Is Dairy Effluent an Alternative for Maize Crop Fertigation in Semiarid Regions? An Approach to Agronomic and Environmental Effects
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
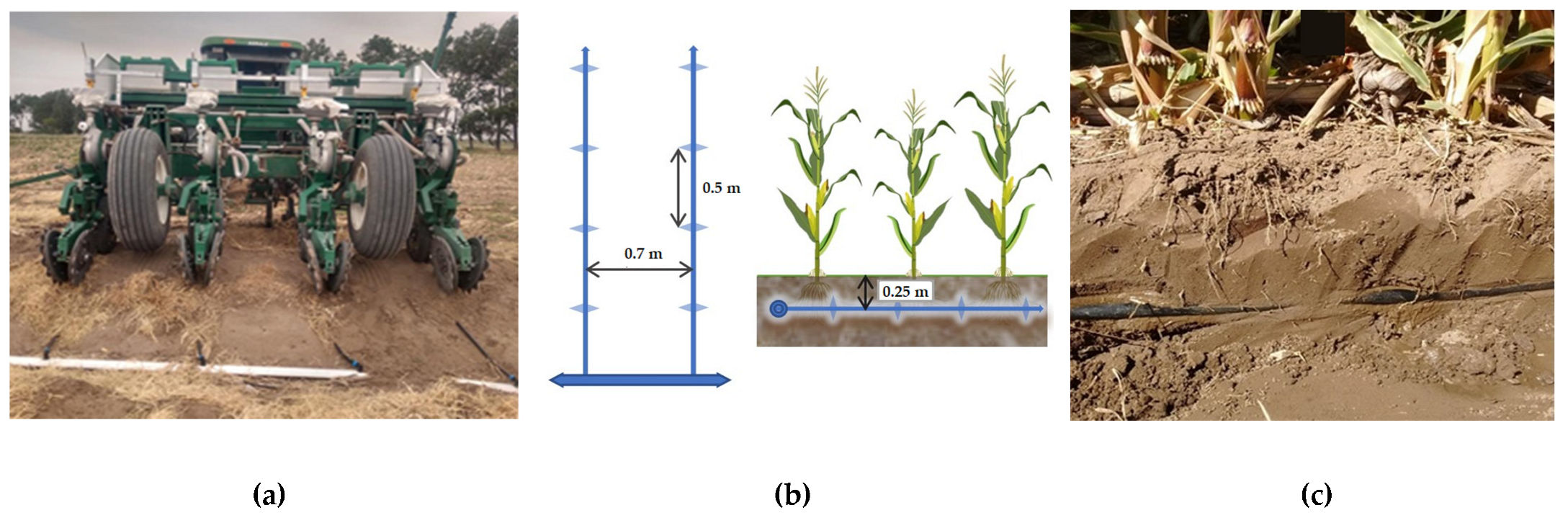
2.1. Experimental Soil and Crop Management
2.2. Weather Conditions and Subsurface Drip Irrigation
2.3. Experimental Design and Treatments
2.4. Dairy farm Description
2.5. GHG Measurements
2.6. Statistical Analysis
3. Results
3.1. Soil Parameters and Maize Yield
3.2. GHG Fluxes
4. Discussion
4.1. Effect of Fertigation Treatments on Soil Properties and Maize Yield
4.2. Effect of Fertigation Treatments on GHG Emissions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahmud, K.; Panday, D.; Mergoum, A.; Missaoui, A. Nitrogen Losses and Potential Mitigation Strategies for a Sustainable Agroecosystem. Sustainability 2021, 13, 2400. [Google Scholar] [CrossRef]
- Monteiro, A.; Santos, S.; Gonçalves, P. Precision Agriculture for Crop and Livestock Farming—Brief Review. Animals 2021, 11, 2345. [Google Scholar] [CrossRef] [PubMed]
- Teenstra, E.D.; Vellinga, T.V.; Aktasaeng, N.; Amatayaku, W.; Ndambi, A.; Pelster, D.; Germer, L.; Jenet, A.; Opio, C.; Andeweg, K. Global Asessment of Manure Management Policies and Practices; Wageningen UR Livestock Research: Wageningen, The Netherlands, 2014; p. 35. [Google Scholar]
- Mordenti, A.L.; Giaretta, E.; Campidonico, L.; Parazza, P.; Formigoni, A. A Review Regarding the Use of Molasses in Animal Nutrition. Animals 2021, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.I.M.; Wassie, S.E.; Joergensen, R.G.; Korir, D.; Goopy, J.P.; Butterbach-Bahl, K.; Merbold, L.; Dickhoefer, U.; Schlecht, E. Feed Quality and Feeding Level Effects on Faecal Composition in East African Cattle Farming Systems. Animals 2021, 11, 564. [Google Scholar] [CrossRef]
- Martín-Marroquín, J.M.; Hidalgo, D. Livestock Waste: Fears and Opportunities. In Environment, Energy and Climate Change I: Environmental Chemistry of Pollutants and Wastes; Springer International Publishing: Cham, Switzerland, 2014; pp. 341–373. ISBN 978-3-319-12907-5. [Google Scholar]
- Owen, J.J.; Silver, W.L. Greenhouse Gas Emissions from Dairy Manure Management: A Review of Field-Based Studies. Glob. Change Biol. 2015, 21, 550–565. [Google Scholar] [CrossRef] [Green Version]
- Sobhi, M.; Gaballah, M.S.; Han, T.; Cui, X.; Li, B.; Sun, H.; Guo, J.; Dong, R. Nutrients Recovery from Fresh Liquid Manure through an Airlift Reactor to Mitigate the Greenhouse Gas Emissions of Open Anaerobic Lagoons. J. Environ. Manag. 2021, 294, 112956. [Google Scholar] [CrossRef]
- Wang, Y.; Ghimire, S.; Wang, J.; Dong, R.; Li, Q. Alternative Management Systems of Beef Cattle Manure for Reducing Nitrogen Loadings: A Case-Study Approach. Animals 2021, 11, 574. [Google Scholar] [CrossRef]
- Menzi, H.; Oenema, O.; Burton, C.; Shipin, O.; Gerber, P.; Robinson, T.; Franceschini, G. Impacts of Intensive Livestock Production and Manure Management on the Environment. In Livestock in a Changing Landscape; Island Press: Washington, DC, USA, 2010; Volume 1, pp. 139–163. [Google Scholar]
- Huang, T.; Gao, B.; Hu, X.-K.; Lu, X.; Well, R.; Christie, P.; Bakken, L.R.; Ju, X.-T. Ammonia-Oxidation as an Engine to Generate Nitrous Oxide in an Intensively Managed Calcareous Fluvo-Aquic Soil. Sci. Rep. 2014, 4, 3950. [Google Scholar] [CrossRef]
- Huang, L.; Riggins, C.W.; Rodríguez-Zas, S.; Zabaloy, M.C.; Villamil, M.B. Long-Term N Fertilization Imbalances Potential N Acquisition and Transformations by Soil Microbes. Sci. Total Environ. 2019, 691, 562–571. [Google Scholar] [CrossRef]
- Scott, B.; Baldwin, A.H.; Yarwood, S.A. Quantification of Potential Methane Emissions Associated with Organic Matter Amendments Following Oxic-Soil Inundation. Biogeosciences 2022, 19, 1151–1164. [Google Scholar] [CrossRef]
- Becerra-Castro, C.; Lopes, A.R.; Vaz-Moreira, I.; Silva, E.F.; Manaia, C.M.; Nunes, O.C. Wastewater Reuse in Irrigation: A Microbiological Perspective on Implications in Soil Fertility and Human and Environmental Health. Environ. Int. 2015, 75, 117–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO. Review of Evidence on Drylands Pastoral Systems and Climate Change; Neely, C., Bunning, S., Wilkes, A., Eds.; Citeseer: Rome, Italy, 2009. [Google Scholar]
- FAO. Water and Cereals in Drylands, 1st ed.; Koohafkan, P., Ed.; Routledge: London, UK, 2008. [Google Scholar]
- Gelaw, A.M.; Singh, B.R.; Lal, R. Soil Organic Carbon and Total Nitrogen Stocks under Different Land Uses in a Semi-Arid Watershed in Tigray, Northern Ethiopia. Agric. Ecosyst. Environ. 2014, 188, 256–263. [Google Scholar] [CrossRef]
- Qadir, M.; Sharma, B.R.; Bruggeman, A.; Choukr-Allah, R.; Karajeh, F. Non-Conventional Water Resources and Opportunities for Water Augmentation to Achieve Food Security in Water Scarce Countries. Agric. Water Manag. 2007, 87, 2–22. [Google Scholar] [CrossRef]
- Lüneberg, K.; Schneider, D.; Siebe, C.; Daniel, R. Drylands Soil Bacterial Community Is Affected by Land Use Change and Different Irrigation Practices in the Mezquital Valley, Mexico. Sci. Rep. 2018, 8, 1413. [Google Scholar] [CrossRef] [PubMed]
- Fuertes, E.; Seradj, A.R.; Maynegre Santaularia, J.; Villalba Mata, D.; de la Fuente Oliver, G.; Balcells Teres, J. Annual Nitrogen Balance from Dairy Barns, Comparison between Cubicle and Compost-Bedded Pack Housing Systems in the Northeast of Spain. Animals 2021, 11, 2136. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Ren, L. Water and Nitrogen Distribution as Affected by Fertigation of Ammonium Nitrate from a Point Source. Irrig. Sci. 2003, 22, 19–30. [Google Scholar] [CrossRef]
- Incrocci, L.; Massa, D.; Pardossi, A. New Trends in the Fertigation Management of Irrigated Vegetable Crops. Horticulturae 2017, 3, 37. [Google Scholar] [CrossRef]
- Sanchez-Martín, L.; Meijide, A.; Garcia-Torres, L.; Vallejo, A. Combination of Drip Irrigation and Organic Fertilizer for Mitigating Emissions of Nitrogen Oxides in Semiarid Climate. Agric. Ecosyst. Environ. 2010, 137, 99–107. [Google Scholar] [CrossRef]
- Rodríguez, D.; Schulz, G.; Moretti, L. Carta de Suelos de la República Argentina: Partido de Villarino: Provincia de Buenos Aires, 1st ed.; Ediciones INTA: Buenos Aires, Argentina, 2018. [Google Scholar]
- Sánchez, R.; Pezzola, N.; Cepeda, J. Caracterización Edafoclimática Del Área de Influencia Del INTA. EEA Hilario Ascasubi. Boletín Divulg. 1998, 18, 72. [Google Scholar]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture; FAO Irrigation and Drainage Paper; FAO: Rome, Italy, 1994. [Google Scholar]
- Sanchez, R.M. Estimación de Los Requerimientos Hídricos de Los Principales Cultivos en El Valle Bonaerense Del Río Colorado. EEA Hilario Ascasubi. Informe Téc. 2013, 40. ISSN 0328-3399. Available online: https://www.fao.org/3/t0234e/t0234e00.htm (accessed on 5 August 2022).
- Ritchie, S.W.; Hanway, J.J.; Benson, G.O. How a Corn Plant Develops; Iowa State University of Science and Technology: Ames, IA, USA, 1986. [Google Scholar]
- Soil Survey Staff. Kellogg Soil Survey Laboratory Methods Manual. In Soil Survey Investigations Report No. 42, Version 5.0; Department of Agriculture, Natural Resources Conservation Service: Washington, DC, USA, 2014; pp. 1–219. [Google Scholar]
- Aboukila, E.; Abdelaty, E. Assessment of Saturated Soil Paste Salinity from 1:2.5 and 1:5 Soil-Water Extracts for Coarse Textured Soils. Alex. Sci. Exch. J. 2017, 38, 722–732. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1996; pp. 961–1010. ISBN 978-0-89118-866-7. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen-Total. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1983; pp. 595–624. ISBN 978-0-89118-977-0. [Google Scholar]
- Iocoli, G.A.; Orden, L.; López, F.M.; Gómez, M.A.; Villamil, M.B.; Zabaloy, M.C. Towards Sustainable Dairy Production in Argentina: Evaluating Nutrient and CO2 Release from Raw and Processed Farm Waste. Agronomy 2021, 11, 2595. [Google Scholar] [CrossRef]
- Parkin, T.B.; Venterea, R.T. Chamber-Based Trace Gas Flux Measurements. In Sampling Protocols; Follett, R.F., Ed.; US Department of Agriculture, Agricultural Research Service: Beltsville, MD, USA, 2010; pp. 3-1–3-39. [Google Scholar]
- Vico, A.; Sáez, J.A.; Pérez-Murcia, M.D.; Martinez-Tomé, J.; Andreu-Rodríguez, J.; Agulló, E.; Bustamante, M.A.; Sanz-Cobena, A.; Moral, R. Production of Spinach in Intensive Mediterranean Horticultural Systems Can Be Sustained by Organic-Based Fertilizers without Yield Penalties and with Low Environmental Impacts. Agric. Syst. 2020, 178, 102765. [Google Scholar] [CrossRef]
- Lombardi, B.; Alvarado, P.I.; Ricci, P.; Guzmán, S.A.; Gonda, H.L.; Juliarena, M.P. Methane and Nitrous Oxide Emissions from Dung Patches Deposited by Grazing Cattle Supplemented with Maize Grain. Anim. Feed Sci. Technol. 2021, 279, 115029. [Google Scholar] [CrossRef]
- Parkin, T.B.; Venterea, R.T.; Hargreaves, S.K. Calculating the Detection Limits of Chamber-Based Soil Greenhouse Gas Flux Measurements. J. Environ. Qual. 2012, 41, 705–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IPCC Chapter 7: The Earth’s Energy Budget, Climate Feedbacks and Climate Sensitivity. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: New York, NY, USA, 2021; p. 132.
- IPCC Volume 4: Agriculture, Forestry and Other Land Use. In 2019 Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Calvo Buendia, E.; Tanabe, K.; Kranjc, A.; Baasansuren, J.; Fukuda, M.; Ngarize, S.; Osako, A.; Pyrozhenko, Y.; Shermanau, P.; Federici, S. (Eds.) IPCC: Geneva, Switzerland, 2019; Volume 4, ISBN 978-4-88788-232-4. [Google Scholar]
- Di Rienzo, J.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat; InfoStat Transfer Center, FCA, Univ. Nac.: Córdoba, Argentina, 2020. [Google Scholar]
- Maas, E.V.; Hoffman, G.J. Crop Salt Tolerance—Current Assessment. J. Irrig. Drain. Div. 1977, 103, 115–134. [Google Scholar] [CrossRef]
- Hawke, R.M.; Summers, S.A. Effects of Land Application of Farm Dairy Effluent on Soil Properties: A Literature Review. N. Z. J. Agric. Res. 2006, 49, 307–320. [Google Scholar] [CrossRef]
- Goss, M.J.; Tubeileh, A.; Goorahoo, D. A Review of the Use of Organic Amendments and the Risk to Human Health. Adv. Agron. 2013, 120, 275–379. [Google Scholar]
- Saggar, S.; Bolan, N.S.; Bhandral, R.; Hedley, C.B.; Luo, J. A Review of Emissions of Methane, Ammonia, and Nitrous Oxide from Animal Excreta Deposition and Farm Effluent Application in Grazed Pastures. N. Z. J. Agric. Res. 2004, 47, 513–544. [Google Scholar] [CrossRef] [Green Version]
- Thompson, T.L.; Roberts, T.; Lazarovitch, N. Managing Soil Surface Salinity with Subsurface Drip Irrigation. In Proceedings of the 19th World Congress of Soil Science, Soil Solutions for a Changing World, Brisbane, Australia, 1—6 August 2010; Volume 6. [Google Scholar]
- Varela, P.; Dunel, L.; Storniolo, R. Dinámica de Las Sales en Un Suelo Bajo Riego Por Goteo Subterráneo. In Proceedings of the VI Congreso de Salinidad, Los diferentes medios salinos y alcalinos y el análisis de su desafío en diferentes escalas de percepción, Buenos Aires, Argentina, 22—25 July 2019; pp. 84–89, ISBN 978-987-46433-1-5. [Google Scholar]
- Mitchell, W.H. Subsurface Irrigation and Fertilization of Field Corn. Agron. J. 1981, 73, 913–916. [Google Scholar] [CrossRef]
- Lamm, F.R.; Trooien, T.P. Subsurface Drip Irrigation for Corn Production: A Review of 10-Years of Research in Kansas. Irrig. Sci. 2003, 22, 195–200. [Google Scholar] [CrossRef]
- Varela, P. Análisis Del Impacto Del Riego Por Goteo Subterráneo Para Maíz en El Valle Bonaerense Del Río Colorado; EEA Hilario Ascasubi. Informe Téc. 2017, 53. ISSN 0328-3399. Available online: https://inta.gob.ar/sites/default/files/inta.ascasubi-riego.goteo_.subterraneo.maiz_.vbrc_.pdf (accessed on 5 August 2022).
- MAGyP. Producción Granaria Argentina Y El Consumo de Fertilizante Por Cultivo; Subsecretaría de Mercados Agropecuarios Ediciones; Buenos Aires, Argentina, 2020; p 32. Available online: https://www.magyp.gob.ar/sitio/areas/ss_mercados_agropecuarios/publicaciones/_archivos/000101_Perfiles/999975_Utilizaci%C3%B3n%20de%20Fertilizantes%20Campa%C3%B1a%202019-2020.pdf (accessed on 5 August 2022).
- Severin, M.; Fuß, R.; Well, R.; Garlipp, F.; Van den Weghe, H. Soil, Slurry and Application Effects on Greenhouse Gas Emissions. Plant Soil Environ. 2015, 61, 344–351. [Google Scholar] [CrossRef] [Green Version]
- Viguria, M.; Sanz-Cobeña, A.; López, D.M.; Arriaga, H.; Merino, P. Ammonia and Greenhouse Gases Emission from Impermeable Covered Storage and Land Application of Cattle Slurry to Bare Soil. Agric. Ecosyst. Environ. 2015, 199, 261–271. [Google Scholar] [CrossRef]
- Velthof, G.L.; Mosquera, J. The Impact of Slurry Application Technique on Nitrous Oxide Emission from Agricultural Soils. Agric. Ecosyst. Environ. 2011, 140, 298–308. [Google Scholar] [CrossRef]
- Sommer, S.G.; Clough, T.J.; Chadwick, D.; Petersen, S.O. Greenhouse Gas Emissions from Animal Manures and Technologies for Their Reduction. In Animal Manure Recycling: Treatment and Management; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 177–194. ISBN 1-118-48853-9. [Google Scholar]
- Sherlock, R.R.; Sommer, S.G.; Khan, R.Z.; Wood, C.W.; Guertal, E.A.; Freney, J.R.; Dawson, C.O.; Cameron, K.C. Ammonia, Methane, and Nitrous Oxide Emission from Pig Slurry Applied to a Pasture in New Zealand. J. Environ. Qual. 2002, 31, 1491–1501. [Google Scholar] [CrossRef] [Green Version]
- Le Mer, J.; Roger, P. Production, Oxidation, Emission and Consumption of Methane by Soils: A Review. Eur. J. Soil Biol. 2001, 37, 25–50. [Google Scholar] [CrossRef]
- Bertilsson, G.O.B.; Kirchmann, H. Sustainable N Fertilizer Production Based on a Loop: Straw—Biogas—‘Haber-Bosch’ Process. Agric. Syst. 2021, 190, 103100. [Google Scholar] [CrossRef]
- McCarty, P.L.; Bae, J.; Kim, J. Domestic Wastewater Treatment as a Net Energy Producer–Can This Be Achieved? Environ. Sci. Technol. 2011, 45, 7100–7106. [Google Scholar] [CrossRef]
- Hoxha, A.; Christensen, B. The Carbon Footprint of Fertiliser Production: Regional Reference Values. In Proceedings of the Proceedings—International Fertiliser Society, Prague, Czech Republic, 8 May 2019; pp. 1–20. [Google Scholar]
- Mainardis, M.; Cecconet, D.; Moretti, A.; Callegari, A.; Goi, D.; Freguia, S.; Capodaglio, A.G. Wastewater Fertigation in Agriculture: Issues and Opportunities for Improved Water Management and Circular Economy. Environ. Pollut. 2022, 296, 118755. [Google Scholar] [CrossRef]
- Jiménez-Benítez, A.; Ferrer, F.J.; Greses, S.; Ruiz-Martínez, A.; Fatone, F.; Eusebi, A.L.; Mondéjar, N.; Ferrer, J.; Seco, A. AnMBR, Reclaimed Water and Fertigation: Two Case Studies in Italy and Spain to Assess Economic and Technological Feasibility and CO2 Emissions within the EU Innovation Deal Initiative. J. Clean. Prod. 2020, 270, 122398. [Google Scholar] [CrossRef]
- Scheierling, S.M.; Bartone, C.R.; Mara, D.D.; Drechsel, P. Towards an Agenda for Improving Wastewater Use in Agriculture. Water Int. 2011, 36, 420–440. [Google Scholar] [CrossRef]
- IPCC Volume 4: Agriculture, Forestry and Other Land Use. In 2006 IPCC Guidelines for National Greenhouse Gas Inventories, Prepared by the National Greenhouse Gas Inventories Programme; Eggleston, H.S.; Buendia, L.; Miwa, K.; Ngara, T.; Tanabe, K. (Eds.) IGES: Kanagawa, Japan, 2006; Volume 4, ISBN 4-88788-032-4. [Google Scholar]
- van der Weerden, T.J.; Cox, N.; Luo, J.; Di, H.J.; Podolyan, A.; Phillips, R.L.; Saggar, S.; de Klein, C.A.M.; Ettema, P.; Rys, G. Refining the New Zealand Nitrous Oxide Emission Factor for Urea Fertiliser and Farm Dairy Effluent. Agric. Ecosyst. Environ. 2016, 222, 133–137. [Google Scholar] [CrossRef]
- Cayuela, M.L.; Aguilera, E.; Sanz-Cobena, A.; Adams, D.C.; Abalos, D.; Barton, L.; Ryals, R.; Silver, W.L.; Alfaro, M.A.; Pappa, V.A.; et al. Direct Nitrous Oxide Emissions in Mediterranean Climate Cropping Systems: Emission Factors Based on a Meta-Analysis of Available Measurement Data. Agric. Ecosyst. Environ. 2017, 238, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, T.L.; Suddick, E.C.; Six, J. Reduced Nitrous Oxide Emissions and Increased Yields in California Tomato Cropping Systems under Drip Irrigation and Fertigation. Agric. Ecosyst. Environ. 2013, 170, 16–27. [Google Scholar] [CrossRef]


| Historical Rainfall | Rainfall | ET | Irrigation | Balance | |
|---|---|---|---|---|---|
| Months | (mm) | ||||
| November | 44.0 | 39.7 | 47.4 | 0.0 | −7.7 |
| December | 52.0 | 68.2 | 149.2 | 81.6 | 0.6 |
| January | 49.4 | 37.6 | 190.4 | 142.0 | −10.8 |
| February | 55.9 | 2.0 | 134.2 | 136.0 | 3.8 |
| March | 62.4 | 18.3 | 29.5 | 20.4 | 9.2 |
| TOTAL | 263.3 | 215.3 | 562.7 | 380.0 | 32.6 |
| pH | EC | Na+ | HCO3− | Cl− | SAR |
|---|---|---|---|---|---|
| (dS m−1) | (meq L−1) | (meq L−1) | (meq L−1) | ||
| 8.21 | 1.89 | 7.61 | 1.87 | 6.58 | 3.87 |
| pH | EC | C | TKN | NO3−-N | NH4+-N | eP | TP | TS | VS | |
|---|---|---|---|---|---|---|---|---|---|---|
| (dS m−1) | (mg L−1) | |||||||||
| DE | 7.8 | 4.73 | 663 | 672 | 10.10 | 215 | 1.52 | 111 | 3070 | 859 |
| N2O | CH4 | CO2eq 1 | |
|---|---|---|---|
| Treatment | (mg m−2) | (mg m−2) | (kg ha−1) |
| Control | 5.3 a | −42 a | 2.9 a |
| Dairy Effluent | 38.5 c | 165 b | 149.9 b |
| Urea | 14.5 b | −53 a | 25.1 a |
| SE2 | 2.7 | 22 | 12.3 |
| p-value | <0.0001 | 0.0001 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombardi, B.; Orden, L.; Varela, P.; Garay, M.; Iocoli, G.A.; Montenegro, A.; Sáez-Tovar, J.; Bustamante, M.Á.; Juliarena, M.P.; Moral, R. Is Dairy Effluent an Alternative for Maize Crop Fertigation in Semiarid Regions? An Approach to Agronomic and Environmental Effects. Animals 2022, 12, 2025. https://doi.org/10.3390/ani12162025
Lombardi B, Orden L, Varela P, Garay M, Iocoli GA, Montenegro A, Sáez-Tovar J, Bustamante MÁ, Juliarena MP, Moral R. Is Dairy Effluent an Alternative for Maize Crop Fertigation in Semiarid Regions? An Approach to Agronomic and Environmental Effects. Animals. 2022; 12(16):2025. https://doi.org/10.3390/ani12162025
Chicago/Turabian StyleLombardi, Banira, Luciano Orden, Patricio Varela, Maximiliano Garay, Gastón Alejandro Iocoli, Agustín Montenegro, José Sáez-Tovar, María Ángeles Bustamante, María Paula Juliarena, and Raul Moral. 2022. "Is Dairy Effluent an Alternative for Maize Crop Fertigation in Semiarid Regions? An Approach to Agronomic and Environmental Effects" Animals 12, no. 16: 2025. https://doi.org/10.3390/ani12162025







