Influence of Spinal Shock on the Neurorehabilitation of ANNPE Dogs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
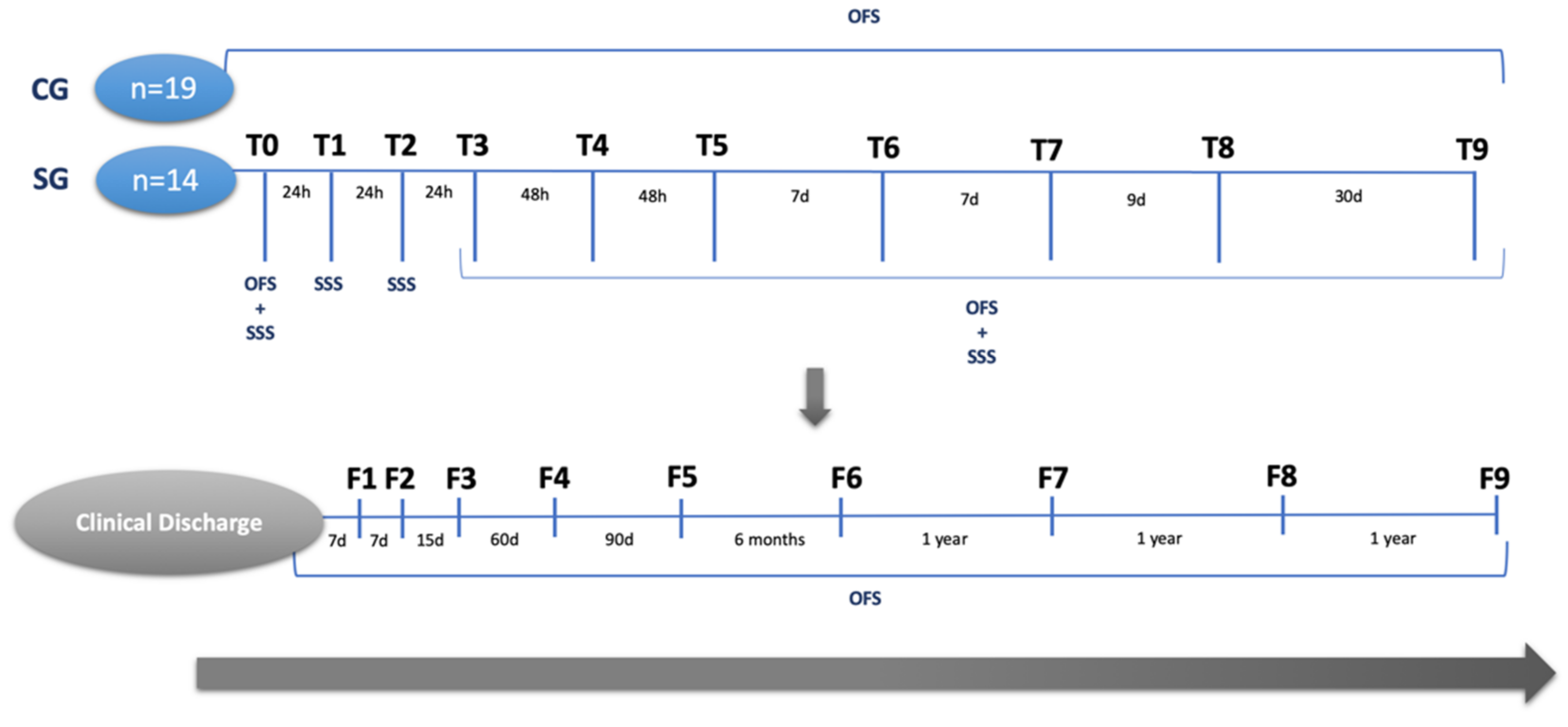
2.1. Study Design
2.2. Procedures
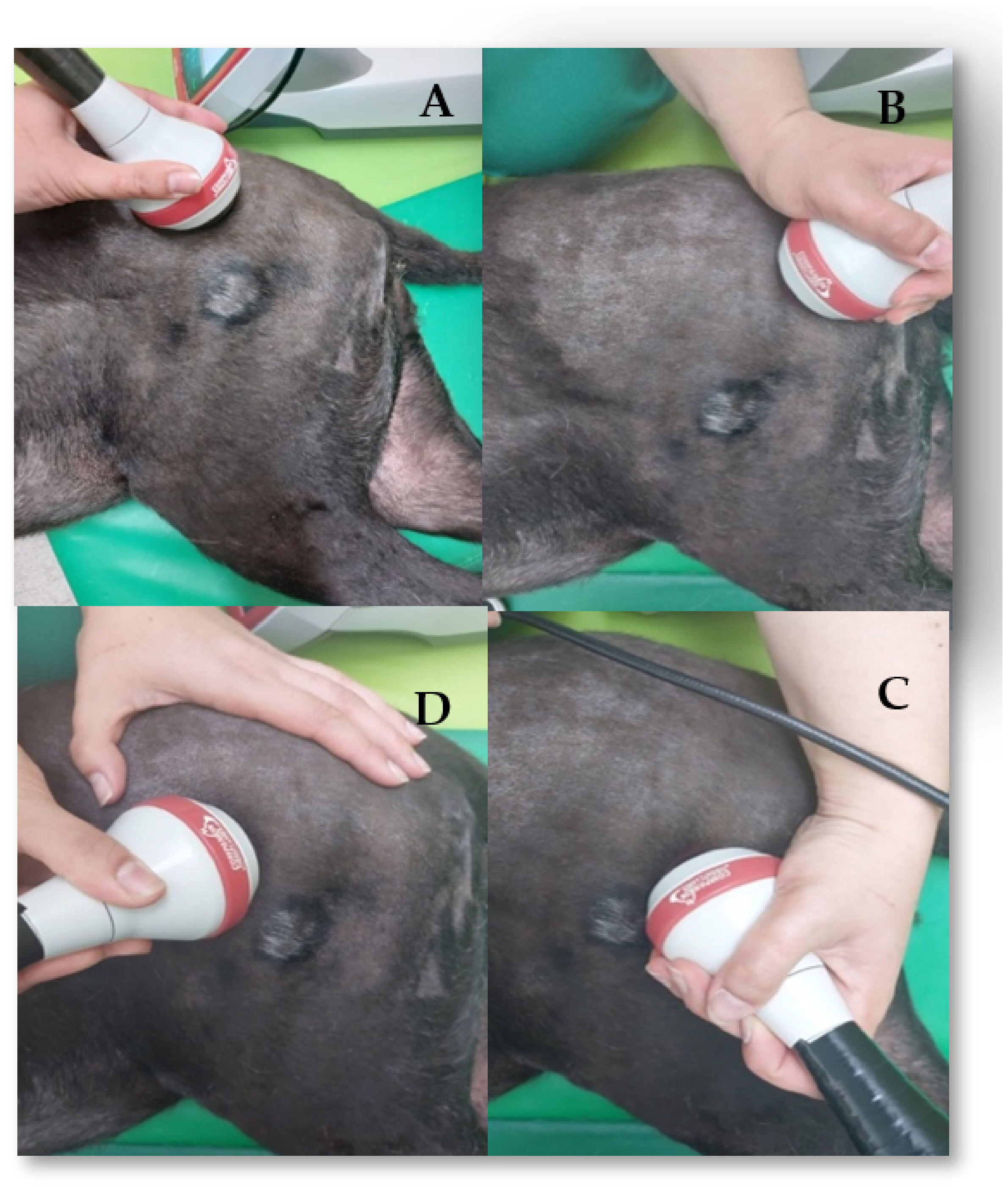
2.2.1. Electrical Stimulation
2.2.2. Class IV Laser Therapy
2.2.3. Kinesiotherapy Protocol
- Locomotor training
- Kinesiotherapy exercises
2.2.4. Supportive Care
2.3. Outcomes and Follow-Ups
2.4. Data Collection
2.5. Statistical Analysis
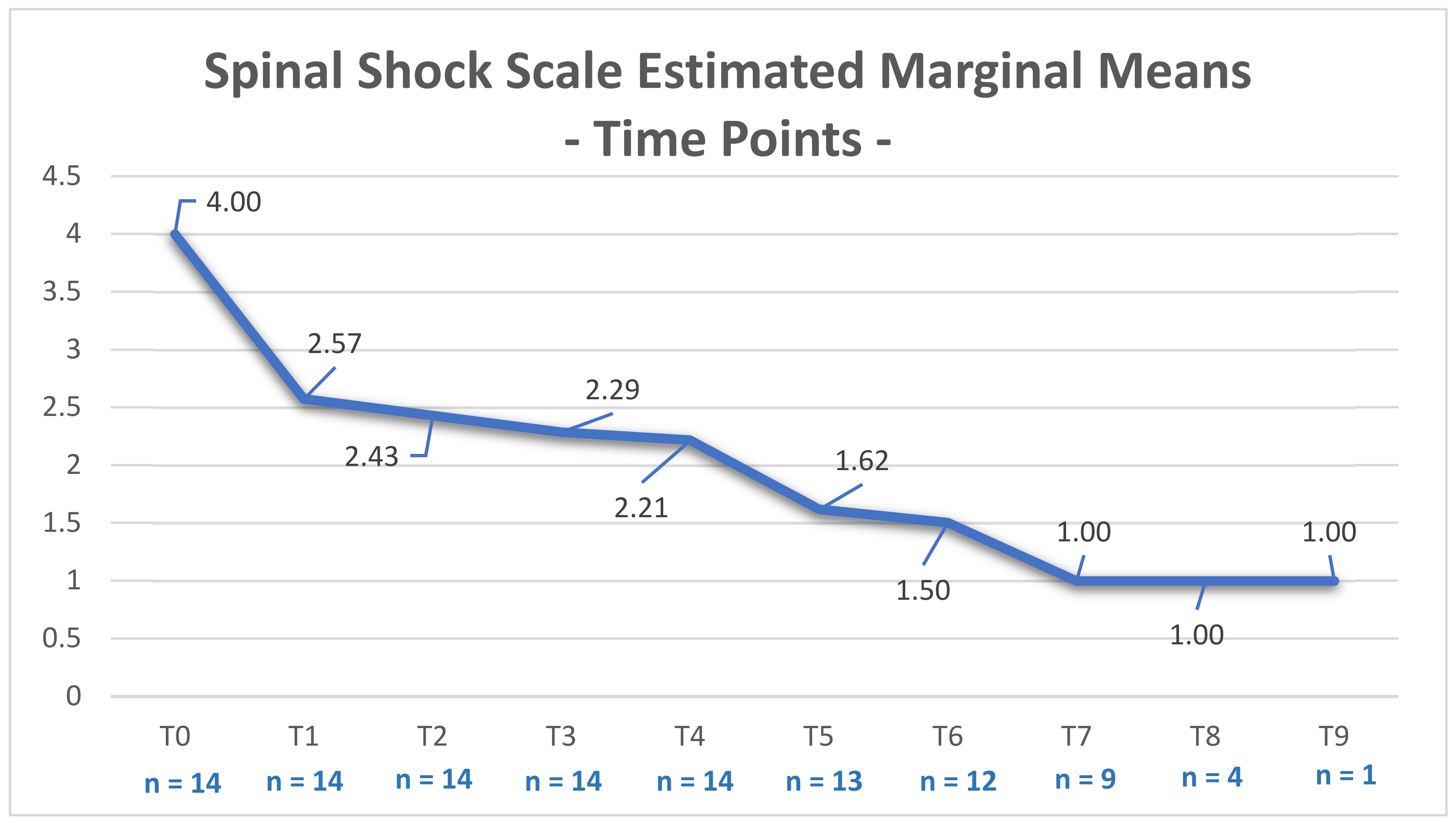
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Da Costa, R.C.; De Decker, S.; Lewis, M.J.; Volk, H.; Cansort, C. Diagnostic imaging in intervertebral disc disease. Front. Vet. Sci. 2020, 7, 782. [Google Scholar] [CrossRef]
- Fenn, J.; Drees, R.; Volk, H.A.; De Decker, S. Comparison of clinical signs and outcomes between dogs with presumptive ischemic myelopathy and dogs with acute noncompressive nucleus pulposus extrusion. J. Am. Vet. Med. Assoc. 2016, 249, 767–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartner, L. Neurological Disease of the Thoracic Limb. In Canine Lameness, 1st ed.; Duerr, F.M., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2020; pp. 255–269. [Google Scholar]
- Freeman, P.M. Paresis, Paralysis, and Proprioceptive Ataxia. In A Practical Approach to Neurology for the Small Animal Practitioner, 1st ed.; Freeman, P.M., Ives, E., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2020; pp. 275–296. [Google Scholar]
- Kim, J.; Kim, H.; Hwang, J.; Eom, K. Preliminary study of presumptive intradural-intramedullary intervertebral disc extrusion in 20 dogs. J. Vet. Sci. 2020, 21, e52. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.M.; Fingeroth, J.M. Historical and current nomenclature associated with intervertebral disc pathology. In Advances in Intervertebral Disc Diseases in Dogs and Cats, 1st ed.; Fingeroth, J.M., Thomas, W.B., Eds.; Wiley-Blackwell: Ames, IA, USA, 2015; pp. 26–31. [Google Scholar]
- Schollum, M.L.; Robertson, P.A.; Broom, N.D. How age influences unravelling morphology of annular lamellae—A study of interfibre cohesivity in the lumbar disc. J. Anat. 2010, 216, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Ives, E.; Freeman, P.M. Neurological Emergencies. In A Practical Approach to Neurology for the Small Animal Practitioner, 1st ed.; Freeman, P.M., Ives, E., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2020; pp. 329–370. [Google Scholar]
- Gandini, G.; Cizinauskas, S.; Lang, J.; Fatzer, R.; Jaggy, A. Fibrocartilaginous embolism in 75 dogs: Clinical findings and factors influencing the recovery rate. J. Small Anim. Pract. 2003, 44, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Snyder, L.A.; Tan, L.; Gerard, C.; Fessler, R.G. Spinal cord trauma. In Bradley’s Neurology in Clinical Practice, 7th ed.; Daroff, R.B., Jankovic, J., Mazziotta, J.C., Pomeroy, S.L., Eds.; Elsevier: St. Louis, MO, USA, 2016; Volume 2, pp. 881–902. [Google Scholar]
- Cardy, T.J.A.; De Decker, S.; Kenny, P.J.; Volk, H.A. Clinical reasoning in canine spinal disease: What combination of clinical information is useful? Vet. Rec. 2015, 177, 171. [Google Scholar] [CrossRef] [Green Version]
- Cauzinille, L.; Kornegay, J.N. Fibrocartilaginous embolism of the spinal cord in dogs: Review of 36 histologically confirmed cases and retrospective study of 26 suspected cases. J. Vet. Intern. Med. 1996, 10, 241–245. [Google Scholar] [CrossRef]
- De Decker, S.; Fenn, J. Acute Herniation of Nondegenerate Nucleus Pulposus, Acute Noncompressive Nucleus Pulposus Extrusion and Compressive Hydrated Nucleus Pulposus Extrusion. Vet. Clin. N. Am. Small Anim. Pract. 2018, 48, 95–109. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, I.R. A syndrome produced by dorso-lateral “explosions” of the cervical intervertebral discs. Vet. Rec. 1970, 87, 737–741. [Google Scholar] [CrossRef]
- Bagley, R.S. Clinical features of diseases—Spinal cord. In Fundamentals of Veterinary Clinical Neurology, 1st ed.; Bagley, R.S., Ed.; Wiley-Blackwell Publishing: Hoboken, NJ, USA, 2005; pp. 174–175. [Google Scholar]
- De Risio, L.; Adams, V.; Dennis, R.; McConnell, F.J. Association of clinical and magnetic resonance imaging findings with outcome in dogs with presumptive acute noncompressive nucleus pulposus extrusion: 42 cases (2000–2007). J. Am. Vet. Med. Assoc. 2009, 234, 495–504. [Google Scholar] [CrossRef]
- De Risio, L.; Platt, S.R. Fibrocartilaginous embolic myelopathy in small animals. Vet. Clin. N. Am. Small Anim. Pract. 2010, 40, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Olby, N.J.; da Costa, R.C.; Levine, J.M.; Stein, V.M. Canine Spinal Cord Injury Consortium (CANSORT SCI). Prognostic Factors in Canine Acute Intervertebral Disc Disease. Front. Vet. Sci. 2020, 7, 596059. [Google Scholar] [CrossRef] [PubMed]
- Specchi, S.; Johnson, P.; Beauchamp, G.; Masseau, I.; Pey, P. Assessment of interobserver agreement and use of selected magnetic resonance imaging variables for differentiation of acute noncompressive nucleus pulposus extrusion and ischemic myelopathy in dogs. J. Am. Vet. Med. Assoc. 2016, 248, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Dennis, R.; Platt, S.R.; Penderis, J. Magnetic resonance imaging of traumatic intervertebral disc extension in dogs. Vet. Rec. 2007, 160, 795–799. [Google Scholar] [CrossRef] [PubMed]
- De Risio, L.; Adams, V.; Dennis, R.; McConnell, F.J.; Platt, S.R. Magnetic resonance imaging findings and clinical associations in 52 dogs with suspected ischemic myelopathy. J. Vet. Intern. Med. 2007, 21, 1290–1298. [Google Scholar] [CrossRef]
- Mari, L.; Behr, S.; Shea, A.; Dominguez, E.; Johnson, P.J.; Ekiri, A.; De Risio, L. Outcome comparison in dogs with presumptive diagnosis of thoracolumbar fibrocartilaginous embolic myelopathy and acute non-compressive nucleus pulposus extrusion. Vet. Rec. 2017, 181, 293. [Google Scholar] [CrossRef]
- De Risio, L. What is fibrocartilaginous embolism and is it related to IVDD. In Advances in Intervertebral Disc. Diseases in Dogs and Cats, 1st ed.; Fingeroth, J.M., Thomas, W.B., Eds.; Wiley-Blackwell: Ames, IA, USA, 2015; pp. 75–87. [Google Scholar]
- Ros, C.; de la Fuente, R.; Rodenas, S.; Novellas, R.; Viu, J.; Añor, S. Myelographic and low-field magnetic resonance imaging findings in dogs with presumptive acute hydrated non-compressive nucleous pulposus extrusion. Vet. Rec. 2017, 181, 594–599. [Google Scholar] [CrossRef]
- De Risio, L. A review of fibrocartilaginous embolic myelopathy and different types of peracute non-compressive intervertebral disk extrusions in dogs and cats. Front. Vet. Sci. 2015, 2, 24. [Google Scholar] [CrossRef] [Green Version]
- Ko, H.-Y.; Ditunno, J.F., Jr.; Graziani, V.; Little, J.W. The pattern of reflex recovery during spinal shock. Spinal Cord 1999, 37, 402–409. [Google Scholar] [CrossRef] [Green Version]
- Santiago, P.; Fessler, R.G. Spinal cord trauma. In Neurology in Clinical Practice: The Neurological Disorders, 4th ed.; Bradley, W.G., Daroff, R.B., Fenichel, G.M., Jankovic, J., Eds.; Elsevier: Philadelphia, PA, USA, 2004; pp. 1149–1178. [Google Scholar]
- De Lahunta, A.; Glass, E. Small animal spinal cord disease. In Veterinary Neuroanatomy and Clinical Neurology, 3rd ed.; de Lahunta, A., Glass, E., Eds.; Saunders: St. Louis, MO, USA, 2009; pp. 248–250. [Google Scholar]
- Smith, P.M.; Jeffery, N.D. Spinal shock—Comparative aspects and clinical relevance. J. Vet. Intern. Med. 2005, 19, 788–793. [Google Scholar]
- Mari, L.; Behr, S.; Shea, A.; Dominguez, E.; Ricco, C.; Alcoverro, E.; Ekiri, A.; Sanchez-Masian, D.; De Risio, L. Predictors of urinary or fecal incontinence in dogs with thoracolumbar acute noncompressive nucleus pulposus extrusion. J. Vet. Intern. Med. 2019, 33, 2693–2700. [Google Scholar] [CrossRef] [PubMed]
- Full, A.M.; Heller, H.L.B.; Mercier, M. Prevalence, clinical presentation, prognosis, and outcome of 17 dogs with spinal shock and acute thoracolumbar spinal cord disease. J. Vet. Emerg. Crit. Care 2016, 26, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Blauch, B. Spinal reflex walking. Vet. Med. Small Anim. Clin. 1977, 72, 169–173. [Google Scholar]
- Little, J.W. Serial recording of reflexes after feline spinal cord transection. Exp. Neurol. 1986, 93, 510–521. [Google Scholar] [CrossRef]
- Ditunno, J.F.; Little, J.W.; Tessler, A.; Burns, A.S. Spinal shock revisited: A four-phase model. Spinal Cord. 2004, 42, 383–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henke, D.; Gorgas, D.; Flegel, T.; Vandevelde, M.; Lang, J.; Doherr, M.G.; Forterre, F. Magnetic resonance imaging findings in dogs with traumatic intervertebral disk extrusion with or without spinal cord compression: 31 cases (2006–2010). J. Am. Vet. Med. Assoc. 2013, 242, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Wessmann, A.; Posporis, C. Compressive hydrated nucleus pulposus extrusion: Is surgery necessary? Vet. Rec. 2017, 181, 622–662. [Google Scholar]
- McKee, W.M.; Downes, C.J.; Pink, J.J.; Gemmill, T.J. Presumptive exercise associated peracute thoracolumbar disc extrusion in 48 dogs. Vet. Rec. 2010, 166, 523–528. [Google Scholar] [CrossRef]
- Yang, X.; Liu, B.; Ouyang, B. Effect of acupuncture combined with rehabilitative training on neural functional recovery of stroke patients during recovery phase: A randomixed controlled trial. World J. Acupunct. Moxibustion 2014, 24, 17–23. [Google Scholar] [CrossRef]
- Draper, W.E.; Schubert, T.A.; Clemmons, R.M.; Miles, S.A. Low-level laser therapy reduces time to ambulation in dogs after hemilaminectomy: A preliminary study. J. Small Anim. Pract. 2012, 53, 465–469. [Google Scholar] [CrossRef]
- De Freitas, G.R.; Santo, C.C.; De Machado-Pereira, N.A.; Bobinski, F.; Dos Santos, A.R.; Ilha, J. Early cyclical neuromuscular electrical stimulation improves strenght and trophism by Akt pathway signaling in partially paralyzed biceps muscle after spinal cord injury in rats. Phys. Ther. 2018, 93, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Canto, S.; Momeni, K.; Ramanujam, A.; Garbarini, E.; Forrest, G.F. Neuromotor response of the leg muscles following a supine, stand retraining with/without neuromuscular electrical stimulation training intervention for individuals with SCI: A case series. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 3143–3146. [Google Scholar]
- Frankel, H.L.; Hancock, D.O.; Hyslop, G.; Melzak, J.; Michaelis, L.S.; Ungar, G.H.; Vernon, J.D.S.; Walsh, J.J. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. Spinal Cord 1969, 7, 179–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olby, N.J.; De Risio, L.; Munana, K.R.; Wosar, M.A.; Skeen, T.M.; Sharp, N.; Keene, B.W. Development of a functional scoring system in dogs with acute spinal cord injuries. Am. J. Vet. Res. 2001, 62, 1624–1628. [Google Scholar] [CrossRef] [PubMed]
- Martins, Â.; Gouveia, D.; Cardoso, A.; Carvalho, C.; Coelho, T.; Silva, C.; Viegas, I.; Gamboa, Ó.; Ferreira, A. A controlled clinical study of intensive neurorehabilitation in post-surgical dogs with severe acute intervertebral disc extrusion. Animals 2021, 11, 3034. [Google Scholar] [CrossRef] [PubMed]
- Martins, Â.; Gouveia, D.; Cardoso, A.; Carvalho, C.; Silva, C.; Coelho, T.; Gamboa, Ó.; Ferreira, A. Functional neurorehabilitation in dogs with an incomplete recovery 3 months following intervertebral disc surgery: A case series. Animals 2021, 11, 2442. [Google Scholar] [CrossRef] [PubMed]
- Liberson, W.T.; Holmquest, H.J.; Scot, D.; Dow, M. Functional electrotherapy stimulation of the peroneal nerve synchronized with the swing phase of the gait of hemiplegic patients. Arch. Phys. Med. Rehabil. 1961, 42, 101–105. [Google Scholar]
- Bennaim, M.; Porrato, M.; Jarleton, A.; Hamon, M.; Carroll, J.D.; Gommeren, K.; Balligand, M. Preliminary evalution of the effects of photobiomodulation therapy and physical rehabilitation on early postoperative recovery of dogs undergoing hemilaminectomy for treatment of thoracolumbar intervertebral disk disease. Am. J. Vet. Res. 2017, 78, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Shaw, K.K.; Brown, L. Modalities Part 2: Laser Therapy. In Physical Rehabilitation for Veterinary Technicians and Nurses, 1st ed.; Goldberg, M.E., Tomlinson, J.E., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2018; pp. 231–240. [Google Scholar]
- Monici, M.; Millis, D.; Ciuperca, I.; McCarthy, D. Laser Therapy. In Essential Facts of Physical Medicine, Rehabilitation and Sports Medicine in Companion Animals, 1st ed.; Bockstahler, B., Wittek, K., Levine, D., Maierl, J., Millis, D., Eds.; VBS GmbH: Babenhausen, Germany, 2019; pp. 245–254. [Google Scholar]
- Fenn, J.; Drees, R.; Volk, H.A.; Decker, S. Inter and intraobserver agreement for diagnosing presumptive ischemic myelopathy and acute noncompressive nucleus pulposus extrusion in dogs using magnetic resonance imaging. Vet. Radiol. Ultrasound 2016, 57, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Dewey, C.W.; da Costa, R.C. Spinal Trauma Management. In Pratical Guide to Canine and Feline Neurology, 3rd ed.; Dewey, C.W., da Costa, R.C., Eds.; Wiley Blackwell: Ames, IA, USA, 2016; pp. 423–433. [Google Scholar]
- Fenn, J.; Ru, H.; Jeffery, N.D.; Moore, S.; Tipold, A.; Soebbeler, F.J.; Wang-Leandro, A.; Mariani, C.L.; Early, P.J.; Muñana, K.R.; et al. Association between anesthesia duration and outcome in dogs with surgically treated acute severe spinal cord injury caused by thoracolumbar intervertebral disk herniation. J. Vet. Intern. Med. 2020, 34, 1507–1513. [Google Scholar] [CrossRef]
- Lewis, M.J.; Howard, J.F.; Olby, N.J. The relationship between trans-lesional conduction, motor neuron pool excitability and motor function in dogs with incomplete recovery from severe spinal cord injury. J. Neurotrauma 2017, 34, 2994–3002. [Google Scholar] [CrossRef]
- Thomas, W.B.; Dewey, C.W. Performing the neurologic examination. In A Pratical Guide to Canine and Feline Neurology, 2nd ed.; Dewey, C.W., Ed.; Wiley-Blackwell: Ames, IA, USA, 2008; p. 64. [Google Scholar]
- Sehl, J.; Gruber, A.D.; Lazzerini, K.; Loderstedt, S. Ischaemic paraspinal myopathy along with myelopathy due to putative fibrocartilaginous embolism in a dog. Vet. Rec. Case Rep. 2017, 5, e000400. [Google Scholar] [CrossRef]
- Sivaramakrishnan, A.; Solomon, J.M.; Manikandan, N. Comparison of transcutaneous electrical nerve stimulation(TENS) and functional electrical stimulation(FES) for spasticity in spinal cord injury—A pilot randomized cross-over trial. J. Spinal Cord Med. 2018, 41, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Sangari, S.; Lundell, H.; Kirshblum, S.; Perez, M.A. Residual descending motor pathways influence spasticity after spinal cord injury. Ann. Neurol. 2019, 86, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Sangari, S.; Perez, M.A. Imbalanced Corticospinal and Reticulospinal Contributions to Spasticty in Spinal Cord Injury. J. Neurosci. 2019, 39, 7872–7881. [Google Scholar] [CrossRef] [Green Version]
- Baker, S.N.; Perez, M.A. Reticulospinal contributions to gross hand function after human spinal cord injury. J. Neurosci. 2017, 37, 9778–9784. [Google Scholar] [CrossRef] [Green Version]
- Botsoglou, R.; Sarpekidou, E.; Patsikas, M.; Kazakos, G. Acute non-compressive nucleus pulposus extrusion in dogs and cats: An overview. J. Hell. Vet. Med. Soc. 2021, 75, 2809–2816. [Google Scholar]




 knuckling position.
knuckling position.
 knuckling position.
knuckling position.



| SG (n = 14) | CG (n = 19) | Total (n = 33) | |
|---|---|---|---|
| Breed | Breed: 64.3% (9/14) | Breed: 89.5% (17/19) | Breed: 78.8% (26/33) |
| Mixed Breed: 35.7% (5/14) | Mixed Breed: 10.5% (2/19) | Mixed Breed: 21.2% (7/33) | |
| Sex | Male: 50% (7/14) | Male: 57.9% (11/19) | Male: 54.5% (18/33) |
| Female: 50% (7/14) | Female: 42.1% (8/19) | Female: 45.5% (15/33) | |
| Age | <7 years old: 71.4% (10/14) | <7 years old: 57.9% (11/19) | <7 years old: 63.6% (21/33) |
| ≥7 years old: 28.6% (4/14) | ≥7 years old: 42.1% (8/19) | ≥7 years old: 36.4% (12/33) Mean: 6 years | |
| Mean: 5.64 years | Mean: 6.42 years | ||
| Weight | ≤15 kg: 35.7% (5/14) | ≤15 kg: 36.8% (7/19) | ≤15 kg: 36.4% (12/33) |
| >15 kg: 64.3% (9/14) | >15 kg: 63.2% (12/19) | >15 kg: 63.6% (21/33) | |
| Mean: 26.86 kg | Mean: 21.89 kg | Mean: 24 kg. | |
| Complementary Exams | MRI: 35.7% (5/14) | MRI: 42.1% (8/19) | MRI: 39.4% (13/33) |
| CT: 64.3% (9/14) | CT: 57.9% (11/19) | CT: 60.6% (20/33) | |
| Neuroanatomical Localization | T12–T13: 14.3% (2/14) | T12–T13: 21.1% (4/19) | T12–T13: 18.2% (6/33) |
| T13–L1: 35.7% (5/14) | T13–L1: 36.8% (7/19) | T13–L1: 36.4% (12/33) | |
| L1–L2: 21.4% (3/14) | L1–L2: 31.6% (6/19) | L1–L2: 27.3% (9/33) | |
| L2–L3: 28.6% (4/14) | L2–L3: 10.5% (2/19) | L2–L3: 18.2% (6/33) | |
| Lateralization signs | Absent: 14.3% (2/14) | Absent: 21.1% (4/19) | Absent: 18.2% (6/33) |
| Present: 85.7% (12/14) | Present: 78.9% (15/19) | Present: 81.8% (27/33) | |
| Spinal discomfort | Absent: 78.6% (11/14) | Absent: 84.2% (16/19) | Absent: 81.8% (27/33) |
| Present: 21.4% (3/14) | Present: 15.8% (3/19) | Present: 18.2% (6/33) | |
| Nociception | Absent: 28.6% (4/14) | Absent: 21.1% (4/19) | Absent: 24.2% (8/33) |
| Present: 71.4% (10/14) | Present: 78.9% (15/19) | Present: 75.8% (25/33) | |
| Modified Frankel Scale | Grade 0: 28.6% (4/14) | Grade 0: 21.1% (4/19) | Grade 0: 24.2% (8/33) |
| Grade 1: 71.4% (10/14) | Grade 1: 78.9% (15/19) | Grade 1: 75.8% (25/33) | |
| Fecal and Urinary Incontinence | Present: 14% (2/14) (progressive myelomalacia) | Absent: 100% (19/19) | Present: 6% (2/33) (progressive myelomalacia) |
| Absent: 86% (12/14) | Absent: 94% (31/33) | ||
| Occurrences | Absent: 28.6% (4/14) Present: 71.4% (10/14) | Absent: 57.9% (11/19) Present: 42.1% (8/19) | Absent: 45.5% (15/33) Present: 54.5% (18/33) (2 progressive myelomalacia) |
| Reduced hindlimb reflexes | Withdrawal reflex | (+1) |
| Patellar reflex | (+1) | |
| Cranial tibial reflex | (+1) | |
| Reduced hindlimb tone | (+1) | |
| Reduced perineal reflex | (+1) | |
| Reduced perineal tone | (+1) | |
| Absent/abnormal cutaneous trunci cut off | (+1) | |
| TOTAL SCORE | ||
| KERRYPNX | Electrical Stimulation | Laser Therapy Class IV | Kinesiotherapy Exercises | ||||
|---|---|---|---|---|---|---|---|
| Land Treadmill | Underwater Treadmill | Exercises | |||||
| Admission to Ambulation | Study Group | DPP Positive | 50 Hz, 10 mA, 150 μs, duty cycle 1:4; | After trichotomy, 12 J/cm2, 2,5 Hz, duty cycle 88%, 3 min, pulsed mode, SID, 15 days. | 3–10 min; | 5–10 min; | Obstacle rails, postural standing: 1–2 min, 3–6 times/day; |
| no slope; | no slope; | bicycles: 2–3 times/day | |||||
| 1.8 km/h | 1.2–2 km/h | alternating floors: 1 min, 3–6 times/day, | |||||
| 10 min; TID; | 4–6 times/day; | SID; | 6 days/week. | ||||
| SSS score < 4; BID. | 6 days/week. | 5 days/week. | |||||
| DPP Negative | 40 Hz; 10–48 mA; 150 μs, | 3–10 min; | 10–20 min; | Obstacle rails, postural standing: 1–2 min, 3–6 times/day; | |||
| no slope; | |||||||
| duty cycle 1:4. | 2–5% slope; 1.5 km/h | 1.2–2 km/h | bicycles: 2–3 times/day; | ||||
| 10 min; TID | 6–8 times/day; | SID; | alternating floors: 1 min, 3–6 times/day, | ||||
| SSS score < 4; BID. | 6 days/week. | 5 days/week. | 6 days/week | ||||
| Control Group | DPP Positive | 50 Hz; 10 mA; 150 μs, duty cycle 1:4; | 3–10 min; | 5–10 min; | Obstacle rails, postural standing: 1–2 min, 3–6 times/day; | ||
| no slope; | no slope; | bicycles: 2–3 times/day; | |||||
| 1.8 km/h | 1.2–2 km/h | alternating floors: 1 min, 3–6 times/day, | |||||
| 4–6 times/day; | SID; | 6 days/week. | |||||
| 10 min; BID. | 6 days/week. | 5 days/week. | |||||
| DPP Negative | 40 Hz; 10–48 mA; 150 μs, | 3–10 min; | 10–20 min; | Obstacles rails, postural standing: 1–2 min, 3–6 times/day; | |||
| 2–5% slope; 1.5 km/h | no slope; | bicycles: 2–3 times/day; | |||||
| 6–8 times/day; | 1.2–2 km/h | alternating floors: 1 min, 3–6 times/day, | |||||
| duty cycle 1:4; | 6 days/week. | SID; | 6 days/week. | ||||
| 10 min; BID. | 5 days/week. | ||||||
| Ambulation to Clinical Discharge | Study Group | DPP Positive | 50 Hz; 10 mA; 150 μs, duty cycle 1:4; | 10–40 min; | 30 min; | Obstacle rails, postural standing: 1–2 min, 2–3 times/day; | |
| 2–5% slope; 2–2.5 km/h | 2–5% slope; | alternating floors: 2 min, 2–3 times/day, | |||||
| 2–3 times/day; | 2–2.5 km/h | 3 days/week. | |||||
| 3 days/week. | SID; | ||||||
| 10 min; SID. | 3 days/week. | ||||||
| DPP Negative | 40 Hz; 10–48 mA; 150 μs, | 10–40 min; | 40 min; | Obstacle rails, postural standing: 1–2 min, 3 times/day; | |||
| duty cycle 1:4; | 2–5% slope; 1.8–2.5 km/h | 5–10% slope; | alternating floors: 2 min, 3 times/day, | ||||
| 10 min; SID. | 2–3 times/day; | 2.8–4.5 km/h | 5 days/week. | ||||
| 5 days/week. | SID; | ||||||
| 5 days/week. | |||||||
| Control Group | DPP Positive | 50 Hz; 10 mA; 150 μs, duty cycle 1:4; | 10–40 min; | Obstacle rails, postural standing: 1–2 min, 2–3 times/day; | |||
| 2–5% slope; 2–2.5 km/h | 30 min; | alternating floors: 2 min, 2–3 times/day, | |||||
| 2–3 times/day; | 2–5% slope; | 3 days/week. | |||||
| 3 days/week. | 2–2.5 km/h | ||||||
| SID; | |||||||
| 10 min; SID. | 3 days/week. | ||||||
| DPP Negative | 40 Hz; 10–48 mA; 150 μs, | 10–40 min; | 40 min; | Obstacle rails, postural standing: 1–2 min, 3 times/day; | |||
| 2–5% slope; 1.8–2.5 km/h | 5–10% slope; | alternating floors: 2 min, 3 times/day, | |||||
| 2–3 times/day; | 2.8–4.5 km/h | 5 days/week. | |||||
| duty cycle 1:4; | 5 days/week. | SID; | |||||
| 10 min; SID. | 5 days/week. | ||||||
| SG (n = 14) | CG (n = 19) | Total (n = 33) | ||
| Age (years) | Mean | 5.64 | 6.42 | 6.09 |
| Median | 5 | 6 | 6 | |
| Variance | 5.94 | 9.48 | 7.898 | |
| SD | 2.437 | 3.079 | 2.81 | |
| Minimum | 2 | 1 | 1 | |
| Maximum | 11 | 12 | 12 | |
| SEM | 0.654 | 0.706 | 0.489 | |
| Shapiro–Wilk Normality Test | 0.55 | 0.326 | 0.088 | |
| Weight (kg) | Mean | 26.86 | 21.89 | 24 |
| Median | 24 | 21 | 21 | |
| Variance | 242.44 | 206.655 | 220.938 | |
| SD | 15.57 | 14.375 | 14.864 | |
| Minimum | 8 | 4 | 4 | |
| Maximum | 60 | 57 | 60 | |
| SEM | 4.161 | 3.298 | 2.587 | |
| Shapiro–Wilk Normality Test | 0.31 | 0.149 | 0.127 | |
| Time Points | T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | |||||||||||
| 1 | 6 | 2 | 2 | 1 | 1 | 1 | |||||
| 2 | 6 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| 3 | 3 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | ||
| 4 | 3 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | |||
| 5 | 3 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | ||
| 6 | 3 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | |||
| 7 | 3 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | ||
| 8 | 3 | 2 | 1 | 1 | 1 | 1 | 1 | ||||
| 9 | 3 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | |||
| 10 | 3 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | |
| 11 | 3 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | |||
| 12 | 3 | 2 | 1 | 1 | 1 | 1 | 1 | ||||
| 13 | 7 | 6 | 6 | 6 | 7 | 7 | 7 | ||||
| 14 | 7 | 6 | 7 | 7 | 7 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gouveia, D.; Cardoso, A.; Carvalho, C.; Gonçalves, A.R.; Gamboa, Ó.; Canejo-Teixeira, R.; Ferreira, A.; Martins, Â. Influence of Spinal Shock on the Neurorehabilitation of ANNPE Dogs. Animals 2022, 12, 1557. https://doi.org/10.3390/ani12121557
Gouveia D, Cardoso A, Carvalho C, Gonçalves AR, Gamboa Ó, Canejo-Teixeira R, Ferreira A, Martins Â. Influence of Spinal Shock on the Neurorehabilitation of ANNPE Dogs. Animals. 2022; 12(12):1557. https://doi.org/10.3390/ani12121557
Chicago/Turabian StyleGouveia, Débora, Ana Cardoso, Carla Carvalho, Ana Rita Gonçalves, Óscar Gamboa, Rute Canejo-Teixeira, António Ferreira, and Ângela Martins. 2022. "Influence of Spinal Shock on the Neurorehabilitation of ANNPE Dogs" Animals 12, no. 12: 1557. https://doi.org/10.3390/ani12121557






