Are Marine Heatwaves Responsible for Mortalities of Farmed Mytilus galloprovincialis? A Pathophysiological Analysis of Marteilia Infected Mussels from Thermaikos Gulf, Greece
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Procedure
2.2. Histopathological Procedure
2.3. SDS-PAGE/Immunoblot and Dot Blot Analysis
2.3.1. Preparation of Tissue Samples
2.3.2. SDS-PAGE/Immunoblot
2.3.3. Dot Blot Analysis
2.4. Molecular Identification of the Pathogen
2.5. RNA Extraction and cDNA Synthesis
2.6. Analysis of Gene Expression with Quantitative PCR
2.7. Statistical Analysis
3. Results
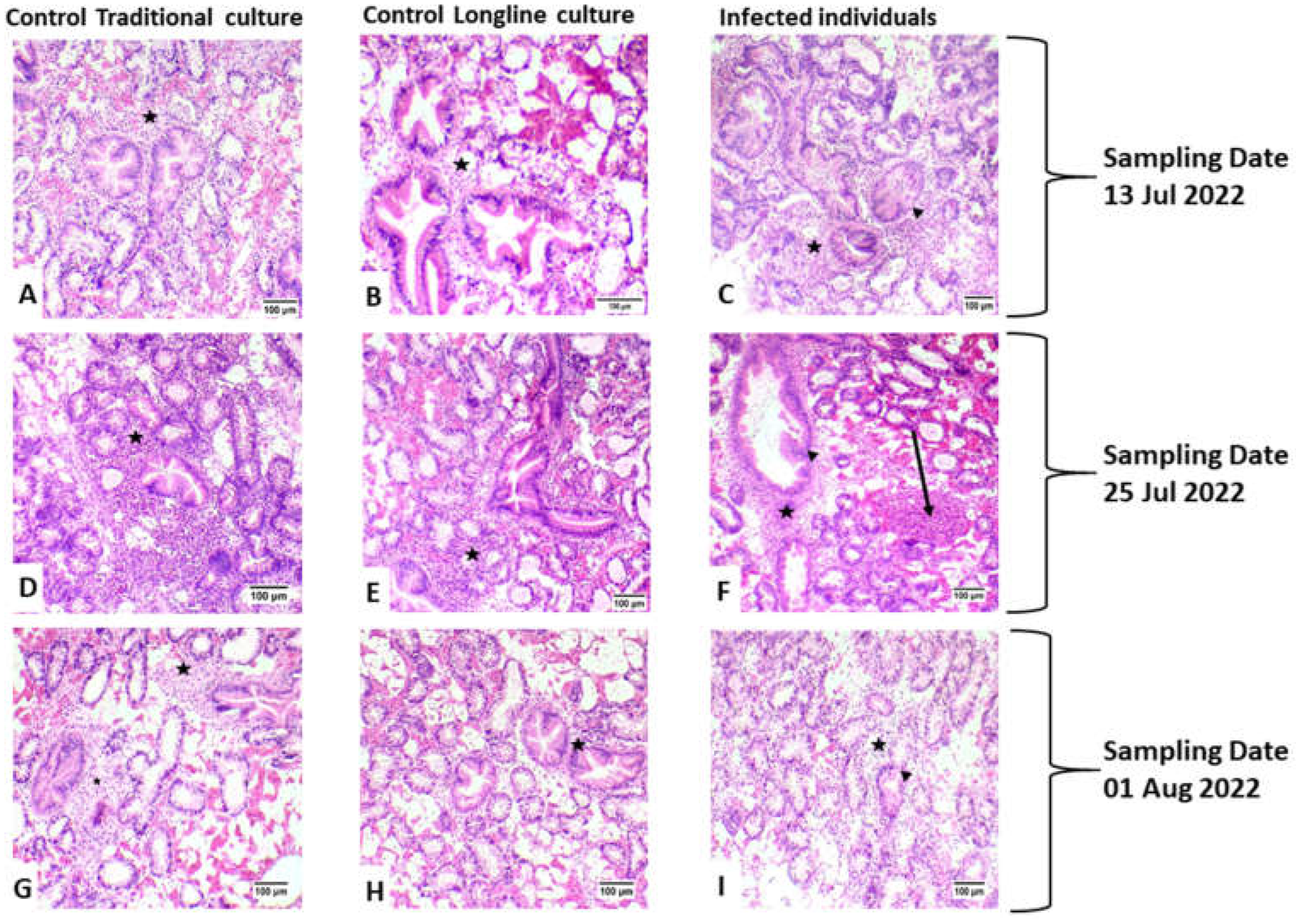
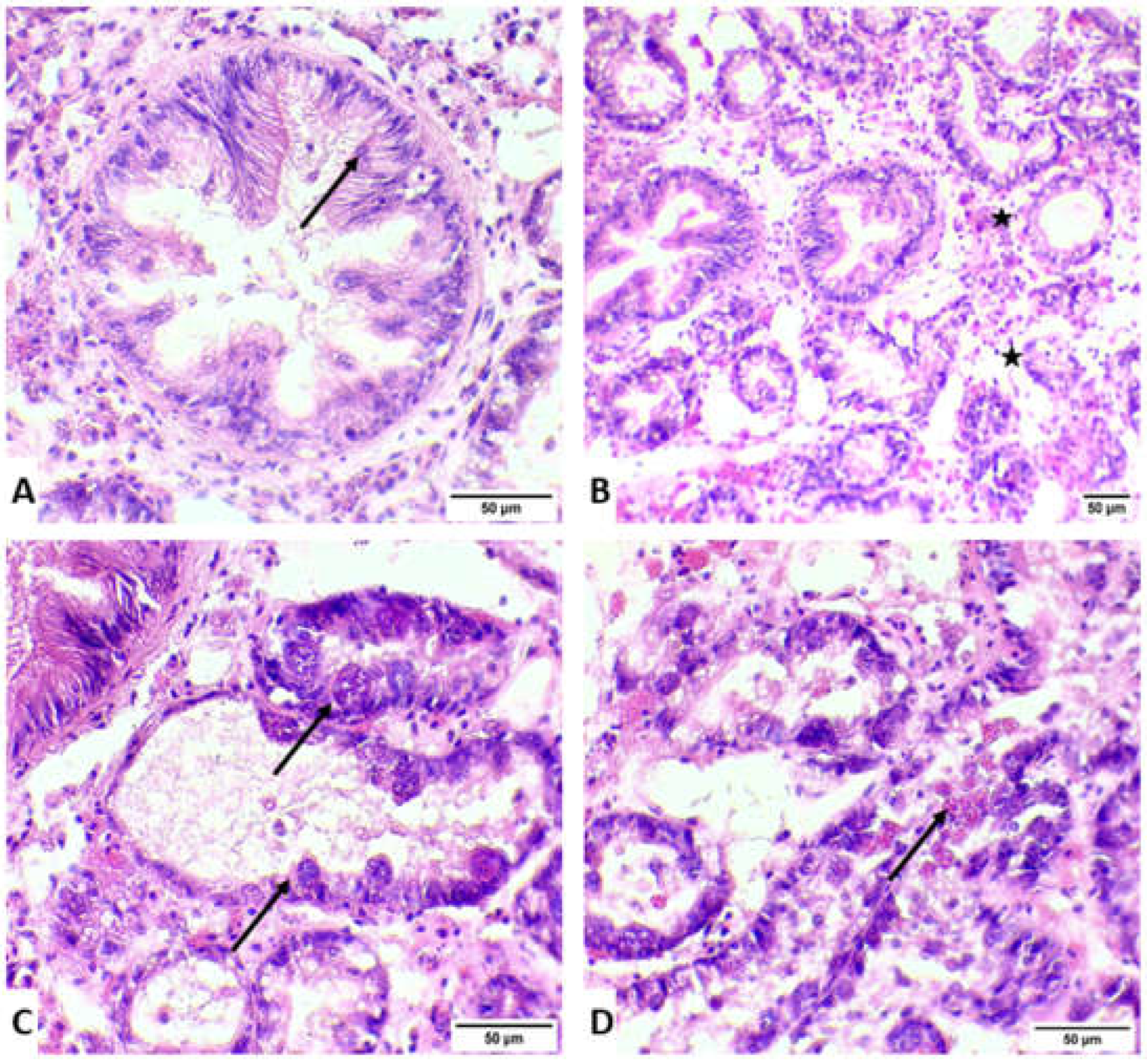
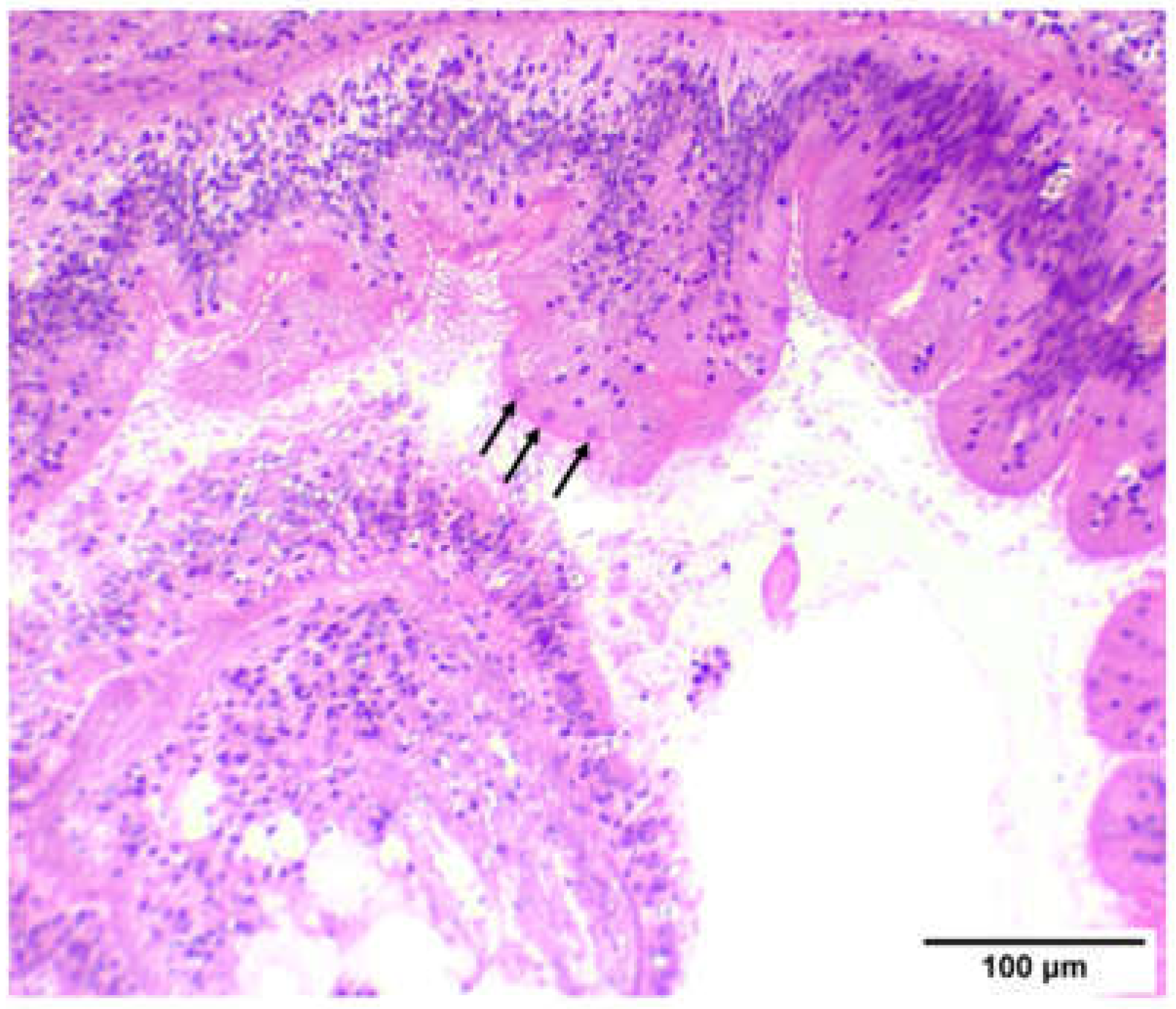
3.1. Histopathology and Molecular Identification of the Pathogen
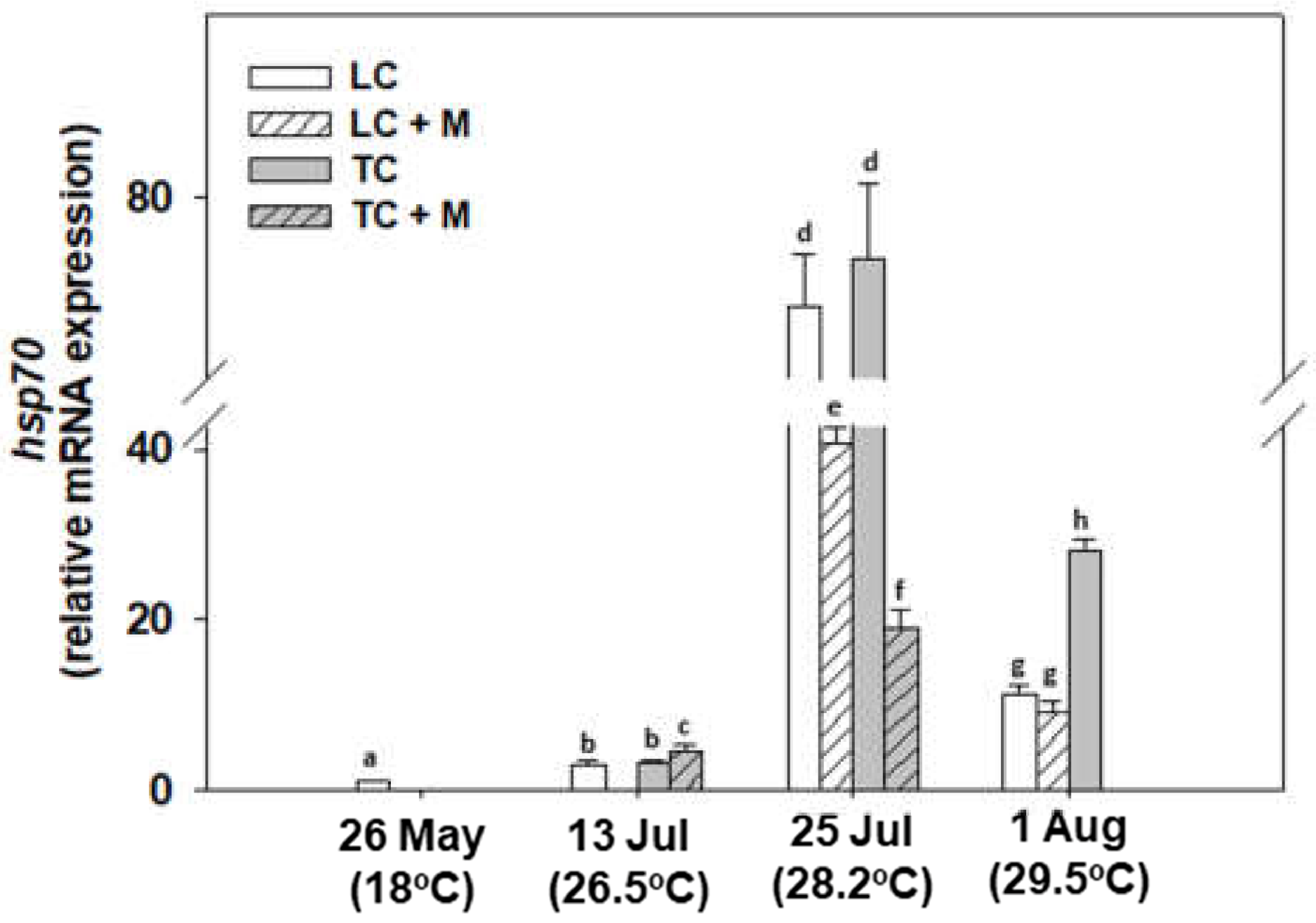
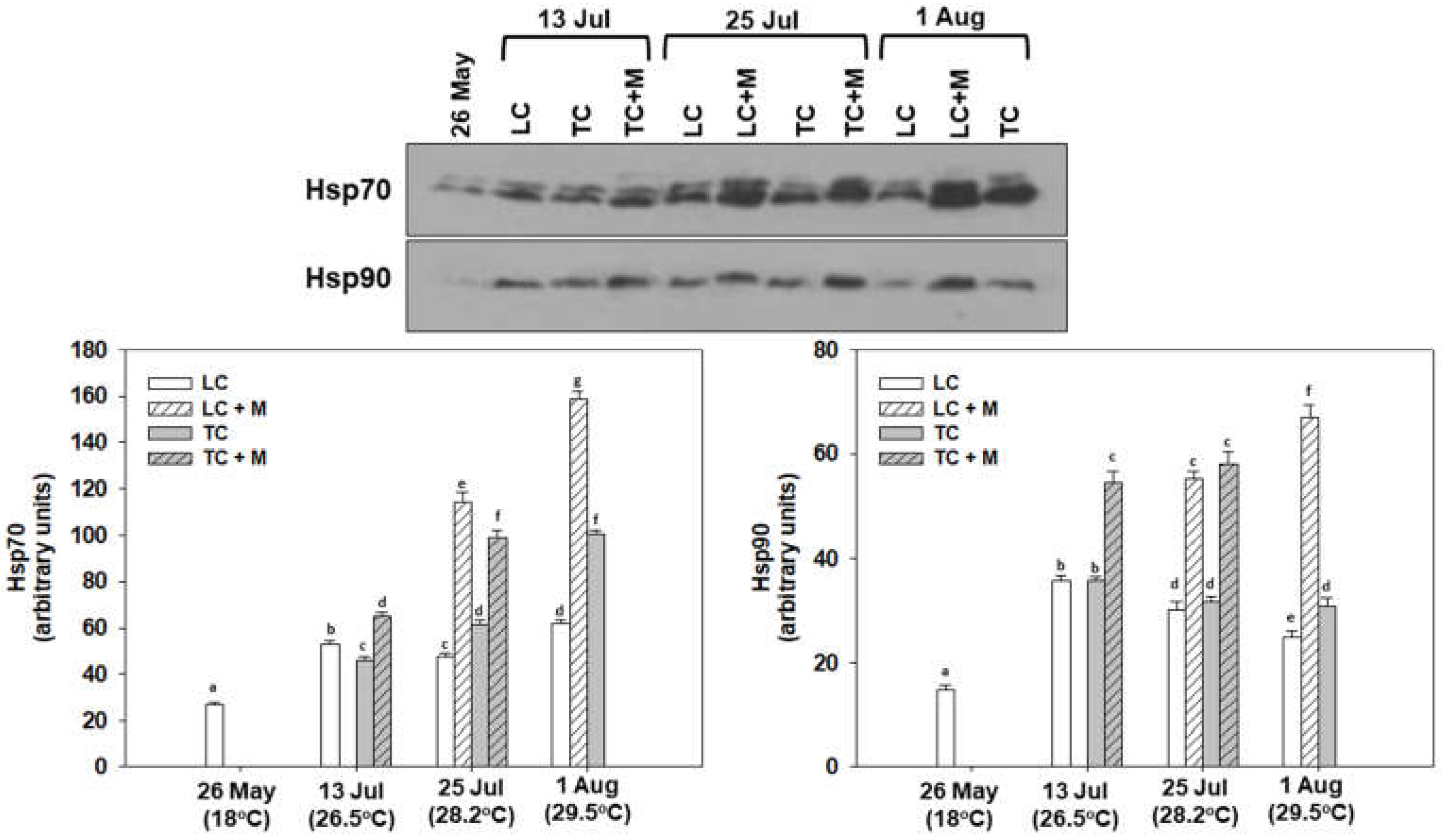
3.2. Heat Shock Response
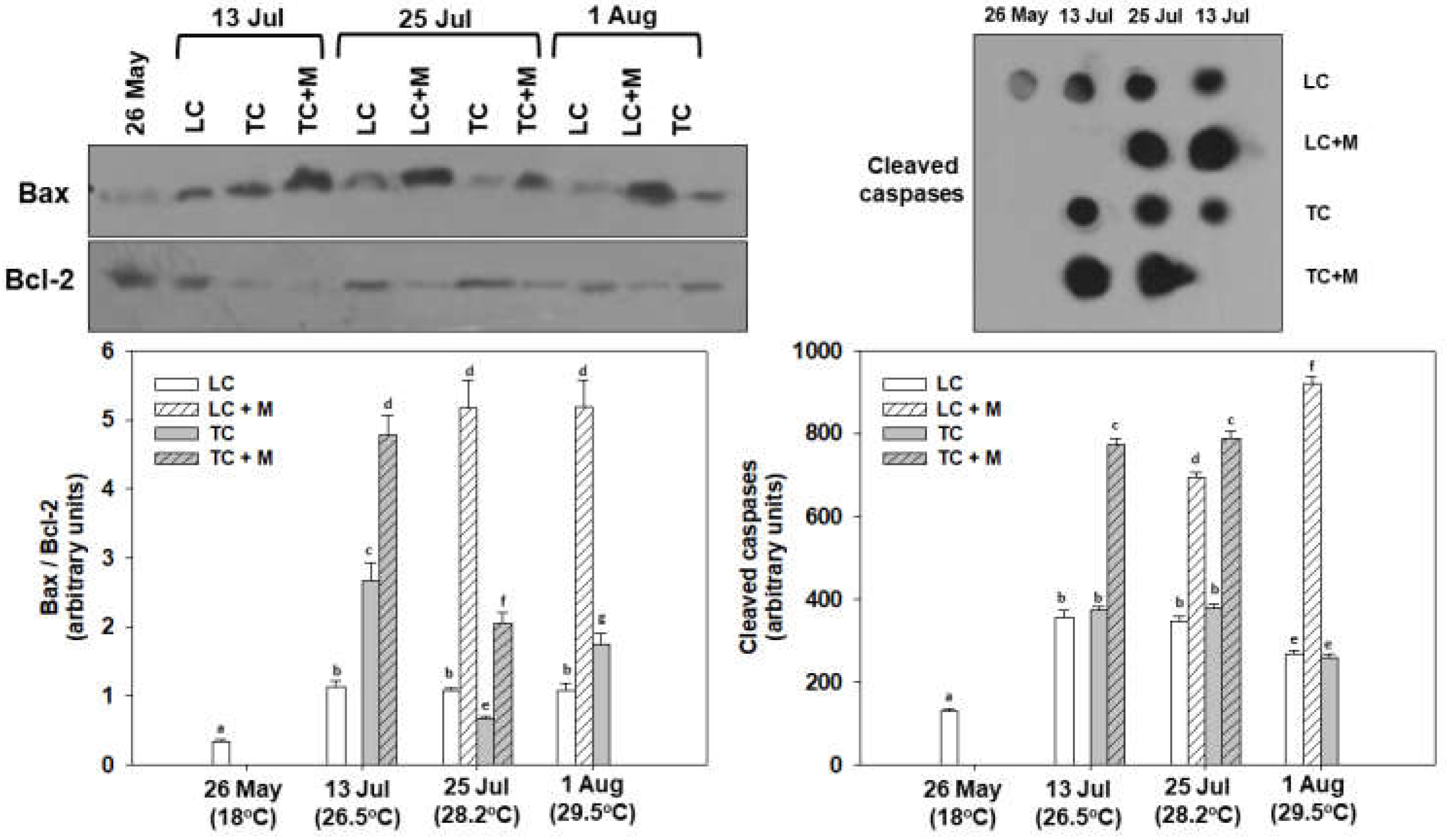
3.3. Apoptosis
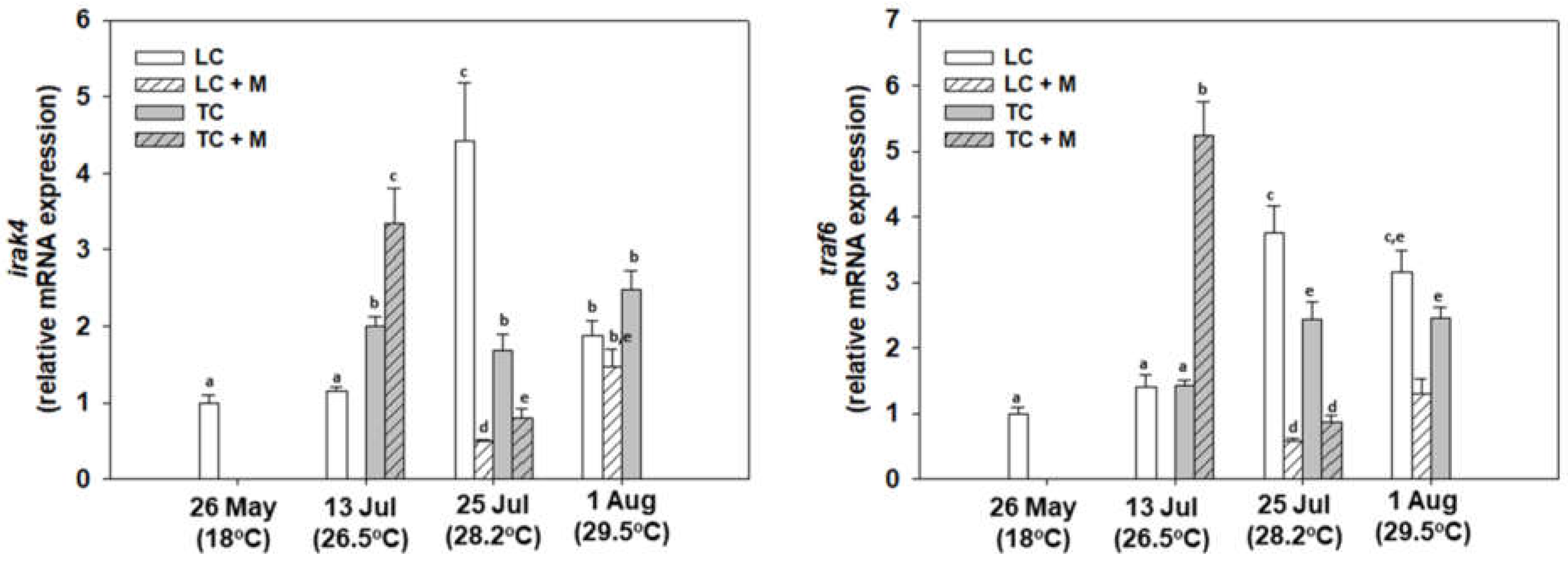
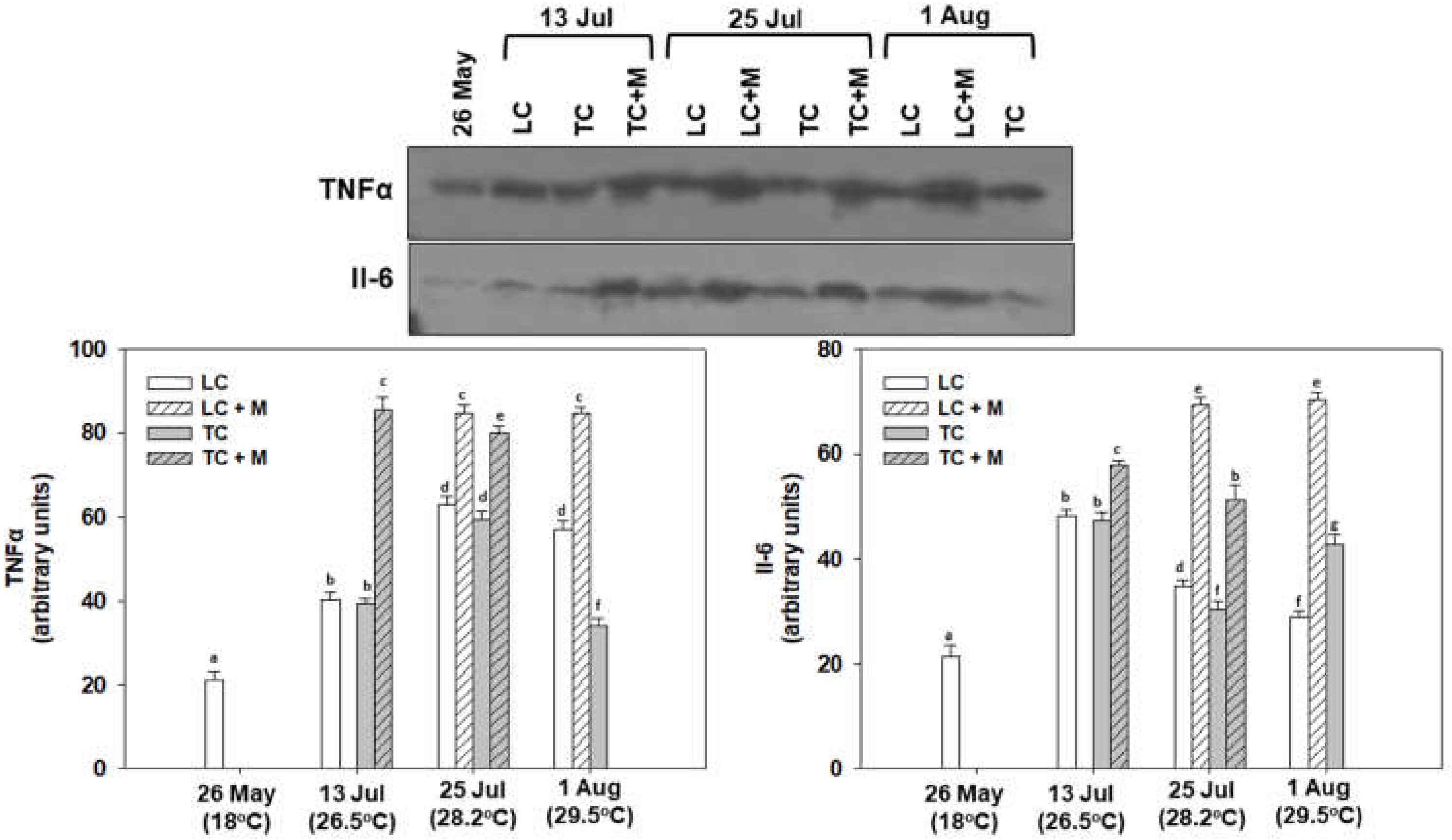
3.4. Immunology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Little, D.C.; Newton, R.W.; Beveridge, M.C.M. Aquaculture: A rapidly growing and significant source of sustainable food? Status, transitions and potential. Proc. Nutr. Soc. 2016, 75, 274–286. [Google Scholar] [CrossRef] [Green Version]
- Subasinghe, R.; Soto, D.; Jia, J. Global aquaculture and its role in sustainable development. Rev. Aquac. 2009, 1, 2–9. [Google Scholar] [CrossRef]
- Tacon, A.G.J. Trends in Global Aquaculture and Aquafeed Production: 2000–2017. Rev. Fish. Sci. Aquac. 2020, 28, 43–56. [Google Scholar] [CrossRef]
- Lucas, J.S. Bivalve Molluscs. In Aquaculture: Farming Aquatic Animals and Plants, 2nd ed.; Willey Blackwell: Hoboken, NJ, USA; pp. 541–566.
- Sun, X.; Dong, J.; Hu, C.; Zhang, Y.; Chen, Y.; Zhang, X. Use of macrofaunal assemblage indices and biological trait analysis to assess the ecological impacts of coastal bivalve aquaculture. Ecol. Indic. 2021, 127, 107713. [Google Scholar] [CrossRef]
- Georgoulis, I.; Feidantsis, K.; Kouvas, D.; Lattos, A.; Delis, G.A.; Theodoridis, A.; Michaelidis, B.; Giantsis, I.A. The effect of seawater physical parameters in bivalve farming: Could systematic monitoring and early warning prevent negative impacts? A review focused on Vistonikos Gulf, North Aegean Sea. Int. J. Agric. Resour. Gov. Ecol. 2022, 18, 22–37. [Google Scholar] [CrossRef]
- Theodorou, J.A.; Perdikaris, C.; Filippopoulos, N.G. Evolution Through Innovation in Aquaculture: A Critical Review of the Greek Mariculture Industry. J. Appl. Aquac. 2015, 27, 160–181. [Google Scholar] [CrossRef]
- Zgouridou, A.; Tripidaki, E.; Giantsis, I.A.; Theodorou, J.A.; Kalaitzidou, M.; Raitsos, D.E.; Lattos, A.; Mavropoulou, A.M.; Sofianos, S.; Karagiannis, D.; et al. The current situation and potential effects of climate change on the microbial load of marine bivalves of the Greek coastlines: An integrative review. Environ. Microbiol. 2022, 24, 1012–1034. [Google Scholar] [CrossRef]
- Giantsis, I.A.; Mucci, N.; Randi, E.; Abatzopoulos, T.J.; Apostolidis, A.P. Microsatellite variation of mussels (Mytilus galloprovincialis) in central and eastern Mediterranean: Genetic panmixia in the Aegean and the Ionian Seas. J. Mar. Biol. Assoc. UK 2014, 94, 797–809. [Google Scholar] [CrossRef]
- Dumbauld, B.R.; Ruesink, J.L.; Rumrill, S.S. The ecological role of bivalve shellfish aquaculture in the estuarine environment: A review with application to oyster and clam culture in West Coast (USA) estuaries. Aquaculture 2009, 290, 196–223. [Google Scholar] [CrossRef]
- Cranford, P.J.; Kamermans, P.; Krause, G.; Mazurié, J.; Buck, B.H.; Dolmer, P.; Fraser, D.; Van Nieuwenhove, K.; O’Beirn, F.X.; Sanchez-Mata, A.; et al. An ecosystem-based approach and management framework for the integrated evaluation of bivalve aquaculture impacts. Aquac. Environ. Interact. 2012, 2, 193–213. [Google Scholar] [CrossRef]
- Avdelas, L.; Avdic-Mravlje, E.; Borges Marques, A.C.; Cano, S.; Capelle, J.J.; Carvalho, N.; Cozzolino, M.; Dennis, J.; Ellis, T.; Fernández Polanco, J.M.; et al. The decline of mussel aquaculture in the European Union: Causes, economic impacts and opportunities. Rev. Aquac. 2021, 13, 91–118. [Google Scholar] [CrossRef]
- Theodorou, J.A.; Tzovenis, I. A framework for risk analysis of the shellfish aquaculture: The case of the Mediterranean mussel farming in Greece. Aquac. Fish. 2021. [Google Scholar] [CrossRef]
- Matozzo, V.; Marin, M.G. Bivalve immune responses and climate changes: Is there a relationship? Invertebr. Surviv. J. 2011, 8, 70–77. [Google Scholar]
- Matozzo, V.; Chinellato, A.; Munari, M.; Finos, L.; Bressan, M.; Marin, M.G. First evidence of immunomodulation in bivalves under seawater acidification and increased temperature. PLoS ONE 2012, 7, e33820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abele, D.; Tesch, C.; Wencke, P.; Pörtner, H.O. How does oxidative stress relate to thermal tolerance in the Antarctic bivalve Yoldia eightsi? Ant. Sci. 2001, 13, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Abele, D.; Heise, K.; Pörtner, H.O.; Puntarulo, S. Temperature-dependence of mitochondrial function and production of reactive oxygen species in the intertidal mud clam Mya arenaria. J. Exp. Biol. 2002, 205, 1831–1841. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, I.M.; Frederich, M.; Bagwe, R.; Lannig, G.; Sukhotin, A.A. Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Mar. Environ. Res. 2012, 79, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Feidantsis, K.; Giantsis, I.A.; Vratsistas, A.; Makri, S.; Pappa, A.Z.; Drosopoulou, E.; Anestis, A.; Mavridou, E.; Exadactylos, A.; Vafidis, D.; et al. Correlation between intermediary metabolism, Hsp gene expression, and oxidative stress-related proteins in long-term thermal-stressed Mytilus galloprovincialis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 319, R264–R281. [Google Scholar] [CrossRef]
- Anestis, A.; Pörtner, H.O.; Karagiannis, D.; Angelidis, P.; Staikou, A.; Michaelidis, B. Response of Mytilus galloprovincialis (L.) to increasing seawater temperature and to marteliosis: Metabolic and physiological parameters. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010, 156, 57–66. [Google Scholar] [CrossRef]
- Rahman, M.A.; Henderson, S.; Miller-ezzy, P.; Li, X.X.; Qin, J.G. Immune response to temperature stress in three bivalve species: Pacific oyster Crassostrea gigas, Mediterranean mussel Mytilus galloprovincialis and mud cockle Katelysia rhytiphora. Fish Shellfish Immunol. 2019, 86, 868–874. [Google Scholar] [CrossRef]
- Dang, V.T.; Speck, P.; Benkendorff, K. Influence of elevated temperatures on the immune response of abalone, Haliotis rubra. Fish Shellfish Immunol. 2012, 32, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Li, L.; Wu, F.; Zhang, G. Effect of chronic temperature exposure on the immunity of abalone, Haliotis discus hannai. Aquac. Res. 2016, 47, 2861–2873. [Google Scholar] [CrossRef]
- Boukadida, K.; Cachot, J.; Morin, B.; Clerandeau, C.; Banni, M. Moderate temperature elevation increase susceptibility of early-life stage of the Mediterranean mussel, Mytilus galloprovincialis to metal-induced genotoxicity. Sci. Total Environ. 2019, 663, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, H.; Yao, C. Molecular and acute temperature stress response characterizations of caspase-8 gene in two mussels, Mytilus coruscus and Mytilus galloprovincialis. Comp. Biochem. Physiol. Part B 2014, 177–178, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Koagouw, W.; Hazell, R.J.; Ciocan, C. Induction of apoptosis in the gonads of Mytilus edulis by metformin and increased temperature, via regulation of HSP70, CASP8, BCL2 and FAS. Mar. Pollut. Bull. 2021, 173, 113011. [Google Scholar] [CrossRef] [PubMed]
- Cavicchioli, R.; Bakken, L.R.; Baylis, M.; Foreman, C.M.; Karl, D.M.; Koskella, B.; Welch, D.B.M.; Martiny, J.B.H.; Moran, M.A.; Rich, V.I.; et al. Scientists’ warning to humanity: Microorganisms and climate change. Nat. Rev. Microbiol. 2019, 17, 569–586. [Google Scholar] [CrossRef] [Green Version]
- Travers, M.; Boettcher, K.; Roque, A.; Friedman, C.S. Bacterial diseases in marine bivalves. J. Invertebr. Pathol. 2015, 131, 11–31. [Google Scholar] [CrossRef] [Green Version]
- Baker-austin, C.; Trinanes, J.; Gonzalez-escalona, N.; Martinez-urtaza, J. Non-Cholera Vibrios: The Microbial Barometer of Climate Change. Trends Microbiol. 2017, 25, 76–84. [Google Scholar] [CrossRef] [Green Version]
- Tubiash, H.S.; Chanley, P.E.; Leifson, E. Bacillary Necrosis, a Disease of Larval and Juvenile Bivalve Mollusks. J. Bacteriol. 1965, 90, 1036–1044. [Google Scholar] [CrossRef] [Green Version]
- Paillard, C.; Le Roux, F.; Borrego, J.J. Bacterial disease in marine bivalves, a review of recent studies: Trends and evolution. Trends Microbiol. 2004, 498, 477–498. [Google Scholar] [CrossRef] [Green Version]
- Paillard, C.; Korsnes, K.; Le Chevalier, P.; Le Boulay, C.; Harkestad, L.; Eriksen, A.G.; Willassen, E.; Bergh, Ø.; Bovo, C.; Skår, C.; et al. Vibrio tapetis -like strain isolated from introduced Manila clams Ruditapes philippinarum showing symptoms of brown ring disease in Norway. Dis. Aquat. Organ. 2008, 81, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Tsutsumi, H.; Hong, J.; Choi, K. Pathology survey of the short-neck clam Ruditapes philippinarum occurring on sandy tidal flats along the coast of Ariake Bay, Kyushu, Japan. J. Invertebr. Pathol. 2008, 99, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Paillard, C. A short-review of brown ring disease, a vibriosis affecting clams, Ruditapes philippinarum and Ruditapes decussatus. Aquat. Living Resour. 2004, 475, 467–475. [Google Scholar] [CrossRef]
- Andree, K.B. Vibrio mediterranei, a potential emerging pathogen of marine fauna: Investigation of pathogenicity using a bacterial challenge in Pinna nobilis and development of a species-specific PCR. J. Appl. Microbiol. 2020, 130, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Prado, P.; Carrasco, N.; Catanese, G.; Grau, A.; Cabanes, P.; Carella, F.; García-march, J.R.; Tena, J.; Roque, A.; Bertomeu, E.; et al. Presence of Vibrio mediterranei associated to major mortality in stabled individuals of Pinna nobilis L. Aquaculture 2020, 519, 734899. [Google Scholar] [CrossRef]
- Lattos, A.; Bitchava, K.; Giantsis, I.A.; Theodorou, J.A.; Batargias, C.; Michaelidis, B. The implication of vibrio bacteria in the winter mortalities of the critically endangered Pinna nobilis. Microorganisms 2021, 9, 922. [Google Scholar] [CrossRef]
- Arzul, I.; Gagnaire, B.; Bond, C.; Chollet, B.; Morga, B.; Ferrand, S.; Robert, M.; Renault, T. Effects of temperature and salinity on the survival of bonamia ostreae, a parasite infecting flat oysters Ostrea edulis. Dis. Aquat. Organ. 2009, 85, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Murray, A.G.; Marcos-lopez, M.; Collet, B.; Munro, L.A. A review of the risk posed to Scottish mollusc aquaculture from Bonamia, Marteilia and oyster herpesvirus. Aquaculture 2012, 370–371, 7–13. [Google Scholar] [CrossRef]
- Soudant, P.; Chu, F.E.; Volety, A. Host—Parasite interactions: Marine bivalve molluscs and protozoan parasites, Perkinsus species. J. Invertebr. Pathol. 2013, 114, 196–216. [Google Scholar] [CrossRef]
- Villalba, A.; Reece, K.S.; Ordás, M.C.; Casas, S.M.; Figueras, A. Perkinsosis in molluscs: A review. Aquat. Living Resour. 2004, 432, 411–432. [Google Scholar] [CrossRef] [Green Version]
- De Montaudouin, X.; Paul-pont, I.; Lambert, C.; Gonzalez, P.; Raymond, N.; Jude, F.; Legeay, A.; Baudrimont, M.; Dang, C.; Le Grand, F.; et al. Bivalve population health: Multistress to identify hot spots. Mar. Pollut. Bull. 2010, 60, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Arzul, I.; Carnegie, R.B. New perspective on the haplosporidian parasites of molluscs. J. Invertebr. Pathol. 2015, 131, 32–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burreson, E.M.; Ford, S.E. A review of recent information on the Haplosporidia, with special reference to Haplosporidium nelsoni (MSX disease). Aquat. Living Resour. 2004, 17, 499–517. [Google Scholar] [CrossRef]
- Carrasco, N.; Green, T.; Itoh, N. Marteilia spp. parasites in bivalves: A revision of recent studies. J. Invert. Pathol. 2015, 131, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Virvilis, C.; Angelidis, P. Presence of the parasite Marteilia sp. in the flat oyster (Ostrea edulis L.) in Greece. Aquaculture 2006, 259, 1–5. [Google Scholar] [CrossRef]
- Karagiannis, D.; Angelidis, P. Infection of cultured mussels Mytilus galloprovincialis by the protozoan Marteilia sp. in the Thermaikos Gulf (N Greece). Bull. Eur. Assoc. Fish Pathol. 2007, 27, 131–141. [Google Scholar]
- Haffnerl, P. Experimental transmission of Marteilia refringens with special consideration of its life cycle. Dis. Aqua. Organ. 1998, 34, 135–144. [Google Scholar]
- Audemard, C.; Roux, F.L.E.; Barnaud, A.; Collins, C.; Sautour, B. Needle in a haystack: Involvement of the copepod Paracartia grani in the life-cycle of the oyster pathogen Marteilia refringens. Parasitology 2002, 124, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Kalaitzidou, M.P.; Alvanou, M.V.; Papageorgiou, K.V.; Lattos, A.; Sofia, M.; Kritas, S.K.; Petridou, E.; Giantsis, I.A. Pollution Indicators and HAB-Associated Halophilic Bacteria Alongside Harmful Cyanobacteria in the Largest Mussel Cultivation Area in Greece. Int. J. Environ. Res. Public Health 2022, 19, 5285. [Google Scholar] [CrossRef]
- Shaw, B.L.; Battle, H.I. the Gross and Microscopic Anatomy of the Digestive Tract of the Oyster Crassostrea Virginica (Gmelin). Can. J. Zool. 1957, 35, 325–347. [Google Scholar] [CrossRef]
- Howard, D.W.; Smith, C.S. Histological Techniques for Marine Bivalve Mollusks and Crustaceans; NOAA, National Ocean Service: Oxford, UK, 1983.
- Lattos, A.; Chaligiannis, I.; Papadopoulos, D.; Giantsis, I.A.; Petridou, E.I.; Vafeas, G.; Staikou, A.; Michaelidis, B. How Safe to Eat Are Raw Bivalves? Host Pathogenic and Public Health Concern Microbes within Mussels, Oysters, and Clams in Greek Markets. Foods 2021, 10, 2793. [Google Scholar] [CrossRef] [PubMed]
- Giannetto, A.; Maisano, M.; Cappello, T.; Oliva, S.; Parrino, V.; Natalotto, A.; De Marco, G.; Fasulo, S. Effects of oxygen availability on oxidative stress biomarkers in the Mediterranean mussel Mytilus galloprovincialis. Mar. Biotechn. 2017, 19, 614–626. [Google Scholar] [CrossRef]
- Estevez-Calvar, N.; Romero, A.; Figueras, A.; Novoa, B. Genes of the mitochondrial apoptotic pathway in Mytilus galloprovincialis. PLoS ONE 2013, 8, e61502. [Google Scholar] [CrossRef]
- Toubiana, M.; Rosani, U.; Giambelluca, S.; Cammarata, M.; Gerdol, M.; Pallavicini, A.; Venier, P.; Roch, P. Toll signal transduction pathway in bivalves: Complete cds of intermediate elements and related gene transcription levels in hemocytes of immune stimulated Mytilus galloprovincialis. Dev. Comp. Immunol. 2014, 45, 300–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreira, R.; Pereiro, P.; Costa, M.M.; Figueras, A.; Novoa, B. Evaluation of reference genes of Mytilus galloprovincialis and Ruditapes philippinarum infected with three bacteria strains for gene expression analysis. Aq. Liv. Res. 2014, 27, 147–152. [Google Scholar] [CrossRef] [Green Version]
- Rayyan, A.; Damianidis, P.; Antoniadou, C.; Chintiroglou, C.C. Protozoan parasites in cultured mussels Mytilus galloprovincialis in the Thermaikos Gulf (north Aegean Sea, Greece). Dis. Aquat. Organ. 2006, 70, 251–254. [Google Scholar] [CrossRef] [Green Version]
- Lattos, A.; Giantsis, I.A.; Karagiannis, D.; Theodorou, J.A.; Michaelidis, B. Gut Symbiotic Microbial Communities in the IUCN Critically Endangered Pinna nobilis Suffering from Mass Mortalities, Revealed by 16S rRNA Amplicon NGS. Pathogens 2020, 9, 1002. [Google Scholar] [CrossRef] [PubMed]
- Lattos, A.; Feidantsis, K.; Georgoulis, I.; Giantsis, I.A.; Karagiannis, D.; Theodorou, J.A.; Staikou, A.; Michaelidis, B. Pathophysiological responses of Pinna nobilis individuals enlightens the etiology of mass mortality situation in the mediterranean populations. Cells 2021, 10, 2838. [Google Scholar] [CrossRef] [PubMed]
- Arzul, I.; Chollet, B.; Boyer, S.; Bonnet, D.; Gaillard, J.; Baldi, Y.; Robert, M.; Joly, J.Â.P.; Garcia, C.; Bouchoucha, M. Contribution to the understanding of the cycle of the protozoan parasite Marteilia refringens. Parasitology 2014, 141, 227–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robledo, J.A.; Figueras, A. The effects of culture-site, depth, season, and stock source on the prevalence of Marteilia refringens in cultured mussels (Mytilus galloprovincialis Lmk.) from Galicia, Spain. J. Parasitol. 1995, 81, 354–363. [Google Scholar] [CrossRef]
- Audemard, C.; Sajus, M.C.; Barnaud, A.; Sautour, B.; Sauriau, P.G.; Berthe, F.J. Infection dynamics of Marteilia refringens in flat oyster Ostrea edulis and copepod Paracartia grani in a claire pond of Marennes-Oleron Bay. Dis. Aquat. Org. 2004, 61, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Elgharsalli, R.; Seguineau, C.; Arzul, I.; Aloui-Bejaoui, N.; Quere, C.; Moal, J. Effect of infection by the protistan parasite Marteilia refringens on the enzyme activity and energy reserves of oyster Ostrea stentina (Payraudeau, 1826) in Tunisia. J. Mar. Biol. Assoc. 2018, 98, 161–170. [Google Scholar] [CrossRef]
- Lattos, A.; Giantsis, I.A.; Karagiannis, D.; Michaelidis, B. First detection of the invasive Haplosporidian and Mycobacteria parasites hosting the endangered bivalve Pinna nobilis in Thermaikos Gulf, North Greece. Mar. Environ. Res. 2020, 155, 104889. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, M.C.; Houston, C.T.; Alcantar, C.Y.; Milshteyn, L.; Brazil, C.A.; Zepeda, O.G. Interactive effects of multiple stressors on the physiological performance of the invasive mussel Mytilus galloprovincialis. Mar. Environ. Res. 2022, 178, 105665. [Google Scholar] [CrossRef] [PubMed]
- Banni, M.; Hajer, A.; Sforzini, S.; Oliveri, C.; Boussetta, H.; Viarengo, A. Transcriptional expression levels and biochemical markers of oxidative stress in Mytilus galloprovincialis exposed to nickel and heat stress. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2014, 160, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.L.; Burnett, N.P.; Ramsey, M.J.; Wagner, K.; Zippay, M.L. Physiological responses to heat stress in an invasive mussel Mytilus galloprovincialis depend on tidal habitat: Habitat-specific stress response in mussels. Mar. Environ. Res. 2020, 154, 104849. [Google Scholar] [CrossRef]
- Munari, M.; Matozzo, V.; Marin, M.G. Combined effects of temperature and salinity on functional responses of haemocytes and survival in air of the clam Ruditapes philippinarum. Fish Shellfish Immunol. 2011, 30, 1024–1030. [Google Scholar] [CrossRef]
- Monari, M.; Matozzo, V.; Foschi, J.; Cattani, O.; Serrazanetti, G.P.; Marin, M.G. Effects of high temperatures on functional responses of haemocytes in the clam Chamelea gallina. Fish Shellfish Immunol. 2007, 22, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Carella, F.; Aceto, S.; Mangoni, O.; Mollica, M.P.; Cavaliere, G.; Trinchese, G.; Aniello, F.; De Vico, G. Assessment of the health status of mussels Mytilus galloprovincialis along the campania coastal areas: A multidisciplinary approach. Front. Physiol. 2018, 9, 683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Box, A.; Capó, X.; Tejada, S.; Catanese, G.; Grau, A.; Deudero, S.; Sureda, A.; Valencia, J.M. Reduced antioxidant response of the fan mussel Pinna nobilis related to the presence of Haplosporidium pinnae. Pathogens 2020, 9, 932. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, D.; Michaelidis, B.; Theodoridis, A.; Angelidis, P.; Feidantsis, K.; Staikou, A. Field studies on the effects of Marteilia sp. on growth of mussel Mytilus galloprovincialis in Thermaikos Gulf. Mar. Environ. Res. 2018, 142, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Karunanithi, S.; Brown, I.R. Heat shock response and homeostatic plasticity. Front. Cell. Neurosci. 2015, 9, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feidantsis, K.; Georgoulis, I.; Giantsis, I.A.; Michaelidis, B. Treatment with ascorbic acid normalizes the aerobic capacity, antioxidant defence, and cell death pathways in thermally stressed Mytilus galloprovincialis. Comp. Biochem. Physiol. Part B 2021, 255, 110611. [Google Scholar] [CrossRef] [PubMed]
- Ammon, H.P.T. Phytomedicine Modulation of the immune system by Boswellia serrata extracts and boswellic acids. Eur. J. Integr. Med. 2010, 17, 862–867. [Google Scholar] [CrossRef]
- Ivanina, A.V.; Taylor, C.; Sokolova, I.M. Effects of elevated temperature and cadmium exposure on stress protein response in eastern oysters Crassostrea virginica (Gmelin). Aquat. Toxicol. 2009, 91, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Ueda, N.; Boettcher, A. Differences in heat shock protein 70 expression during larval and early spat development in the Eastern oyster, Crassostrea virginica (Gmelin, 1791). Cell Stress Chaperones 2009, 14, 439–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cellura, C.; Toubiana, M.; Parrinello, N.; Roch, P. HSP70 gene expression in Mytilus galloprovincialis hemocytes is triggered by moderate heat shock and Vibrio anguillarum, but not by V. splendidus or Micrococcus lysodeikticus. Dev. Comp. Immunol. 2006, 30, 984–997. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Rahman, S. Effects of elevated temperature on prooxidant-antioxidant homeostasis and redox status in the American oyster: Signaling pathways of cellular apoptosis during heat stress. Environ. Res. 2021, 196, 110428. [Google Scholar] [CrossRef] [PubMed]
- Nash, S.; Johnstone, J.; Rahman, M.S. Elevated temperature attenuates ovarian functions and induces apoptosis and oxidative stress in the American oyster, Crassostrea virginica: Potential mechanisms and signaling pathways. Cell Stress Chaperones 2019, 24, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Kolkman, A.; Daran-Lapujade, P.; Fullaondo, A.; Olsthoorn, M.M.A.; Pronk, J.T.; Slijper, M.; Heck, A.J.R. Proteome analysis of yeast response to various nutrient limitations. Mol. Syst. Biol. 2006, 2, 2006–0026. [Google Scholar] [CrossRef] [PubMed]
- Brockmann, R.; Beyer, A.; Heinisch, J.J.; Wilhelm, T. Post-transcriptional expression regulation: What determines translation rates? PLoS Comput. Biol. 2007, 3, 0531–0539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingolia, N.T.; Ghaemmaghami, S.; Newman, J.R.; Weissman, J.S. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science 2009, 324, 218–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, K.C.; Guo, X.L.; White, C.W. Induction of thioredoxin and thioredoxin reductase gene expression in lungs of newborn primates by oxygen. Am. J. Physiol. 1999, 276, L530–L539. [Google Scholar] [CrossRef] [PubMed]
- Das, K.C.; Pahl, P.M.B.; Guo, X.L.; White, C.W. Induction of peroxiredoxin gene expression by oxygen in lungs of newborn primates. Am. J. Respir. Cell Mol. Biol. 2001, 25, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I.; Lushchak, L.P.; Mota, A.A.; Hermes-Lima, M. Oxidative stress and antioxidant defenses in goldfish Carassius auratus during anoxia and reoxygenation. Am. J. Physiol. 2001, 280, 100–107. [Google Scholar] [CrossRef] [Green Version]
- Schwanhäusser, Β.; Busse, D.; Li, N.; Dittmar, G.; Schuchhardt, J.; Wolf, J.; Chen, W.; Selbach, M. Global quantification of mammalian gene expression control. Nature 2011, 473, 337–342. [Google Scholar] [CrossRef] [PubMed]



| Target Gene | Forward Primer (5′-3′) | Amplicon (bp) | GenBank Accession | Reference |
|---|---|---|---|---|
| Reverse Primer (5′-3′) | No. | |||
| hsp70 | CGGAGGCAAGCCAAAACTAC | 109 | AB180909.1 | Giannetto et al. [54] |
| AGCCTCGGCAGTTTCTTTCA | ||||
| bax | CCAACAGGTCCACCATTAGAAC | 151 | KC545830.1 | Estevez-Calvar et al. [55] |
| CTCTTGGCCACAGTTAGGAATG | ||||
| bcl-2 | AGATAACGGTGGTTGGCAAG TAACGCCATTGCGCCTAT | 127 | KC545829.1 | Estevez-Calvar et al. [55] |
| irak4 | TTTGAGGAAGATGCTAAACCTG | 127 | KC994891.1 | Toubiana et al. [56] |
| CAACTGAGAAACCCAAGAAAG | ||||
| traf6 | GAAGGCTGTAAAGTGATAGAAGTT | 135 | KC994893.1 | Toubiana et al. [56] |
| CTGAGATAGATGATGAGGTAAGTC | ||||
| β-actin | CGACTCTGGAGATGGTGTCA | 153 | AF157491.1 | Moreira et al. [57] |
| GCGGTGGTTGTGAATGAGTA | ||||
| EF-1α | GATATGCGCCAGTCTTGGAT | 223 | AB162021 | Moreira et al. [57] |
| CTCATGTCTCGGACAGCAAA |
| Sampling Date | Infection Rate | ||
|---|---|---|---|
| Heavy | Moderate | Light | |
| 13 July 2022 | + | − | − |
| 25 July 2022 | + | − | + |
| 1 August 2022 | − | − | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lattos, A.; Papadopoulos, D.K.; Feidantsis, K.; Karagiannis, D.; Giantsis, I.A.; Michaelidis, B. Are Marine Heatwaves Responsible for Mortalities of Farmed Mytilus galloprovincialis? A Pathophysiological Analysis of Marteilia Infected Mussels from Thermaikos Gulf, Greece. Animals 2022, 12, 2805. https://doi.org/10.3390/ani12202805
Lattos A, Papadopoulos DK, Feidantsis K, Karagiannis D, Giantsis IA, Michaelidis B. Are Marine Heatwaves Responsible for Mortalities of Farmed Mytilus galloprovincialis? A Pathophysiological Analysis of Marteilia Infected Mussels from Thermaikos Gulf, Greece. Animals. 2022; 12(20):2805. https://doi.org/10.3390/ani12202805
Chicago/Turabian StyleLattos, Athanasios, Dimitrios K. Papadopoulos, Konstantinos Feidantsis, Dimitrios Karagiannis, Ioannis A. Giantsis, and Basile Michaelidis. 2022. "Are Marine Heatwaves Responsible for Mortalities of Farmed Mytilus galloprovincialis? A Pathophysiological Analysis of Marteilia Infected Mussels from Thermaikos Gulf, Greece" Animals 12, no. 20: 2805. https://doi.org/10.3390/ani12202805
APA StyleLattos, A., Papadopoulos, D. K., Feidantsis, K., Karagiannis, D., Giantsis, I. A., & Michaelidis, B. (2022). Are Marine Heatwaves Responsible for Mortalities of Farmed Mytilus galloprovincialis? A Pathophysiological Analysis of Marteilia Infected Mussels from Thermaikos Gulf, Greece. Animals, 12(20), 2805. https://doi.org/10.3390/ani12202805








