Characteristics of tRNA-Derived Small RNAs and microRNAs Associated with Immunocompromise in an Intrauterine Growth-Restricted Pig Model
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Sample Collection
2.3. Peripheral Blood T-lymphocyte Subset Analysis and Cytokine Tests
2.4. miRNA and tsRNA Sequencing
2.5. Reverse Transcription-Quantitative PCR (RT-qPCR)
2.6. Target Gene Prediction and GO- and KEGG-Enrichment Analysis
2.7. Statistical Analysis
3. Results
3.1. Spleen and Immune Indicators of Intrauterine Growth-Restricted Pig Model
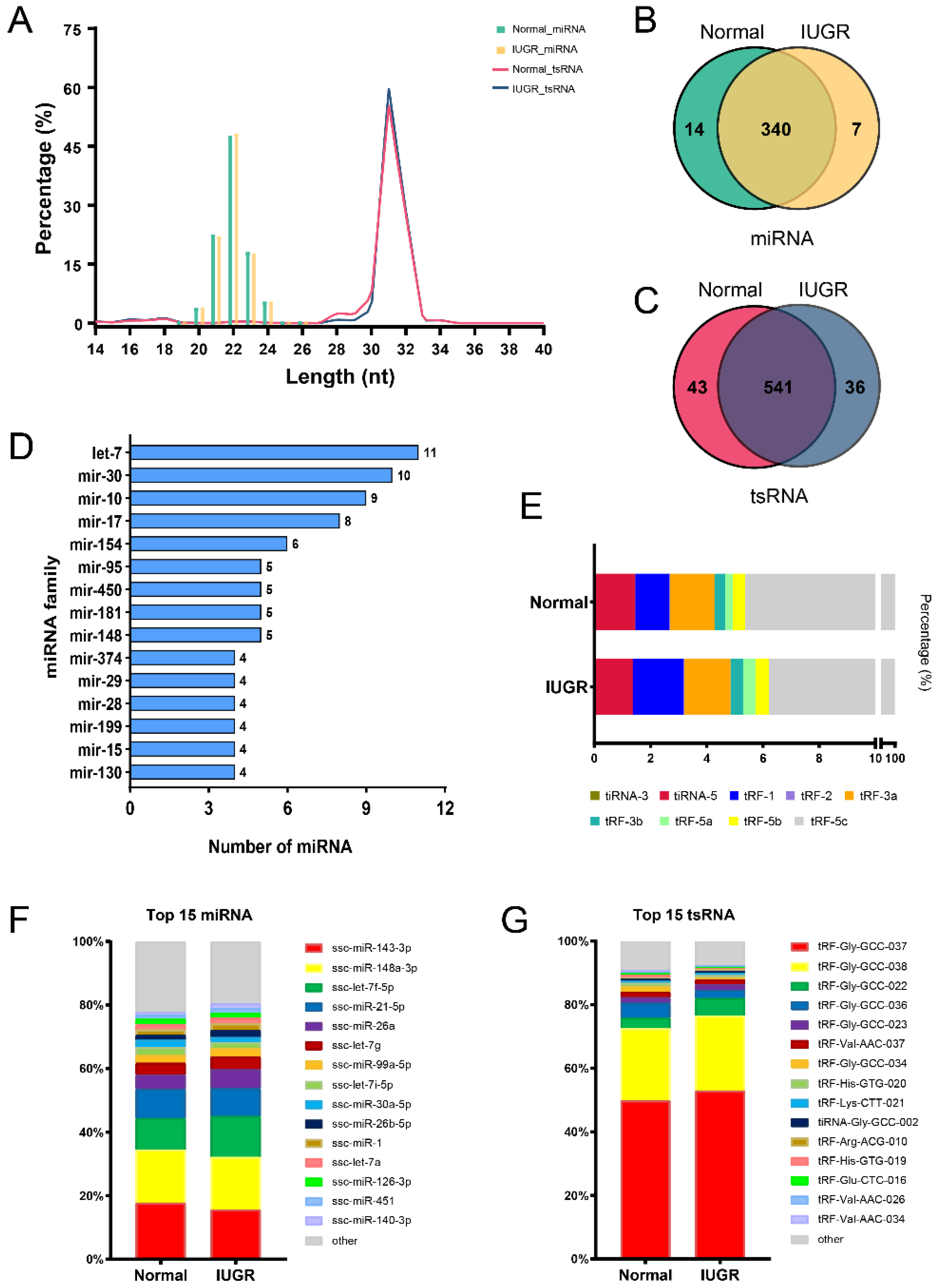
3.2. Characteristics of miRNA and tsRNA Profiling of Pig Spleen
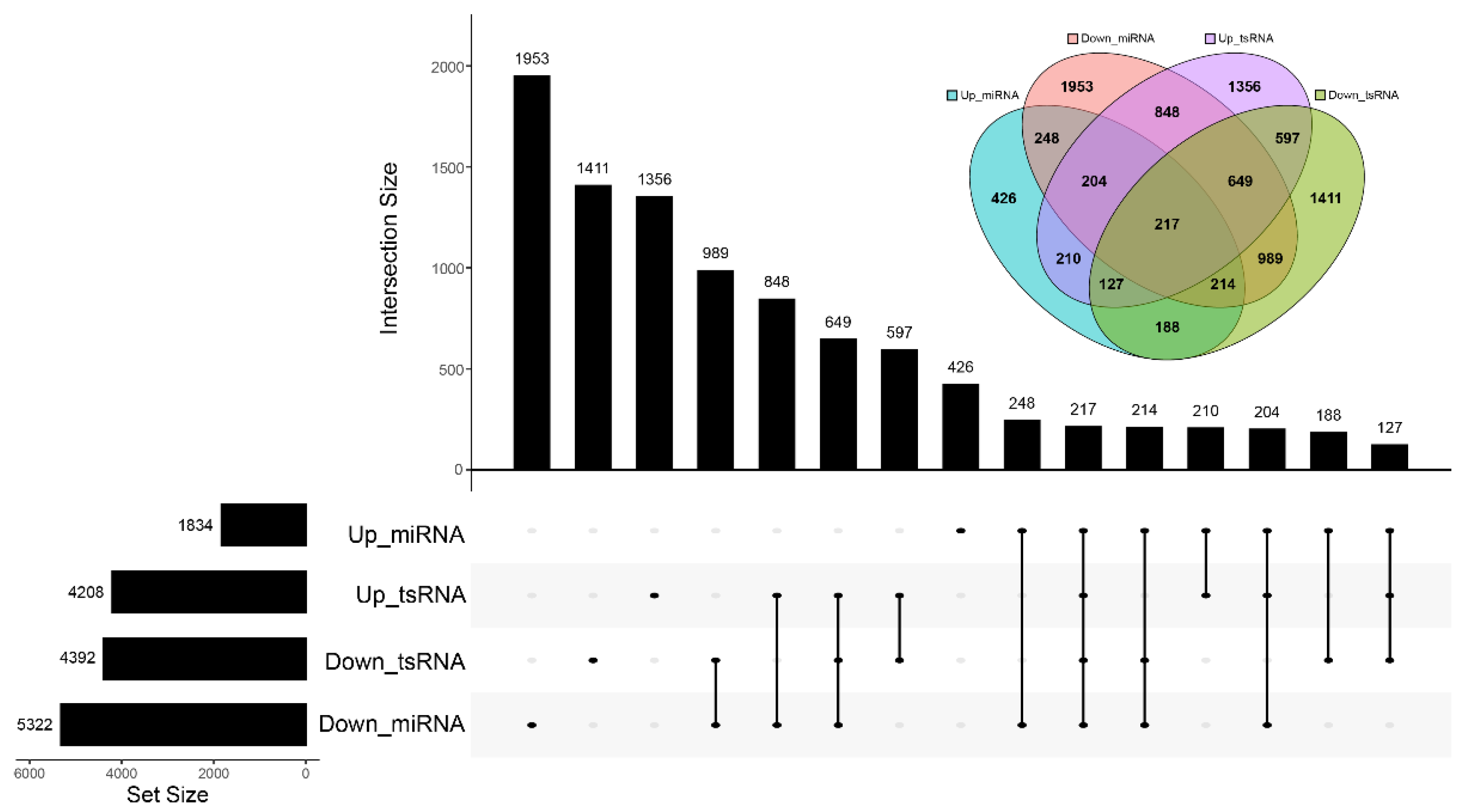
3.3. Differentially Expressed miRNAs and tsRNAs between IUGR and Normal Pig Spleen
3.4. Prediction of Target Genes and Functional Enrichment Analysis of Differentially Expressed miRNAs and tsRNAs
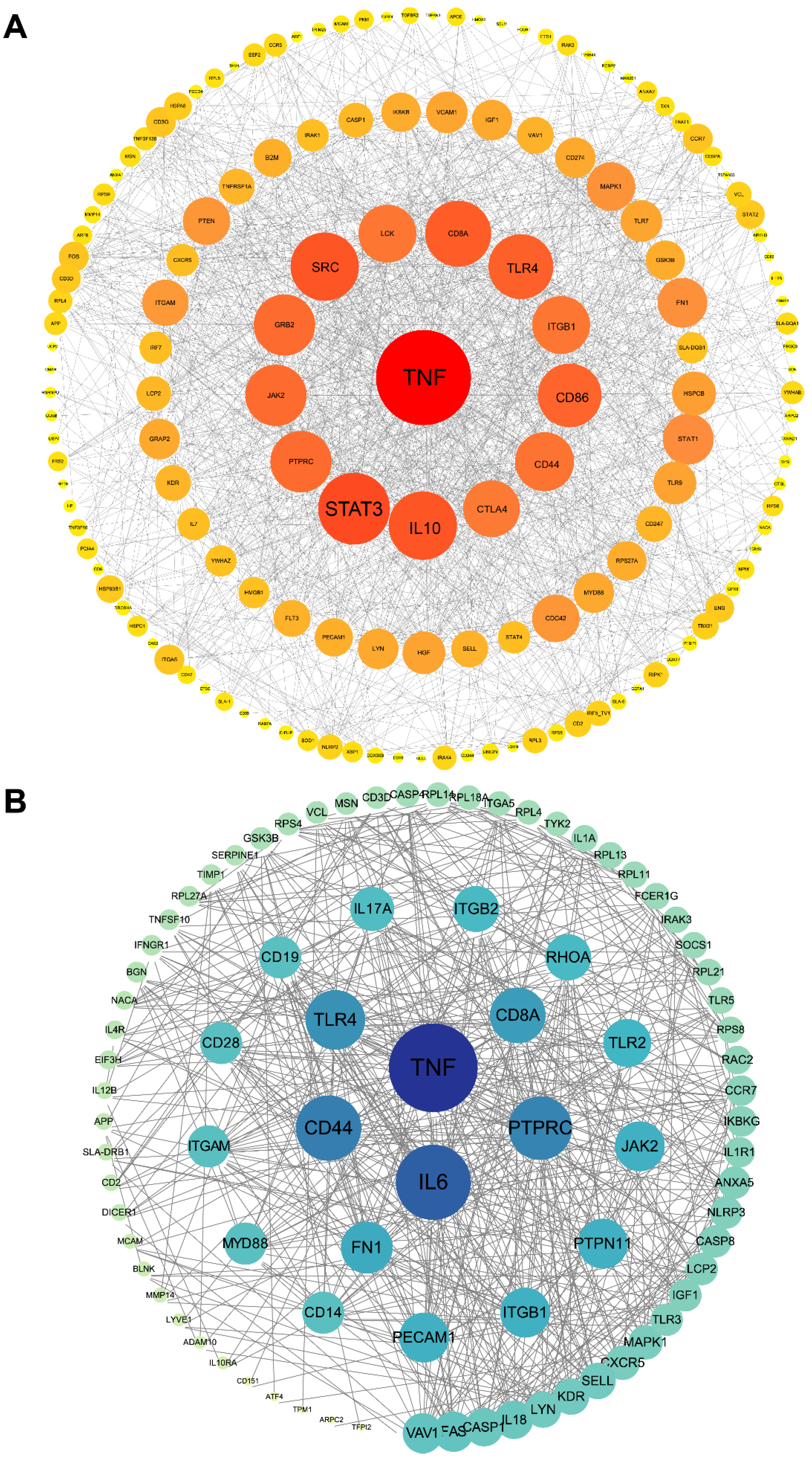
3.5. Protein–Protein Interaction (PPI) Network Construction of Target Genes
3.6. Pathway Regulation Network Related to the Immune System of IUGR Pigs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, J.; Fang, M.; Zhuang, S.; Qiao, Y.; Huang, W.; Gong, Q.; Xu, D.; Zhang, Y.; Wang, H. Prenatal Dexamethasone Exposure Exerts Sex-Specific Effect on Placental Oxygen and Nutrient Transport Ascribed to the Differential Expression of IGF2. Ann. Transl. Med. 2020, 8, 233. [Google Scholar] [CrossRef] [PubMed]
- Barut, F.; Barut, A.; Gun, B.D.; Kandemir, N.O.; Harma, M.I.; Harma, M.; Aktunc, E.; Ozdamar, S.O. Intrauterine Growth Restriction and Placental Angiogenesis. Diagn. Pathol. 2010, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Darendeliler, F. IUGR: Genetic Influences, Metabolic Problems, Environmental Associations/Triggers, Current and Future Management. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101260. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, M.M.; Araújo, F.d.V.; de Carvalho, P.R.N.; de Sá, R.A.M. Aortic Isthmus Doppler Velocimetry in Fetuses with Intrauterine Growth Restriction: A Literature Review. Rev. Bras. Ginecol. Obs. 2020, 42, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bazer, F.W.; Wallace, J.M.; Spencer, T.E. BOARD-INVITED REVIEW: Intrauterine Growth Retardation: Implications for the Animal Sciences. J. Anim. Sci. 2006, 84, 2316–2337. [Google Scholar] [CrossRef]
- Pallotto, E.K.; Kilbride, H.W. Perinatal Outcome and Later Implications of Intrauterine Growth Restriction. Clin. Obstet. Gynecol. 2006, 49, 257–269. [Google Scholar] [CrossRef]
- Aly, H.; Davies, J.; El-Dib, M.; Massaro, A. Renal Function Is Impaired in Small for Gestational Age Premature Infants. J. Matern. -Fetal Neonatal Med. 2013, 26, 388–391. [Google Scholar] [CrossRef]
- Zanardo, V.; Bertin, M.; de Luca, F.; Zaninotto, M.; Trevisanuto, D.; Cosmi, E. Albuminuria and Sodiuria in IUGR Children. J. Matern. Fetal Neonatal Med. 2015, 28, 362–365. [Google Scholar] [CrossRef]
- Hu, L.; Liu, Y.; Yan, C.; Peng, X.; Xu, Q.; Xuan, Y.; Han, F.; Tian, G.; Fang, Z.; Lin, Y.; et al. Postnatal Nutritional Restriction Affects Growth and Immune Function of Piglets with Intra-Uterine Growth Restriction. Br. J. Nutr. 2015, 114, 53–62. [Google Scholar] [CrossRef]
- Stojanovska, V.; Sharma, N.; Dijkstra, D.J.; Scherjon, S.A.; Jäger, A.; Schorle, H.; Plösch, T. Placental Insufficiency Contributes to Fatty Acid Metabolism Alterations in Aged Female Mouse Offspring. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R1107–R1114. [Google Scholar] [CrossRef]
- Chen, L.; Yue, J.; Han, X.; Li, J.; Hu, Y. Ouabain Rescues Rat Nephrogenesis during Intrauterine Growth Restriction by Regulating the Complement and Coagulation Cascades and Calcium Signaling Pathway. J. Dev. Orig. Health Dis. 2016, 7, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Cromi, A.; Ghezzi, F.; Raffaelli, R.; Bergamini, V.; Siesto, G.; Bolis, P. Ultrasonographic Measurement of Thymus Size in IUGR Fetuses: A Marker of the Fetal Immunoendocrine Response to Malnutrition. Ultrasound Obstet. Gynecol. 2009, 33, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Liu, Y.; Zhang, C.; Zhang, Z.; Song, S. Effect of Intrauterine Growth Restriction during Late Pregnancy on the Growth Performance, Blood Components, Immunity and Anti-Oxidation Capability of Ovine Fetus. Livest. Sci. 2013, 155, 435–441. [Google Scholar] [CrossRef]
- Ding, D.; Mou, D.; Zhao, L.; Jiang, X.; Che, L.; Fang, Z.; Xu, S.; Lin, Y.; Zhuo, Y.; Li, J.; et al. Maternal Organic Selenium Supplementation Alleviates LPS Induced Inflammation, Autophagy and ER Stress in the Thymus and Spleen of Offspring Piglets by Improving the Expression of Selenoproteins. Food Funct. 2021, 12, 11214–11228. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, H.; Meng, X.; Hao, L.; Fu, Y.; Fang, L.; Shen, D.; Yu, X.; Li, J. Immunomodulatory Effects of Cinobufagin on Murine Lymphocytes and Macrophages. Evid. Based Complementary Altern. Med. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bai, K.; Jiang, L.; Li, Q.; Zhang, J.; Zhang, L.; Wang, T. Dietary Dimethylglycine Sodium Salt Supplementation Alleviates Redox Status Imbalance and Intestinal Dysfunction in Weaned Piglets with Intrauterine Growth Restriction. Anim. Nutr. 2022, 10, 188–197. [Google Scholar] [CrossRef]
- Tang, X.; Xiong, K. Intrauterine Growth Retardation Affects Intestinal Health of Suckling Piglets via Altering Intestinal Antioxidant Capacity, Glucose Uptake, Tight Junction, and Immune Responses. Oxidative Med. Cell. Longev. 2022, 2022, 1–12. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Pathophysiology of Placental-Derived Fetal Growth Restriction. Am. J. Obstet. Gynecol. 2018, 218, S745–S761. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, L.; Wang, L.; Liu, X.; Hou, X.; Zhao, F.; Yan, H.; Wang, L. Liver Transcriptome Profiling and Functional Analysis of Intrauterine Growth Restriction (IUGR) Piglets Reveals a Genetic Correction and Sexual-Dimorphic Gene Expression during Postnatal Development. BMC Genom. 2020, 21, 701. [Google Scholar] [CrossRef]
- Kakadia, J.H.; Jain, B.B.; Biggar, K.; Sutherland, A.; Nygard, K.; Li, C.; Nathanielsz, P.W.; Jansson, T.; Gupta, M.B. Hyperphosphorylation of Fetal Liver IGFBP-1 Precedes Slowing of Fetal Growth in Nutrient-Restricted Baboons and May Be a Mechanism Underlying IUGR. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E614–E628. [Google Scholar] [CrossRef]
- Ali, A.; Murani, E.; Hadlich, F.; Liu, X.; Wimmers, K.; Ponsuksili, S. Prenatal Skeletal Muscle Transcriptome Analysis Reveals Novel MicroRNA-MRNA Networks Associated with Intrauterine Growth Restriction in Pigs. Cells 2021, 10, 1007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, Y.; Wang, M.; Elsabagh, M.; Loor, J.J.; Wang, H. Dietary Supplementation of L-Arginine and N-Carbamylglutamate Enhances Duodenal Barrier and Mitochondrial Functions and Suppresses Duodenal Inflammation and Mitophagy in Suckling Lambs Suffering from Intrauterine-Growth-Restriction. Food Funct. 2020, 11, 4456–4470. [Google Scholar] [CrossRef] [PubMed]
- Bai, K.; Jiang, L.; Li, Q.; Zhang, J.; Zhang, L.; Wang, T. Dietary Dimethylglycine Sodium Salt Supplementation Improves Growth Performance, Redox Status, and Skeletal Muscle Function of Intrauterine Growth-Restricted Weaned Piglets. J. Anim. Sci. 2021, 99, skab186. [Google Scholar] [CrossRef]
- Chen, Y.; Hong, T.; Wang, S.; Mo, J.; Tian, T.; Zhou, X. Epigenetic Modification of Nucleic Acids: From Basic Studies to Medical Applications. Chem. Soc. Rev. 2017, 46, 2844–2872. [Google Scholar] [CrossRef]
- Dong, Y.; Xu, S.; Liu, J.; Ponnusamy, M.; Zhao, Y.; Zhang, Y.; Wang, Q.; Li, P.; Wang, K. Non-Coding RNA-Linked Epigenetic Regulation in Cardiac Hypertrophy. Int. J. Biol. Sci. 2018, 14, 1133–1141. [Google Scholar] [CrossRef]
- Rupaimoole, R.; Slack, F.J. MicroRNA Therapeutics: Towards a New Era for the Management of Cancer and Other Diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, W.; Yuan, T.; Fu, L.; Zhou, L.; Lin, G.; Zhao, S.; Zhou, H.; Wu, G.; Wang, J. MicroRNA-29a Mediates the Impairment of Intestinal Epithelial Integrity Induced by Intrauterine Growth Restriction in Pig. Am. J. Physiol. -Gastrointest. Liver Physiol. 2017, 312, G434–G442. [Google Scholar] [CrossRef]
- Saget, S.; Cong, R.; Decourtye, L.; Endale, M.-L.; Martinerie, L.; Girardet, C.; Perret, C.; Clemessy, M.; Leneuve, P.; Dinard, L.; et al. Changes in Circulating MiRNA19a-3p Precede Insulin Resistance Programmed by Intra-Uterine Growth Retardation in Mice. Mol. Metab. 2020, 42, 101083. [Google Scholar] [CrossRef]
- Shen, L.; Tan, Z.; Gan, M.; Li, Q.; Chen, L.; Niu, L.; Jiang, D.; Zhao, Y.; Wang, J.; Li, X.; et al. TRNA-Derived Small Non-Coding RNAs as Novel Epigenetic Molecules Regulating Adipogenesis. Biomolecules 2019, 9, 274. [Google Scholar] [CrossRef]
- Borek, E.; Baliga, B.S.; Gehrke, C.W.; Kuo, C.W.; Belman, S.; Troll, W.; Waalkes, T.P. High Turnover Rate of Transfer RNA in Tumor Tissue. Cancer Res. 1977, 37, 3362–3366. [Google Scholar]
- Speerm, J.; Gehrkep, C.; Kuo, K.; Phillipwaalkesm, T. TRNA Breakdown Products as Markers for Cancer. Cancer 1979, 44, 2120–2123. [Google Scholar] [CrossRef]
- Goodarzi, H.; Liu, X.; Nguyen, H.C.B.; Zhang, S.; Fish, L.; Tavazoie, S.F. Endogenous TRNA-Derived Fragments Suppress Breast Cancer Progression via YBX1 Displacement. Cell 2015, 161, 790–802. [Google Scholar] [CrossRef]
- Couvillion, M.T.; Bounova, G.; Purdom, E.; Speed, T.P.; Collins, K. A Tetrahymena Piwi Bound to Mature TRNA 3′ Fragments Activates the Exonuclease Xrn2 for RNA Processing in the Nucleus. Mol. Cell 2012, 48, 509–520. [Google Scholar] [CrossRef]
- Lyons, S.M.; Gudanis, D.; Coyne, S.M.; Gdaniec, Z.; Ivanov, P. Identification of Functional Tetramolecular RNA G-Quadruplexes Derived from Transfer RNAs. Nat. Commun. 2017, 8, 1127. [Google Scholar] [CrossRef]
- Venkatesh, T.; Suresh, P.S.; Tsutsumi, R. TRFs: MiRNAs in Disguise. Gene 2016, 579, 133–138. [Google Scholar] [CrossRef]
- Zong, T.; Yang, Y.; Zhao, H.; Li, L.; Liu, M.; Fu, X.; Tang, G.; Zhou, H.; Aung, L.H.H.; Li, P.; et al. TsRNAs: Novel Small Molecules from Cell Function and Regulatory Mechanism to Therapeutic Targets. Cell Prolif. 2021, 54, e12977. [Google Scholar] [CrossRef]
- Whyte, J.J.; Prather, R.S. Genetic Modifications of Pigs for Medicine and Agriculture: GENETIC MODIFICATIONS OF PIGS. Mol. Reprod. Dev. 2011, 78, 879–891. [Google Scholar] [CrossRef]
- Morise, A.; Sève, B.; Macé, K.; Magliola, C.; Le Huërou-Luron, I.; Louveau, I. Impact of Intrauterine Growth Retardation and Early Protein Intake on Growth, Adipose Tissue, and the Insulin-Like Growth Factor System in Piglets. Pediatr. Res. 2009, 65, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Bulnes, A.; Astiz, S.; Parraguez, V.H.; Garcia-Contreras, C.; Vazquez-Gomez, M. Empowering Translational Research in Fetal Growth Restriction: Sheep and Swine Animal Models. CPB 2016, 17, 848–855. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, X.; Jiang, G.; Zhang, H.; Ge, L.; Chen, F.; Li, J.; Liu, H.; Wang, H. 5′-TRF-GlyGCC: A TRNA-Derived Small RNA as a Novel Biomarker for Colorectal Cancer Diagnosis. Genome Med. 2021, 13, 20. [Google Scholar] [CrossRef]
- Gan, M.; Liu, L.; Zhang, S.; Guo, Z.; Tan, Y.; Luo, J.; Yang, Q.; Pan, H.; Li, X.; Wang, J.; et al. Expression Characteristics of MicroRNA in Pig Umbilical Venous Blood and Umbilical Arterial Blood. Animals 2021, 11, 1563. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.P.; Lowe, T.M. GtRNAdb 2.0: An Expanded Database of Transfer RNA Genes Identified in Complete and Draft Genomes. Nucleic Acids Res. 2016, 44, D184–D189. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.P.; Lin, B.Y.; Mak, A.J.; Lowe, T.M. TRNAscan-SE 2.0: Improved Detection and Functional Classification of Transfer RNA Genes. Nucleic Acids Res. 2021, 49, 9077–9096. [Google Scholar] [CrossRef]
- Kim, H.K.; Fuchs, G.; Wang, S.; Wei, W.; Zhang, Y.; Park, H.; Roy-Chaudhuri, B.; Li, P.; Xu, J.; Chu, K.; et al. A Transfer-RNA-Derived Small RNA Regulates Ribosome Biogenesis. Nature 2017, 552, 57–62. [Google Scholar] [CrossRef]
- Qin, C.; Xu, P.-P.; Zhang, X.; Zhang, C.; Liu, C.-B.; Yang, D.-G.; Gao, F.; Yang, M.-L.; Du, L.-J.; Li, J.-J. Pathological Significance of TRNA-Derived Small RNAs in Neurological Disorders. Neural Regen. Res. 2020, 15, 212. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Shastri, S.; Sharma, P. Intrauterine Growth Restriction: Antenatal and Postnatal Aspects. Clin. Med. Insights Pediatr. 2016, 10, CMPed.S40070. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Gan, M.; Zhang, S.; Ma, J.; Tang, G.; Jiang, Y.; Li, M.; Wang, J.; Li, X.; Che, L.; et al. Transcriptome Analyses Reveal Adult Metabolic Syndrome With Intrauterine Growth Restriction in Pig Models. Front. Genet. 2018, 9, 291. [Google Scholar] [CrossRef] [PubMed]
- Crispi, F.; Miranda, J.; Gratacós, E. Long-Term Cardiovascular Consequences of Fetal Growth Restriction: Biology, Clinical Implications, and Opportunities for Prevention of Adult Disease. Am. J. Obstet. Gynecol. 2018, 218, S869–S879. [Google Scholar] [CrossRef]
- Lunney, J.K.; Van Goor, A.; Walker, K.E.; Hailstock, T.; Franklin, J.; Dai, C. Importance of the Pig as a Human Biomedical Model. Sci. Transl. Med. 2021, 13, eabd5758. [Google Scholar] [CrossRef]
- Ferenc, K.; Pietrzak, P.; Godlewski, M.M.; Piwowarski, J.; Kilianczyk, R.; Guilloteau, P.; Zabielski, R. Intrauterine Growth Retarded Piglet as a Model for Humans—Studies on the Perinatal Development of the Gut Structure and Function. Reprod. Biol. 2014, 14, 51–60. [Google Scholar] [CrossRef]
- Raqib, R.; Alam, D.S.; Sarker, P.; Ahmad, S.M.; Ara, G.; Yunus, M.; Moore, S.E.; Fuchs, G. Low Birth Weight Is Associated with Altered Immune Function in Rural Bangladeshi Children: A Birth Cohort Study. Am. J. Clin. Nutr. 2007, 85, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Rosace, D.; López, J.; Blanco, S. Emerging Roles of Novel Small Non-Coding Regulatory RNAs in Immunity and Cancer. RNA Biol. 2020, 17, 1196–1213. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Yang, T.; Gao, S.; Cheng, M.; Shao, Y.; Xi, Y.; Guo, L.; Zhang, D.; Gao, W.; Zhang, G.; et al. MiR-148a-3p Silences the CANX/MHC-I Pathway and Impairs CD8 + T Cell-Mediated Immune Attack in Colorectal Cancer. FASEB J. 2021, 35. [Google Scholar] [CrossRef] [PubMed]
- Ashizawa, M.; Okayama, H.; Ishigame, T.; Thar Min, A.K.; Saito, K.; Ujiie, D.; Murakami, Y.; Kikuchi, T.; Nakayama, Y.; Noda, M.; et al. MiRNA-148a-3p Regulates Immunosuppression in DNA Mismatch Repair–Deficient Colorectal Cancer by Targeting PD-L1. Mol. Cancer Res. 2019, 17, 1403–1413. [Google Scholar] [CrossRef]
- Gao, X.; Huang, X.; Yang, Q.; Zhang, S.; Yan, Z.; Luo, R.; Wang, P.; Wang, W.; Xie, K.; Gun, S. MicroRNA-21-5p Targets PDCD4 to Modulate Apoptosis and Inflammatory Response to Clostridium Perfringens Beta2 Toxin Infection in IPEC-J2 Cells. Dev. Comp. Immunol. 2021, 114, 103849. [Google Scholar] [CrossRef]
- Kochhar, P.; Dwarkanath, P.; Ravikumar, G.; Thomas, A.; Crasta, J.; Thomas, T.; Kurpad, A.V.; Mukhopadhyay, A. Placental Expression of MiR-21-5p, MiR-210-3p and MiR-141-3p: Relation to Human Fetoplacental Growth. Eur. J. Clin. Nutr. 2022, 76, 730–738. [Google Scholar] [CrossRef]
- Kim, H.K.; Yeom, J.-H.; Kay, M.A. Transfer RNA-Derived Small RNAs: Another Layer of Gene Regulation and Novel Targets for Disease Therapeutics. Mol. Ther. 2020, 28, 2340–2357. [Google Scholar] [CrossRef]
- Kumar, P.; Kuscu, C.; Dutta, A. Biogenesis and Function of Transfer RNA-Related Fragments (TRFs). Trends Biochem. Sci. 2016, 41, 679–689. [Google Scholar] [CrossRef]
- Cui, H.; Li, H.; Wu, H.; Du, F.; Xie, X.; Zeng, S.; Zhang, Z.; Dong, K.; Shang, L.; Jing, C.; et al. A Novel 3’tRNA-Derived Fragment TRF-Val Promotes Proliferation and Inhibits Apoptosis by Targeting EEF1A1 in Gastric Cancer. Cell Death Dis. 2022, 13, 471. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Luo, L.; Yang, X.; Zhang, J.; Xie, Y.; Liang, R.; Wang, W.; Lu, S. Screening and Potential Role of TRFs and TiRNAs Derived from TRNAs in the Carcinogenesis and Development of Lung Adenocarcinoma. Oncol. Lett. 2021, 22, 506. [Google Scholar] [CrossRef]
- Yang, W.; Gao, K.; Qian, Y.; Huang, Y.; Xiang, Q.; Chen, C.; Chen, Q.; Wang, Y.; Fang, F.; He, Q.; et al. A Novel TRNA-Derived Fragment AS-TDR-007333 Promotes the Malignancy of NSCLC via the HSPB1/MED29 and ELK4/MED29 Axes. J. Hematol. Oncol. 2022, 15, 53. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Hong, X.; Zhou, W.; Zhang, Y. Expression Profiles of TRNA-Derived Fragments and Their Potential Roles in Ovarian Endometriosis. Epigenomics 2020, 12, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Lai, H.; Yang, Y.; Hu, J.; Li, Z.; Ma, B.; Xu, W.; Liu, W.; Wei, W.; Li, D.; et al. A 5′-TRNA Halve, TiRNA-Gly Promotes Cell Proliferation and Migration via Binding to RBM17 and Inducing Alternative Splicing in Papillary Thyroid Cancer. J. Exp. Clin. Cancer Res. 2021, 40, 222. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Peng, H.; Cui, Q.; Zhou, Y. TRFTar: Prediction of TRF-Target Gene Interactions via Systemic Re-Analysis of Argonaute CLIP-Seq Datasets. Methods 2021, 187, 57–67. [Google Scholar] [CrossRef]
- Green, J.A.; Ansari, M.Y.; Ball, H.C.; Haqqi, T.M. TRNA-Derived Fragments (TRFs) Regulate Post-Transcriptional Gene Expression via AGO-Dependent Mechanism in IL-1β Stimulated Chondrocytes. Osteoarthr. Cartil. 2020, 28, 1102–1110. [Google Scholar] [CrossRef]
- Falconi, M.; Giangrossi, M.; Zabaleta, M.E.; Wang, J.; Gambini, V.; Tilio, M.; Bencardino, D.; Occhipinti, S.; Belletti, B.; Laudadio, E.; et al. A Novel 3′-TRNA (Glu) -Derived Fragment Acts as a Tumor Suppressor in Breast Cancer by Targeting Nucleolin. FASEB J. 2019, 33, 13228–13240. [Google Scholar] [CrossRef]
- Niu, Y.; Zhao, Y.; He, J.; Yun, Y.; Shen, M.; Gan, Z.; Zhang, L.; Wang, T. Dietary Dihydroartemisinin Supplementation Alleviates Intestinal Inflammatory Injury through TLR4/NOD/NF-ΚB Signaling Pathway in Weaned Piglets with Intrauterine Growth Retardation. Anim. Nutr. 2021, 7, 667–678. [Google Scholar] [CrossRef]
- Zhou, Y.; Ji, J.; Chen, C.; Hong, F. Retardation of Axonal and Dendritic Outgrowth Is Associated with the MAPK Signaling Pathway in Offspring Mice Following Maternal Exposure to Nanosized Titanium Dioxide. J. Agric. Food Chem. 2019, 67, 2709–2715. [Google Scholar] [CrossRef]
- Teufel, C.; Horvath, E.; Peter, A.; Ercan, C.; Piscuoglio, S.; Hall, M.N.; Finke, D.; Lehmann, F.M. MTOR Signaling Mediates ILC3-Driven Immunopathology. Mucosal Immunol. 2021, 14, 1323–1334. [Google Scholar] [CrossRef]
- Shibutani, S.T.; Saitoh, T.; Nowag, H.; Münz, C.; Yoshimori, T. Autophagy and Autophagy-Related Proteins in the Immune System. Nat. Immunol. 2015, 16, 1014–1024. [Google Scholar] [CrossRef]
- Zarate, M.A.; De Dios, R.K.; Balasubramaniyan, D.; Zheng, L.; Sherlock, L.G.; Rozance, P.J.; Wright, C.J. The Acute Hepatic NF-ΚB-Mediated Proinflammatory Response to Endotoxemia Is Attenuated in Intrauterine Growth-Restricted Newborn Mice. Front. Immunol. 2021, 12, 706774. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.Y.; Moldenhauer, L.M.; Groome, H.M.; Schjenken, J.E.; Robertson, S.A. Toll-like Receptor-4 Null Mutation Causes Fetal Loss and Fetal Growth Restriction Associated with Impaired Maternal Immune Tolerance in Mice. Sci. Rep. 2021, 11, 16569. [Google Scholar] [CrossRef]
- Liu, N.; Wang, X.; Steer, C.J.; Song, G. MicroRNA-206 Promotes the Recruitment of CD8 + T Cells by Driving M1 Polarisation of Kupffer Cells. Gut 2021, 71, 1642–1655. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.D.; Nistala, K.; Petermann, F.; Saran, N.; Chennupati, V.; Schmitz, S.; Korn, T.; Wedderburn, L.R.; Förster, R.; Krueger, A.; et al. Expression of MiRNAs MiR-133b and MiR-206 in the Il17a/f Locus Is Co-Regulated with IL-17 Production in Aβ and Γδ T Cells. PLoS ONE 2011, 6, e20171. [Google Scholar] [CrossRef] [PubMed]

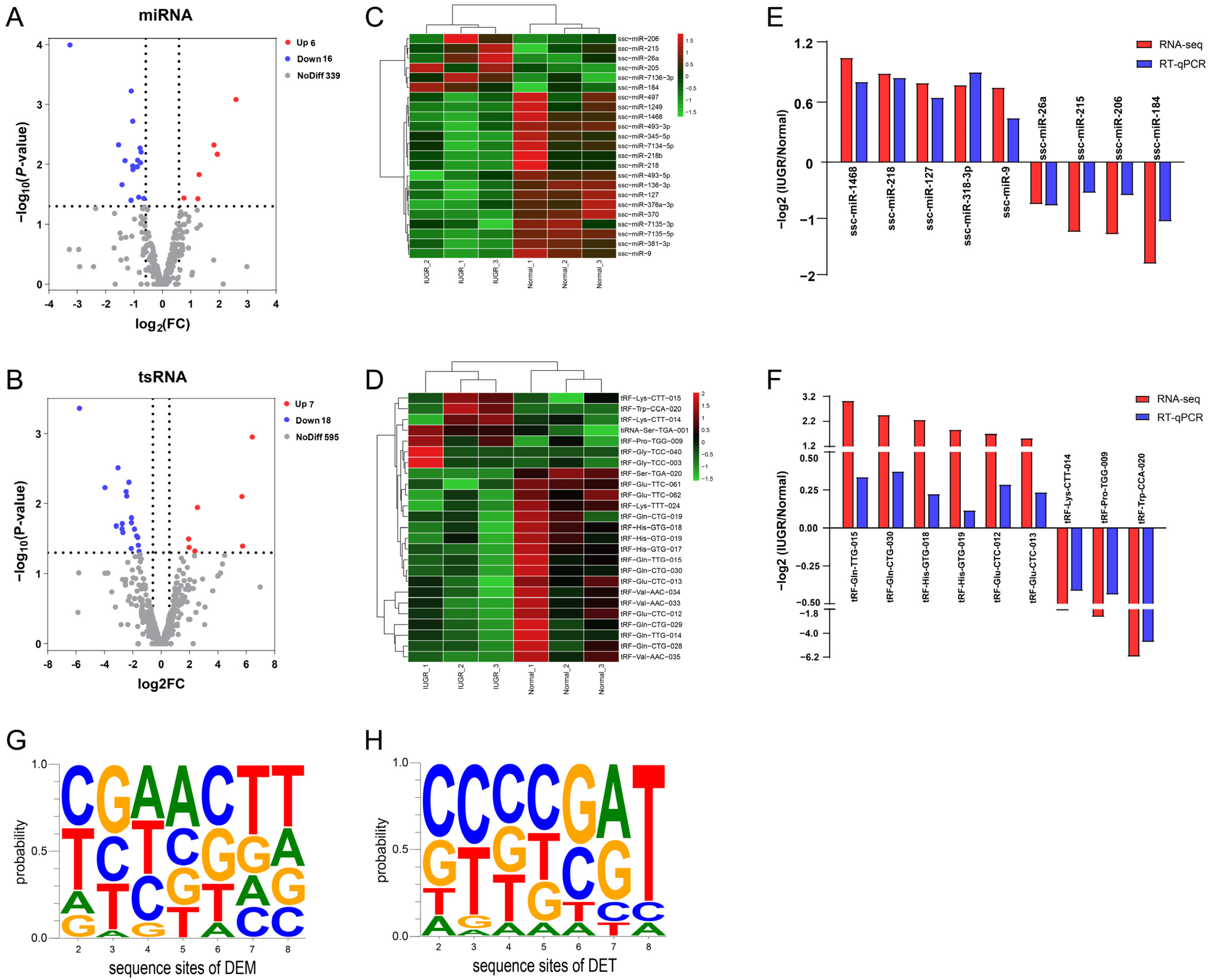




| Type | miRNA-ID | Normal-CMP | IUGR-CMP | log2FC | p-Value |
|---|---|---|---|---|---|
| Up-regulated | ssc-miR-205 | 0.1883 | 1.0705 | 2.5905 | 0.000825628 |
| ssc-miR-7136-3p | 0.5789 | 1.7741 | 1.9331 | 0.006799598 | |
| ssc-miR-184 | 6.299 | 19.002 | 1.8141 | 0.004753366 | |
| ssc-miR-206 | 17.486 | 32.298 | 1.2932 | 0.014926584 | |
| ssc-miR-215 | 15.401 | 28.950 | 1.2511 | 0.037612553 | |
| ssc-miR-26a | 44735 | 58503 | 0.7552 | 0.036464342 | |
| Down-regulated | ssc-miR-7135-5p | 1.7123 | 0.1257 | −3.2535 | 0.000101718 |
| ssc-miR-1249 | 3.3335 | 0.8842 | −1.5453 | 0.004733207 | |
| ssc-miR-493-5p | 4.4964 | 1.2656 | −1.4257 | 0.021899034 | |
| ssc-miR-376a-3p | 4.4698 | 1.3902 | −1.3168 | 0.008688021 | |
| ssc-miR-7135-3p | 4.0429 | 1.5163 | −1.1024 | 0.039710544 | |
| ssc-miR-370 | 39.167 | 14.533 | −1.0981 | 0.000597613 | |
| ssc-miR-345-5p | 16.812 | 6.615 | −1.0461 | 0.010609881 | |
| ssc-miR-1468 | 282.04 | 105.31 | −1.0445 | 0.00191462 | |
| ssc-miR-493-3p | 4.7686 | 1.8293 | −1.0411 | 0.012239605 | |
| ssc-miR-218 | 1788.05 | 750.36 | −0.8888 | 0.01102262 | |
| ssc-miR-136-3p | 25.72 | 11.29 | −0.8684 | 0.008511165 | |
| ssc-miR-497 | 40.94 | 17.7 | −0.8377 | 0.035318563 | |
| ssc-miR-127 | 319.23 | 147.31 | −0.7924 | 0.005334554 | |
| ssc-miR-381-3p | 6486.27 | 3045.71 | −0.7757 | 0.009420996 | |
| ssc-miR-9 | 640.91 | 300.39 | −0.7493 | 0.006231918 | |
| ssc-miR-7134-5p | 107.070 | 54.293 | −0.6539 | 0.037300766 |
| Type | tsRNA-ID | Normal-CMP | IUGR-CMP | log2FC | p-Value |
|---|---|---|---|---|---|
| Up-regulated | tRF-Trp-CCA-020 | 0 | 48.6523 | 6.4292 | 0.001116986 |
| tRF-Gly-TCC-040 | 0 | 18.0276 | 5.7421 | 0.040442328 | |
| tRF-Gly-TCC-003 | 10.90 | 408.74 | 5.7054 | 0.007916416 | |
| tRF-Pro-TGG-009 | 6.263 | 40.205 | 2.5519 | 0.011326413 | |
| tRF-Lys-CTT-015 | 2.098 | 21.47 | 2.3787 | 0.047462016 | |
| tiRNA-Ser-TGA-001 | 6.282 | 26.298 | 1.9759 | 0.042137556 | |
| tRF-Lys-CTT-014 | 14.178 | 72.018 | 1.9456 | 0.031865939 | |
| Down-regulated | tRF-Ser-TGA-020 | 23.454 | 0 | −5.7568 | 0.000436346 |
| tRF-Gln-CTG-028 | 70.232 | 2.902 | −3.9634 | 0.00590216 | |
| tRF-Gln-CTG-029 | 233.094 | 18.174 | −3.1648 | 0.020822792 | |
| tRF-Gln-TTG-015 | 300.411 | 29.932 | −3.0391 | 0.003070212 | |
| tRF-Gln-TTG-014 | 232.12 | 25.72 | −2.7517 | 0.022836512 | |
| tRF-Gln-CTG-019 | 98.760 | 10.323 | −2.7407 | 0.019339968 | |
| tRF-Glu-TTC-062 | 21.424 | 3.230 | −2.6897 | 0.025766303 | |
| tRF-Gln-CTG-030 | 964.27 | 145.702 | −2.4727 | 0.006773031 | |
| tRF-Val-AAC-033 | 713 | 106 | −2.425 | 0.007834966 | |
| tRF-His-GTG-018 | 1198 | 223 | −2.2756 | 0.004945062 | |
| tRF-Glu-TTC-061 | 33.508 | 8.631 | −2.1028 | 0.043496743 | |
| tRF-Val-AAC-034 | 4270 | 789 | −2.0977 | 0.018732543 | |
| tRF-Val-AAC-035 | 85.789 | 20.367 | −2.0953 | 0.016049707 | |
| tRF-His-GTG-019 | 6449 | 1594 | −1.8803 | 0.02306075 | |
| tRF-Glu-CTC-012 | 2202 | 621 | −1.7247 | 0.028886881 | |
| tRF-His-GTG-017 | 490 | 144 | −1.6523 | 0.030190001 | |
| tRF-Lys-TTT-024 | 112.961 | 41.084 | −1.6095 | 0.039361651 | |
| tRF-Glu-CTC-013 | 3520.92 | 1146.79 | −1.5432 | 0.048273186 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Gan, M.; Chen, J.; Chen, L.; Zhao, Y.; Zhu, Y.; Niu, L.; Zhang, S.; Jiang, Y.; Guo, Z.; et al. Characteristics of tRNA-Derived Small RNAs and microRNAs Associated with Immunocompromise in an Intrauterine Growth-Restricted Pig Model. Animals 2022, 12, 2102. https://doi.org/10.3390/ani12162102
Ma J, Gan M, Chen J, Chen L, Zhao Y, Zhu Y, Niu L, Zhang S, Jiang Y, Guo Z, et al. Characteristics of tRNA-Derived Small RNAs and microRNAs Associated with Immunocompromise in an Intrauterine Growth-Restricted Pig Model. Animals. 2022; 12(16):2102. https://doi.org/10.3390/ani12162102
Chicago/Turabian StyleMa, Jianfeng, Mailin Gan, Jingyun Chen, Lei Chen, Ye Zhao, Yan Zhu, Lili Niu, Shunhua Zhang, Yanzhi Jiang, Zongyi Guo, and et al. 2022. "Characteristics of tRNA-Derived Small RNAs and microRNAs Associated with Immunocompromise in an Intrauterine Growth-Restricted Pig Model" Animals 12, no. 16: 2102. https://doi.org/10.3390/ani12162102







