S.; Bae, Y.C.; Jeon, E.J.; Kwon, Y.K.; Joh, S.J. Multiplex PCR analysis of virulence genes and their influence on antibiotic resistance in Enterococcus spp. isolated from broiler chicken. J. Vet. Sci. 2019, 20, e26. [Google Scholar] [CrossRef]
- Yoon, S.; Lee, Y.J. Molecular characteristics of enterococcus faecalis and enterococcus faecium from bulk tank milk in Korea. Animals 2021, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Korea Agro-Fisheries & Food Trade Corporation Food Information Statistics System (ATFIS). Available online: https://www.atfis.or.kr/home/M000000000/index.do# (accessed on 18 May 2021).
- Washburn, S.P.; White, S.L.; Green, J.T.; Benson, G.A. Reproduction, mastitis, and body condition of seasonally calved holstein and jersey cows in confinement or pasture systems. J. Dairy Sci. 2002, 85, 105–111. [Google Scholar] [CrossRef]
- Animal and Plant Quarantine Agency (APQA). Antimicrobial Use and Monitoring in Animals and Andimal Products; APQA: Gimcheon, Korea, 2019.
- Shahini Shams Abadi, M.; Taji, A.; Salehi, F.; Kazemian, H.; Heidari, H. High-level Gentamicin Resistance among Clinical Isolates of Enterococci in Iran: A Systematic Review and Meta-analysis. Folia Med. 2021, 63, 15–23. [Google Scholar] [CrossRef]
- Jackson, C.R.; Fedorka-Cray, P.J.; Barrett, J.B.; Ladely, S.R. High-level aminoglycoside resistant enterococci isolated from swine. Epidemiol. Infect. 2005, 133, 367–371. [Google Scholar] [CrossRef]
- Riboldi, G.P.; Frazzon, J.; D’Azevedo, P.A.; Frazzon, A.P.G. Antimicrobial resistance profile of Enterococcus spp. isolated from food in southern Brazil. Braz. J. Microbiol. 2009, 40, 125–128. [Google Scholar] [CrossRef]
- Kim, Y.B.; Seo, K.W.; Son, S.H.; Noh, E.B.; Lee, Y.J. Genetic characterization of high-level aminoglycoside-resistant Enterococcus faecalis and Enterococcus faecium isolated from retail chicken meat. Poult. Sci. 2019, 98, 5981–5988. [Google Scholar] [CrossRef]
- Özdemir, R.; Tuncer, Y. Detection of antibiotic resistance profiles and aminoglycoside-modifying enzyme (AME) genes in high-level aminoglycoside-resistant (HLAR) enterococci isolated from raw milk and traditional cheeses in Turkey. Mol. Biol. Rep. 2020, 47, 1703–1712. [Google Scholar] [CrossRef]
- Hegstad, K.; Mikalsen, T.; Coque, T.M.; Werner, G.; Sundsfjord, A. Mobile genetic elements and their contribution to the emergence of antimicrobial resistant Enterococcus faecalis and Enterococcus faecium. Clin. Microbiol. Infect. 2010, 16, 541–554. [Google Scholar] [CrossRef]
- Hollenbeck, B.L.; Rice, L.B. Intrinsic and acquired resistance mechanisms in enterococcus. Virulence 2012, 3, 421–569. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.J.; Rather, P.N.; Hare, R.S.; Miller, G.H. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol. Rev. 1993, 57, 138–163. [Google Scholar] [CrossRef]
- Udo, E.E.; Al-Sweih, N.; John, P.; Jacob, L.E.; Mohanakrishnan, S. Characterization of high-level aminoglycoside-resistant enterococci in Kuwait hospitals. Microb. Drug Resist. 2004, 10, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Bartash, R.; Nori, P. Beta-lactam combination therapy for the treatment of Staphylococcus aureus and Enterococcus species bacteremia: A summary and appraisal of the evidence. Int. J. Infect. Dis. 2017, 63, 7–12. [Google Scholar] [CrossRef]
- Livermore, D.M.; Winstanley, T.G.; Shannon, K.P. Interpretative reading: Recognizing the unusual and inferring resistance mechanisms from resistance phenotypes. J. Antimicrob. Chemother. 2001, 48, 87–102. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Davies, J.; Wright, G.D. Bacterial resistance to aminoglycoside antibiotics. Trends Microbiol. 1997, 5, 234–240. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, S.; Shang, X.; Wang, X.; Yan, Z.; Li, H.; Li, J. Short communication: Antimicrobial resistance and virulence genes of Enterococcus faecalis isolated from subclinical bovine mastitis cases in China. J. Dairy Sci. 2019, 102, 140–144. [Google Scholar] [CrossRef]
- Choi, J.M.; Woo, G.J. Transfer of Tetracycline Resistance Genes with Aggregation Substance in Food-Borne Enterococcus faecalis. Curr. Microbiol. 2015, 70, 476–484. [Google Scholar] [CrossRef]
- Agersø, Y.; Pedersen, A.G.; Aarestrup, F.M. Identification of Tn5397-like and Tn916-like transposons and diversity of the tetracycline resistance gene tet(M) in enterococci from humans, pigs and poultry. J. Antimicrob. Chemother. 2006, 57, 832–839. [Google Scholar] [CrossRef]
- Ministry of Food and Drug Safety (MFDS). Processing Standards and Ingredient Specifications for Livestock Products; NIFDS: Cheongju, Korea, 2018.
- Gao, X.; Fan, C.; Zhang, Z.; Li, S.; Xu, C.; Zhao, Y.; Han, L.; Zhang, D.; Liu, M. Enterococcal isolates from bovine subclinical and clinical mastitis: Antimicrobial resistance and integron-gene cassette distribution. Microb. Pathog. 2019, 129, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Tatsing Foka, F.E.; Ateba, C.N.; Lourenco, A. Detection of virulence genes in multidrug resistant enterococci isolated from feedlots dairy and beef cattle: Implications for human health and food safety. BioMed Res. Int. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.I.; Prichula, J.; Santesteva, N.A.; d’Azevedo, P.A.; Motta, A.d.S.; Frazzon, A.P.G. Virulence Profiles in Enterococcus spp. Isolated from Raw Buffalo’s Milk in South Brazil. Res. J. Microbiol. 2017, 12, 248–254. [Google Scholar] [CrossRef][Green Version]
- Shafeek, M.; El-Malt, L.; Abdel Hameed, K.; El-Zamkan, M. Some Virulence Genes of Pathogenic Enterococci Isolated from Raw Milk and Some Milk Products. SVU Int. J. Vet. Sci. 2018, 1, 102–113. [Google Scholar] [CrossRef]
| Locus | Target Gene | Sequence (5′-3′) | Size (pb) | Reference |
|---|---|---|---|---|
| Aminoglycoside-modifying enzymes | aac(6″)Ie-aph(2″)-1la | F: CAGAGCCTTGGGAAGATGAAG | 348 | [17] |
| R: CCTCGTGTAATTCATGTTCTGGC | ||||
| ant(6)-Ia | F: ACTGGCTTAATCAATTTGGG | 597 | [15] | |
| R: GCCTTTCCGCCACCTCACCG | ||||
| aph(2″)-Ic | F: CCACAATGATAATGACTCAGTTCCC | 444 | [17] | |
| R: CCACAGCTTCCGATAGCAAGAG | ||||
| aph(2″)-Id | F: GTGGTTTTTACAGGAATGCCATC | 641 | [17] | |
| R: CCCTCTTCATACCAATCCATATAACC | ||||
| Macrolide resistance | ermA | F: TAACATCAGTACGGATATTG | 200 | [19] |
| R: AGTCTACACTTGGCTTAGG | ||||
| ermB | F: CCGAACACTAGGGTTGCTC | 139 | [19] | |
| R: ATCTGGAACATCTGTGGTATG | ||||
| mef | F: AGTATCATTAATCACTAGTGC | 348 | [19] | |
| R: TTCTTCTGGTACTAAAAGTGG | ||||
| Oxazolidinone resistance | optrA | F: AGGTGGTCAGCGAACTAA | 1395 | [20] |
| R: ATCAACTGTTCCCATTCA | ||||
| poxtA | F: TCCACAAAGGATGGGTTATG | 1336 | [22] | |
| R: ATGCCCGTATTGGTTATCTC | ||||
| Phenicol resistance | catA | F: GGATATGAAATTTATCCCTC | 486 | [21] |
| R: CAATCATCTACCCTATGAAT | ||||
| catB | F: TGAACACCTGGAACCGCAGAG | 482 | [21] | |
| R: GCCATAGTAAACACCGGAGCA | ||||
| cfr | F: TGAAGTATAAAGCAGGTTGGGAGTCA | 746 | [18] | |
| R: ACCATATAATTGACCACAAGCAGC | ||||
| fexA | F: GTACTTGTAGGTGCAATTACGGCTGA | 1272 | [18] | |
| R: CGCATCTGAGTAGGACATAGCGTC | ||||
| Tetracycline resistance | tetL | F: ATAAATTGTTTCGGGTCGGTAAT | 1077 | [16] |
| R: AACCAGCCAACTAATGACAATGAT | ||||
| tetM | F: GTTAAATAGTGTTCTTGGAG | 657 | [16] | |
| R: CTAAGATATGGCTCTAACAA | ||||
| tetO | F: CAATATCACCAGAGCAGGCT | 614 | [16] | |
| R: TCC CAC TGT TCC ATA TCG TCA | ||||
| Virulence gene | ace | F: GGAATGACCGAGAACGATGGC | 616 | [23] |
| R: GCTTGATGTTGGCCTGCTTCCG | ||||
| asa1 | F: CACGCTATTACGAACTATGA | 375 | [23] | |
| R: TAAGAAAGAACATCACCACGA | ||||
| cad1 | F: TTCCAA AACTACGCACAACA | 423 | [24] | |
| R: CTTTTTCAGCAGCATTCACTAATT | ||||
| cylA | F: GACTCGGGGATTGATAGGC | 688 | [23] | |
| R: GCTGCTAAAGCTGCGCTTAC | ||||
| efaA | F: CGTGAGAAAGAAATGGAGGA | 499 | [23] | |
| R: CTACTAACACGTCACGAATG | ||||
| esp | F: AGATTTCATCTTTGATTCTTG | 510 | [23] | |
| R: AATTGATTCTTTAGCATCTGG | ||||
| gelE | F: TATGACAATGCTTTTTGGGAT | 213 | [23] | |
| R: AGATGCACCCGAAATAATATA |
| Company (No. of Farms) | No. of E. faecalis | No. of MDR 1 (%) | No. of HLAR 2 (%) |
|---|---|---|---|
| A (106) | 52 | 37 (71.2) a | 36 (69.2) |
| B (47) | 39 | 20 (51.3) a,b | 28 (71.8) |
| C (120) | 86 | 41 (47.7) b | 54 (62.8) |
| D (122) | 124 | 51 (41.1) b | 67 (54.0) |
| Total (395) | 301 | 149 (49.5) | 185 (61.5) |
| No. (%) of Antimicrobial-Resistant HLAR E. faecalis by Company | |||||
|---|---|---|---|---|---|
| Antimicrobials | A (n = 36) * | B (n = 28) | C (n = 54) | D (n = 67) | Total (n = 185) |
| Ampicillin | 0 (0.0) | 1 (3.6) | 0 (0.0) | 1 (1.5) | 2 (1.1) A,B |
| Chloramphenicol | 27 (75.0) a | 14 (50.0) a,b | 19 (35.2) b,c | 15 (22.4) c | 75 (40.5) C |
| Ciprofloxacin | 0 (0.0) | 0 (0.0) | 2 (3.7) | 0 (0.0) | 2 (1.1) A,B |
| Doxycycline | 24 (66.7) a,b | 20 (71.4) a,b | 31 (57.4) b | 56 (83.6) a | 131(70.8) D |
| Erythromycin | 30 (83.3) | 18 (64.3) | 43 (79.6) | 42 (62.7) | 133 (71.9) D |
| Penicillin | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) B |
| Rifampin | 4 (11.1) | 0 (0.0) | 5 (9.3) | 2 (3.0) | 11 (5.9) A |
| Tetracycline | 33 (91.7) | 25 (89.3) | 50 (92.6) | 65 (97.0) | 173 (93.5) E |
| Vancomycin | 0 (0.0) | 1 (3.6) | 0 (0.0) | 0 (0.0) | 1 (0.5) A,B |
| Genes | No. (%) of Isolates with Antimicrobial Resistance Gene(s) by Company | ||||
|---|---|---|---|---|---|
| A (n = 36) * | B (n = 28) | C (n = 54) | D (n = 67) | Total (n = 185) | |
| Aminoglycoside-modifying enzymes | |||||
| aac(6′)Ie-aph(2″)-la | 2 (5.6) | 1 (3.6) | 6 (11.1) | 4 (6.0) | 13 (7.0) A |
| ant(6)-Ia | 8 (22.2) a,b | 10 (35.7) b | 13 (24.1) a,b | 5 (7.5) a | 36 (19.5) B |
| aph(2″)-Ic | 0 (0.0) | 1 (3.6) | 1 (1.9) | 0 (0.0) | 2 (1.1) A,C |
| aph(2″)-Id | 1 (2.8) | 2 (7.1) | 2 (3.7) | 8 (11.9) | 13 (7.0) A |
| aac(6″)Ie-aph(2″)-la, ant(6)-Ia | 12 (33.3) a,b | 6 (21.4) b | 18 (33.3) a,b | 36 (53.7) a | 72 (38.9) D |
| aac(6″)Ie-aph(2″)-la, aph(2″)-Id | 0 (0.0) | 0 (0.0) | 1 (1.9) | 0 (0.0) | 1 (0.5) C |
| aph(2″)-Ic, aph(2″)-Id | 0 (0.0) | 0 (0.0) | 1 (1.9) | 0 (0.0) | 1 (0.5) C |
| aph(2″)-Ic, ant(6)-Ia | 1 (2.8) | 0 (0.0) | 1 (1.9) | 1 (1.5) | 3 (1.6) A,C |
| aph(2″)-Id, ant(6)-Ia | 1 (2.8) | 3 (10.7) | 5 (9.3) | 3 (4.5) | 12 (6.5) A,C |
| aph(2″)-Ic, aph(2″)-Id, ant(6)-Ia | 0 (0.0) | 0 (0.0) | 1 (1.9) | 0 (0.0) | 1 (0.5) C |
| Macrolides | |||||
| ermA | 0 (0.0) | 1 (3.6) | 0 (0.0) | 0 (0.0) | 1 (0.5) A |
| ermB | 29 (80.6) | 18 (64.3) | 43 (79.6) | 42 (62.7) | 132 (71.4) B |
| mef | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) A |
| Oxazolidinones | |||||
| optrA | 0 (0.0) | 1 (3.6) | 0 (0.0) | 1 (1.5) | 2 (1.1) A |
| poxtA | 0 (0.0) | 0 (0.0) | 1 (1.9) | 0 (0.0) | 1 (0.5) A |
| Phenicols | |||||
| catA | 11 (30.6) a | 3 (10.7) a,b | 8 (14.8) a,b | 5 (7.5) b | 27 (14.6) B |
| catB | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) A |
| cfr | 2 (5.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (1.1) A |
| fexA | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) A |
| Tetracyclines | |||||
| tetL | 0 (0.0) | 1 (3.6) | 3 (5.6) | 2 (3.0) | 6 (3.2) A |
| tetM | 19 (52.8) a | 3 (10.7) b | 30 (55.6) a | 15 (22.4) b | 67 (36.2) B |
| tetO | 0 (0.0) | 2 (7.1) | 0 (0.0) | 0 (0.0) | 2 (1.1) A |
| tetM + tetL | 11 (30.6) a | 15 (53.6) a,b | 16 (29.6) a | 44 (65.7) b | 86 (46.5) B |
| Genes | No. (%) of Isolates with Virulence Gene(s) by Company | ||||
|---|---|---|---|---|---|
| A (n = 36) | B (n = 28) | C (n = 54) | D (n = 67) | Total (n = 185) | |
| ace | 36 (100) | 27 (96.4) | 54 (100) | 67 (100) | 184 (99.5) A |
| asa1 | 24 (66.7) | 17 (60.7) | 28 (51.9) | 45 (67.2) | 114 (61.6) B |
| cad1 | 36 (100) | 28 (100) | 53 (98.1) | 65 (97.0) | 182 (98.4) A |
| cylA | 5 (13.9) a | 2 (7.1) a,b | 5 (9.3) a,b | 0 (0) b | 12 (6.5) C |
| efaA | 36 (100) a | 26 (92.9) b | 54 (100) a | 67 (100) a | 183 (98.9) A |
| esp | 4 (11.1) | 4 (14.3) | 11 (20.4) | 4 (6.0) | 23 (12.4) C |
| gelE | 31 (86.1) a,b | 23 (82.1) a,b | 40 (74.1) b | 65 (97.0) a | 159 (85.9) D |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, H.J.; Yoon, S.; Kim, K.; Lee, Y.J. Characteristics of High-Level Aminoglycoside-Resistant Enterococcus faecalis Isolated from Bulk Tank Milk in Korea. Animals 2021, 11, 1724. https://doi.org/10.3390/ani11061724
Kang HJ, Yoon S, Kim K, Lee YJ. Characteristics of High-Level Aminoglycoside-Resistant Enterococcus faecalis Isolated from Bulk Tank Milk in Korea. Animals. 2021; 11(6):1724. https://doi.org/10.3390/ani11061724
Chicago/Turabian StyleKang, Hyo Jung, Sunghyun Yoon, Koeun Kim, and Young Ju Lee. 2021. "Characteristics of High-Level Aminoglycoside-Resistant Enterococcus faecalis Isolated from Bulk Tank Milk in Korea" Animals 11, no. 6: 1724. https://doi.org/10.3390/ani11061724
APA StyleKang, H. J., Yoon, S., Kim, K., & Lee, Y. J. (2021). Characteristics of High-Level Aminoglycoside-Resistant Enterococcus faecalis Isolated from Bulk Tank Milk in Korea. Animals, 11(6), 1724. https://doi.org/10.3390/ani11061724







