Evaluation of Ultrasound Measurement of Subcutaneous Fat Thickness in Dairy Jennies during the Periparturient Period
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Inclusion Criteria
2.3. Study Design
2.4. RTU Assessment of SF Thickness
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thiemann, A.; Foxcroft, A. Working across Europe to improve donkey welfare. Vet. Rec. 2016, 179, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Muraro, M.A.; Giampietro, P.G.; Galli, E. Soy formulas and nonbovine milk. Ann. Allergy Asthma Immunol. 2002, 89, 97–101. [Google Scholar] [CrossRef]
- Lisuzzo, A.; Bonelli, F.; Sgorbini, M.; Nocera, I.; Cento, G.; Mazzotta, E.; Turini, L.; Martini, M.; Salari, F.; Morgante, M.; et al. Differences of the Plasma Total Lipid Fraction from Pre-Foaling to Post-Foaling Period in Donkeys. Animals 2022, 12, 304. [Google Scholar] [CrossRef] [PubMed]
- Turini, L.; Bonelli, F.; Nocera, I.; Battaglia, F.; Meucci, V.; Panzani, D.; Mele, M.; Sgorbini, M. Evaluation of jennies’ colostrum: IgG concentrations and absorption in the donkey foals. A preliminary study. Heliyon 2020, 6, e04598. [Google Scholar] [CrossRef]
- Sgorbini, M.; Bonelli, F.; Rota, A.; Baragli, P.; Marchetti, V.; Corazza, M. Hematology and clinical chemistry in Amiata donkey foals from birth to 2 months of age. J. Equine Vet. Sci. 2013, 33, 35–39. [Google Scholar] [CrossRef]
- Bonelli, F.; Nocera, I.; Conte, G.; Panzani, D.; Sgorbini, M. Relation between APGAR scoring and physical parameters in 44 newborn Amiata foals at birth. Theriogenology 2019, 142, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Sgorbini, M.; Bonelli, F.; Percacini, G.; Pasquini, A.; Rota, A. Maternal and Neonatal Evaluation of Derived Reactive Oxygen Metabolites and Biological Antioxidant Potential in Donkey Mares and Foals. Animals 2021, 11, 2885. [Google Scholar] [CrossRef]
- Vitale, V.; Nocera, I.; Sgorbini, M.; Aliboni, B.; Laus, F.; Mannini, A.; Bazzano, M. Ultrasonography Evaluation of Umbilical Structures in Clinically Healthy Donkey Foals during the First Week of Life. Animals 2021, 11, 1650. [Google Scholar] [CrossRef]
- Liao, Q.; Li, Z.; Han, Y.; Deng, L. Comparative Analysis of Serum Mineral and Biochemical Parameter Profiles between Late Pregnant and Early Lactating Jennies. J. Equine Vet. Sci. 2021, 99, 103401. [Google Scholar] [CrossRef]
- Fazio, E.; Medica, P.; Galvano, E.; Cravana, C.; Ferlazzo, A. Changes in the cortisol and some biochemical patterns of pregnant and barren jennies (Equus asinus). Vet. Arh. 2011, 81, 563–574. [Google Scholar]
- Quaresma, M.; Payan-Carreira, R.; Silva, S.R. Relationship between ultrasound measurements of body fat reserves and body condition score in female donkeys. Vet. J. 2013, 197, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Maśko, M.; Wierzbicka, M.; Zdrojkowski, Ł.; Jasiński, T.; Pawliński, B.; Domino, M. Characteristics of the Donkey’s Dorsal Profile in Relation to Its Functional Body Condition Assessment. Animals 2021, 11, 3095. [Google Scholar] [CrossRef] [PubMed]
- Valle, E.; Raspa, F.; Giribaldi, M.; Barbero, R.; Bergagna, S.; Antoniazzi, S.; Mc Lean, K.A.; Minero, M.; Cavallarin, L. A functional approach to the body condition assessment of lactating donkeys as a tool for welfare evaluation. PeerJ 2017, 5, e3001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schröder, U.J.; Staufenbiel, R. Invited review: Methods to determine body fat reserves in the dairy cow with special regard to ultrasonographic measurement of backfat thickness. Int. J. Dairy Sci. 2006, 89, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Fiore, E.; Fabbri, G.; Gallo, L.; Morgante, M.; Muraro, M.; Boso, M.; Gianesella, M. Application of texture analysis of b-mode ultrasound images for the quantification and prediction of intramuscular fat in living beef cattle: A methodological study. Res. Vet. Sci. 2020, 131, 254–258. [Google Scholar] [CrossRef]
- Dugdale, A.H.; Grove-White, D.; Curtis, G.C.; Harris, P.A.; Argo, C.M. Body condition scoring as a predictor of body fat in horses and ponies. Vet. J. 2012, 194, 173–178. [Google Scholar] [CrossRef]
- Silva, S.R.; Payan-Carreira, R.; Quaresma, M.; Guedes, C.M.; Santos, A.S. Relationships between body condition score and ultrasound skin-associated subcutaneous fat depth in equids. Acta Vet. Scand. 2016, 58, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Domino, M.; Romaszewski, M.; Jasiński, T.; Maśko, M. Comparison of the surface thermal patterns of horses and donkeys in infrared thermography images. Animals 2020, 10, 2201. [Google Scholar] [CrossRef]
- National Research Council Donkey and Other Equids. Nutrient Requirements of Horses; National Academies Press: Washington, DC, USA, 2007; pp. 268–279. ISBN 9780309102124. [Google Scholar]
- Carluccio, A.; Gloria, A.; Veronesi, M.C.; De Amicis, I.; Noto, F.; Contri, A. Factors affecting pregnancy length and phases of parturition in Martina Franca jennies. Theriogenology 2015, 84, 650–655. [Google Scholar] [CrossRef]
- Pearson, R.A.; Ouassat, M. Guide to Body Condition Scoring and Live Weight Estimation of Donkeys; Thomson Colour Printers Ltd.: Glasgow, UK, 2000; p. 21. [Google Scholar]
- Costa, L.R.R.; Paradis, M.R. Manual of Clinical Procedures in the Horse, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 416–426. [Google Scholar]
- Domecq, J.J.; Skidmore, A.L.; Lloyd, J.W.; Kaneene, J.B. Validation of body condition scores with ultrasound measurements of subcutaneous fat of dairy cows. J. Dairy Sci. 1995, 78, 2308–2313. [Google Scholar] [CrossRef]
- Kidd, J.A.; Lu, K.G.; Frazer, M.L. Atlas of Equine Ultrasonography; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Salari, F.; Roncoroni, C.; Altomonte, I.; Boselli, C.; Brajon, G.; Martini, M. Effects of the Physiological Status and Diet on Blood Metabolic Parameters in Amiata Dairy Donkeys. Animals 2021, 11, 3292. [Google Scholar] [CrossRef] [PubMed]
- Drackley, J.K.; Wallace, R.L.; Graugnard, D.; Vasquez, J.; Richards, B.F.; Loor, J.J. Visceral adipose tissue mass in nonlactating dairy cows fed diets differing in energy density1. J. Dairy Sci. 2014, 97, 3420–3430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cellini, M.; Hussein, H.A.; Elsayed, H.K.; Sayed, A.S. The association between metabolic profile indices, clinical parameters, and ultrasound measurement of backfat thickness during the periparturient period of dairy cows. Comp. Clin. Pathol. 2019, 28, 711–723. [Google Scholar] [CrossRef]
- Cosentino, C.; Paolino, R.; Freschi, P.; Calluso, A.M. Jenny milk production and qualitative characteristics. Int. J. Dairy Sci. 2012, 95, 2910–2915. [Google Scholar] [CrossRef]
- Gentry, L.R.; Thompson, D.; Gentry, G.T., Jr.; Del Vecchio, R.P.; Davis, K.A.; Del Vecchio, P.M. The relationship between body condition score and ultrasonic fatmeasurements in mares of high versus low body condition. J. Equine Vet. Sci. 2004, 24, 198–203. [Google Scholar] [CrossRef]

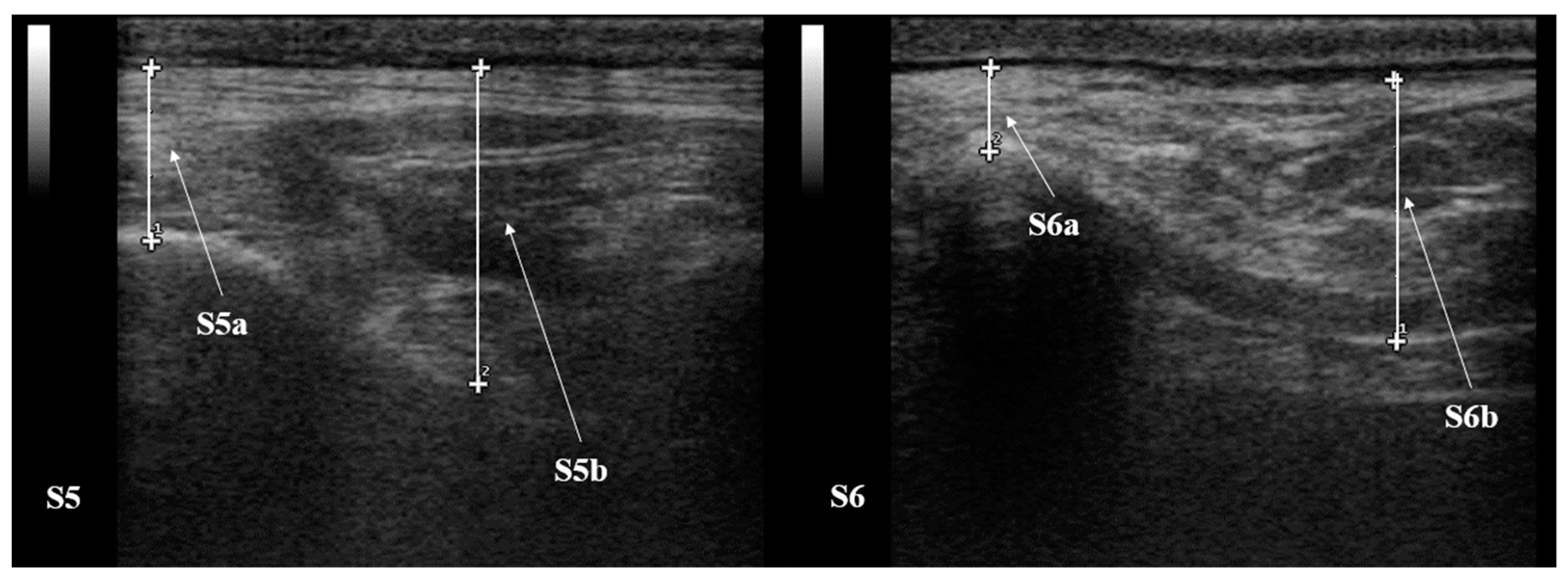
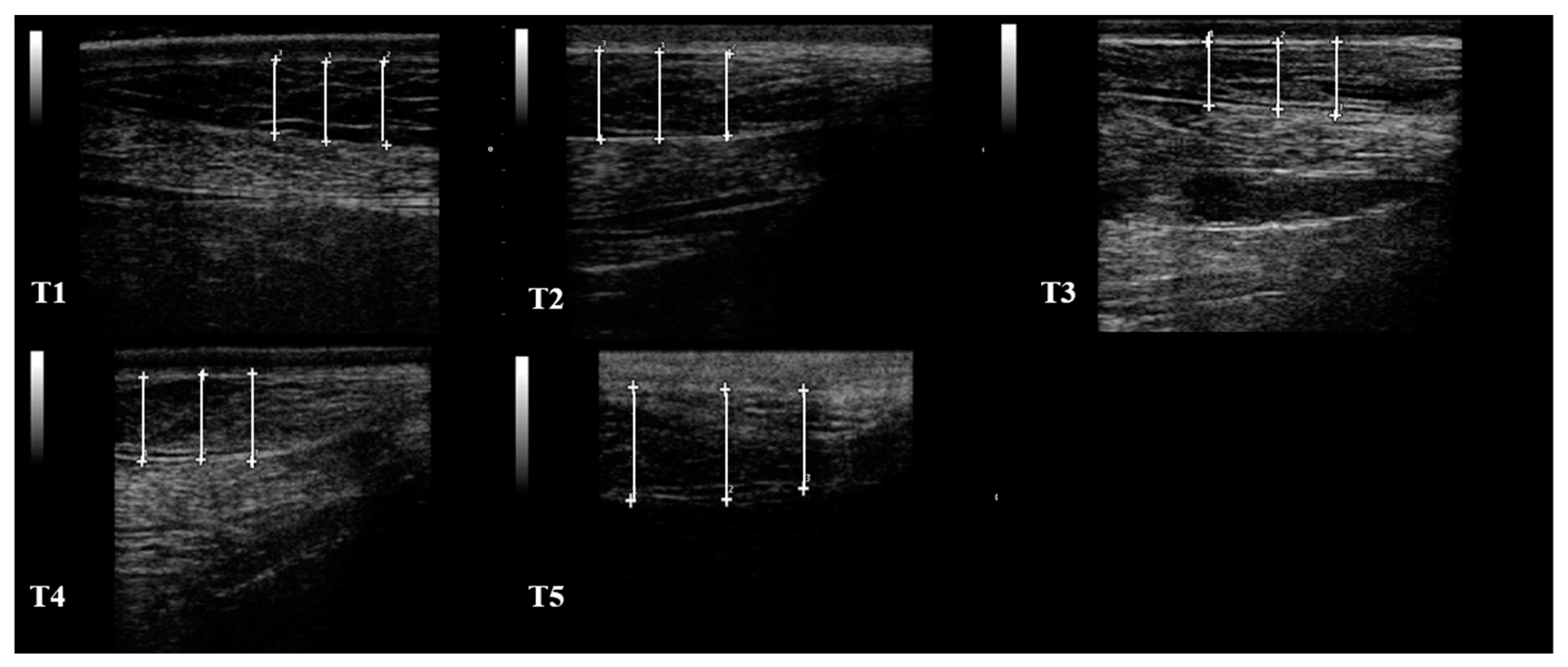

| T1 | T2 | T3 | T4 | T5 | ||
|---|---|---|---|---|---|---|
| S1 (mm) | 10.95 ± 3.12 | 12.03 ± 3.09 | 10.87 ± 3.01 | 12.32 ± 1.75 | 10.72 ± 1.29 | |
| S2 (mm) | 3.60 ± 0.86 | 4.23 ± 1.18 | 3.50 ± 1.18 | 2.85 ± 0.64 | 2.12 ± 0.41 | |
| S3 (mm) | 9.17 ± 7.17 | 7.30 ± 3.10 | 7.47 ± 2.96 | 6.90 ± 3.37 | 5.92 ± 3.10 | |
| S4 (mm) | 11.70 ± 1.37 | 12.45 ± 1.23 | 14.90 ± 1.75 | 15.80 ± 2.40 | 15.35 ± 1.73 | |
| S5 (mm) | a | 10.25 ± 2.86 | 11.17 ± 3.19 | 10.70 ± 4.17 | 10.02 ± 3.37 | 9.17 ± 3.82 |
| b | 20.83 ± 13.51 | 20.18 ± 1.49 | 17.78 ± 4.30 | 21.15 ± 2.10 | 18.92 ± 4.58 | |
| S6 (mm) | a | 10.55 ± 3.12 | 8.53 ± 2.38 | 8.47 ± 3.00 | 6.25 ± 2.76 | 4.72 ± 2.60 |
| b | 20.28 ± 4.32 | 18.10 ± 2.42 | 20.17 ± 3.70 | 16.50 ± 3.56 | 17.58 ± 4.15 | |
| BCS | 6.5 ± 0.63 | 6.33 ± 0.98 | 6.08 ± 1.39 | 6.17 ± 1.32 | 5.67 ± 1.29 |
| Prediction Equations | Constant | S1 | S2 | S3 | S4 | S5a | S5b | S6a | S6b | p-Value | Ac. R2% |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Y = a + b1X1 + b2X2 + b3X3 + b4X4 + b5X5 + b6X6 + b7X7 + b8X8 | 3.00 | 0.03 | −0.05 | −0.10 | 0.12 | 0.02 | 0.10 | 0.30 | −0.13 | 0.01 | 0.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nocera, I.; Bonelli, F.; Turini, L.; Madrigali, A.; Aliboni, B.; Sgorbini, M. Evaluation of Ultrasound Measurement of Subcutaneous Fat Thickness in Dairy Jennies during the Periparturient Period. Animals 2022, 12, 1359. https://doi.org/10.3390/ani12111359
Nocera I, Bonelli F, Turini L, Madrigali A, Aliboni B, Sgorbini M. Evaluation of Ultrasound Measurement of Subcutaneous Fat Thickness in Dairy Jennies during the Periparturient Period. Animals. 2022; 12(11):1359. https://doi.org/10.3390/ani12111359
Chicago/Turabian StyleNocera, Irene, Francesca Bonelli, Luca Turini, Alessio Madrigali, Benedetta Aliboni, and Micaela Sgorbini. 2022. "Evaluation of Ultrasound Measurement of Subcutaneous Fat Thickness in Dairy Jennies during the Periparturient Period" Animals 12, no. 11: 1359. https://doi.org/10.3390/ani12111359







