Glycine Alleviated Intestinal Injury by Inhibiting Ferroptosis in Piglets Challenged with Diquat
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Design
2.2. Sample Collection
2.3. Intestinal Morphology
2.4. Disaccharidase Activities of the Intestinal Mucosa
2.5. Protein, DNA, and RNA Contents of the Intestinal mucosa
2.6. Antioxidative Capacity of the Intestinal Mucosa
2.7. Gene Expression Analysis
2.8. Protein Abundance Analysis
2.9. Statistical Analyses
3. Results
3.1. Intestinal Morphology
3.2. Disaccharidases Activities of the Intestinal Mucosa
3.3. Protein, DNA, and RNA Contents of the Intestinal Mucosa
3.4. Antioxidative Capacity of the Intestinal Mucosa
3.5. Intestinal Mucosal Gene Expressions of the Key Genes Related to Ferroptosis
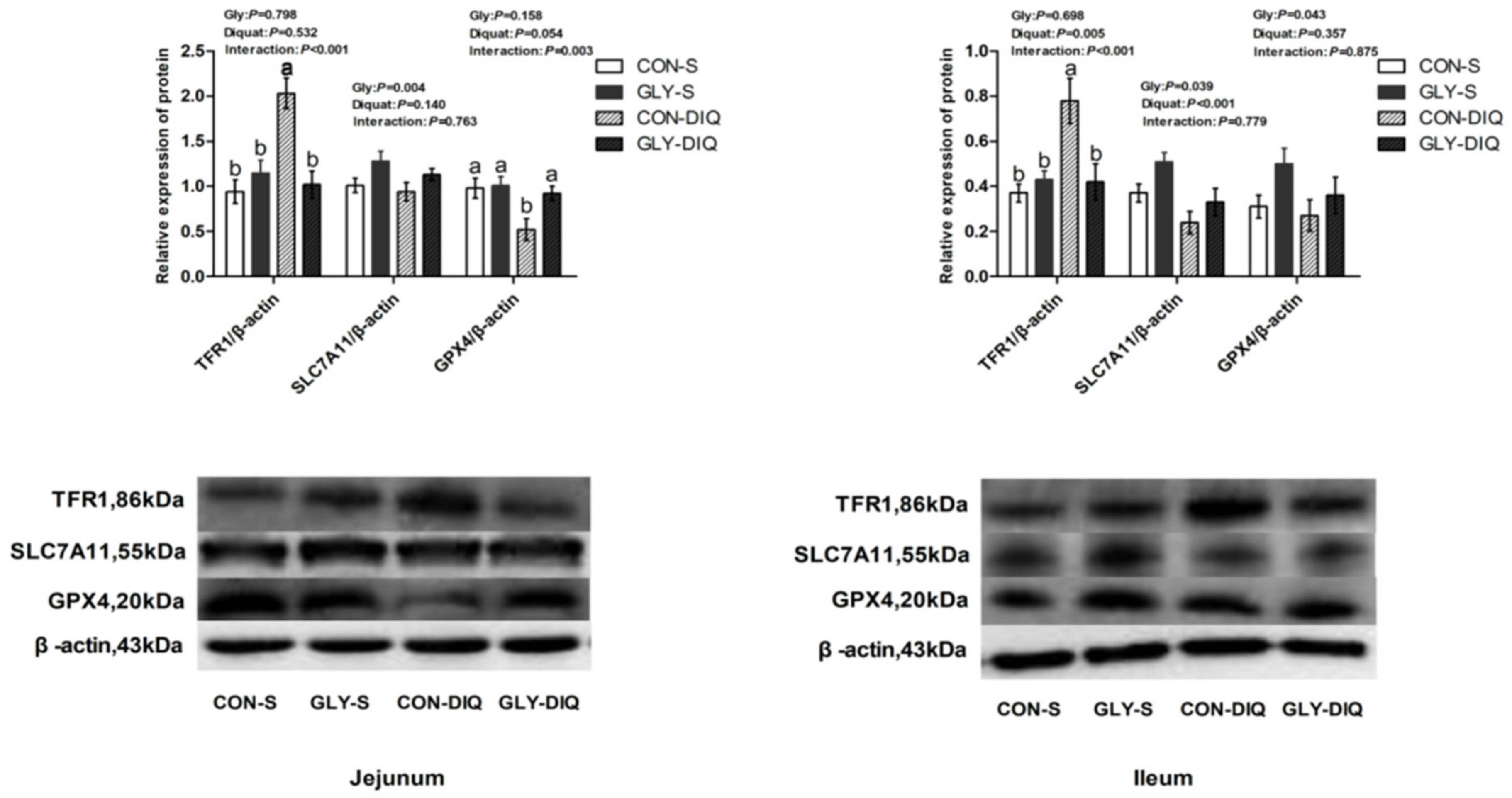
3.6. Intestinal Mucosal Protein Abundance of the Key Proteins Related to Ferroptosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, P.W.; Hua, H.W.; Tian, W.; Zhu, H.L.; Liu, Y.L.; Xu, X. Holly (Ilex latifolia Thunb.) polyphenols extracts alleviate hepatic damage by regulating ferroptosis following diquat challenge in a piglet model. Front. Nutr. 2020, 7, 604328. [Google Scholar] [CrossRef] [PubMed]
- Circu, M.L.; Aw, T.Y. Intestinal redox biology and oxidative stress. Semin. Cell Dev. Biol. 2012, 23, 729–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueira, T.R.; Barros, M.H.; Camargo, A.A.; Castilho, R.F.; Ferreira, J.C.B.; Kowaltowski, A.J.; Sluse, F.E.; Souza-Pinto, N.C.; Vercesi, A.E. Mitochondria as a source of reactive oxygen and nitrogen species: From molecular mechanisms to human health. Antioxid. Redox Signal. 2013, 18, 2029–2074. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Kim, K.J.; Gaschler, M.M.; Patel, M.; Shchepinov, M.S.; Stockwell, B.R. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci. USA 2016, 113, 4966–4975. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Wu, M.M.; Li, Y.Y.; Ren, W.K.; Xiao, H.; Chen, S.; Li, C.Y.; Tan, B.; Ni, H.J.; Xiong, X. Toxicity assessment of hydrogen peroxide on Toll-like receptor system, apoptosis, and mitochondrial respiration in piglets and IPEC-J2 cells. Oncotarget 2017, 8, 3124–3131. [Google Scholar] [CrossRef] [Green Version]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [Green Version]
- Stockwell, B.R.; Angeli, J.P.F.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascon, S.; Hatzios, S.K.; Kagan, V.E. Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [Green Version]
- Dixon, S.J.; Stockwell, B.R. The Hallmarks of Ferroptosis. Annu. Rev. Cancer Biol. 2019, 3, 35–54. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Z.; Dai, Z.; Yang, Y.; Wang, J.; Wu, G. Glycine metabolism in animals and humans: Implications for nutrition and health. Amino Acids. 2013, 45, 463–477. [Google Scholar] [CrossRef]
- Wang, W.; Dai, Z.; Wu, Z.; Lin, G.; Jia, S.; Hu, S.; Dahanayaka, S.; Wu, G. Glycine is a nutritionally essential amino acid for maximal growth of milk-fed young pigs. Amino Acids 2014, 46, 2037–2045. [Google Scholar] [CrossRef]
- Wu, G. Dietary requirements of synthesizable amino acids by animals: A paradigm shift in protein nutrition. J. Anim. Sci. Biotechno. 2014, 5, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flynn, N.E.; Knabe, D.A.; Mallick, B.K.; Wu, G. Postnatal changes of plasma amino acids in suckling pigs. J. Anim. Sci. 2000, 78, 2369–2375. [Google Scholar] [CrossRef]
- Barakat, H.; Hamza, A.H. Glycine alleviates liver injury induced by deficiency in methionine and or choline in rats. Aust. J. Basic Appl. Sci. 2011, 5, 1061–1070. [Google Scholar]
- Senthilkumar, R.; Viswanathan, P.; Nalini, N. Effect of glycine on oxidative stress in rats with alcohol induced liver injury. Pharmazie. 2004, 59, 55–60. [Google Scholar]
- Marsh, D.C.; Vreugdenhil, P.K.; Mack, V.E.; Belzer, F.O.; Southard, J.H. Glycine protects hepatocytes from injury caused by anoxia, cold ischemia and mitochondrial inhibitors, but not injury caused by calcium ionophores or oxidative stress. Hepatology 2010, 17, 91–98. [Google Scholar] [CrossRef]
- Angeli, J.P.F.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E.; et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014, 16, 1180–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doan, N.; Liu, Y.; Xiong, X.; Kim, K.; Wu, Z.; Bravo, D.M.; Blanchard, A.; Ji, P. Organic selenium supplement partially alleviated diquat-induced oxidative insults and hepatic metabolic stress in nursery pigs. Br. J. Nutr. 2020, 124, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Wang, A.N.; Cai, C.J.; Zeng, X.F.; Zhang, F.R.; Zhang, G.L.; Thacker, P.A.; Wang, J.J.; Qiao, S.Y. Dietary supplementation with Lactobacillus fermentum I5007 improves the anti-oxidative activity of weanling piglets challenged with diquat. J. Appl. Micorbiol. 2013, 114, 1582–1591. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.K.; Wang, H.; Yang, H.; Tan, B.; Zhou, S.; Guan, G. Effects of dietary carboxymethyl pachyman on oxidative stress and inflammation in weaned piglets challenged with diquat. Anim. Feed Sci. Technol. 2021, 276, 114922. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Swine, 11th ed.; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Pi, D.; Liu, Y.; Shi, H.; Li, S.; Odle, J.; Lin, X.; Zhu, H.; Chen, F.; Hou, Y.; Leng, W. Dietary supplementation of aspartate enhances intestinal integrity and energy status in weanling piglets after lipopolysaccharide challenge. J. Nutr. Biochem. 2014, 25, 456–462. [Google Scholar] [CrossRef]
- Liu, Y.L.; Huang, J.J.; Hou, Y.Q.; Zhu, H.L.; Zhao, S.J.; Ding, B.Y.; Yin, Y.L.; Yi, G.F.; Shi, J.X.; Fan, W. Dietary arginine supplementation alleviates intestinal mucosal disruption induced by Escherichia coli lipopolysaccharide in weaned pigs. Br. J. Nutr. 2008, 100, 552–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Chen, S.K.; Wang, H.B.; Tu, Z.X.; Wang, S.H.; Wang, X.Y.; Zhu, H.L.; Wang, C.W.; Zhu, J.D.; Liu, Y.L. Medium-chain TAG improve intestinal integrity by suppressing toll-like receptor 4, nucleotide-binding oligomerisation domain proteins and necroptosis signaling in weanling piglets challenged with lipopolysaccharide. Br. J. Nutr. 2018, 119, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.L.; Liu, Y.L.; Chen, S.K.; Wang, X.Y.; Pi, D.A.; Leng, W.B.; Chen, F.; Zhang, J.; Kang, P. Fish oil enhances intestinal barrier function and inhibits corticotropin-releasing hormone/corticotropin-releasing hormone receptor 1 signalling pathway in weaned pigs after lipopolysaccharide challenge. Br. J. Nutr. 2016, 115, 1947–1957. [Google Scholar] [CrossRef] [Green Version]
- Hong, Q.H.; Li, X.; Lin, Q.; Shen, Z.J.; Feng, J.; Hu, C.H. Resveratrol improves intestinal morphology and anti-oxidation ability in deoxynivalenol-challenged piglets. Animals 2022, 12, 311. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Li, C.Y.; Yin, Y.L.; Zhang, S.; Li, X.Z.; Sun, Q.P.; Wan, D. Effects of zinc oxide/zeolite on intestinal morphology, intestinal microflora, and diarrhea rates in weaned piglets. Biol. Trace Elem. Res. 2021, 199, 1405–1413. [Google Scholar] [CrossRef]
- Tsukahara, T.; Inoue, R.; Nakatani, M.; Fukuta, K.; Kishino, E.; Ito, T.; Ushida, K. Influence of weaning age on the villous height and disaccharidase activities in the porcine small intestine. Anim. Sci. J. 2016, 87, 67–75. [Google Scholar] [CrossRef]
- Hartke, J.L.; Monaco, M.H.; Wheeler, M.B.; Donovan, S.M. Effect of a short-term fast on intestinal disaccharidase activity and villus morphology of piglets suckling insulin-like growth factor-I transgenic sows. J. Anim. Sci. 2005, 83, 2404–2413. [Google Scholar] [CrossRef]
- Scharl, M.; Paul, G.; Barrett, K.E.; McCole, D.F. AMP-activated protein kinase mediates the interferon-gamma-induced decrease in intestinal epithelial barrier function. J. Biol. Chem. 2010, 284, 27952–27963. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Wang, X.Y.; Wu, H.T.; Zhu, H.L.; Liu, C.C.; Hou, Y.Q.; Dai, B.; Liu, X.T.; Liu, Y.L. Glycine relieves intestinal injury by maintaining MTOR signaling and suppressing AMPK, TLR4, and NOD signaling in weaned piglets after lipopolysaccharide challenge. Int. J. Mol. Sci. 2018, 19, 1980. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.X.; Huang, R.; Wang, N.; Deng, Y.K.; Tan, B.; Yin, Y.L.; Qi, M.; Wang, J. Ellagic acid alleviates oxidative stress by mediating Nrf2 signaling pathways and protects against paraquat-induced intestinal injury in piglets. Antioxidants 2022, 11, 252. [Google Scholar] [CrossRef]
- Xu, X.; Wei, Y.; Hua, H.W.; Jing, X.Q.; Zhu, H.L.; Xiao, K.; Zhao, J.C.; Liu, Y.L. Polyphenols sourced from Ilex Latifolia Thunb. relieve intestinal injury via modulating ferroptosis in weanling piglets under oxidative stress. Antioxidants 2022, 11, 966. [Google Scholar] [CrossRef]
- Yang, Y.; Fan, X.X.; Ji, Y.; Li, J.; Dai, Z.; Wu, Z.L. Glycine represses endoplasmic reticulum stress-related apoptosis and improves intestinal barrier by activating mammalian target of rapamycin complex 1 signaling. Anim. Nutr. 2022, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Yu, B.; Mao, X.B.; Zheng, P.; He, J.; Chen, D.W. Responses of growth performance and tryptophan metabolism to oxidative stress induced by diquat in weaned pigs. Animal 2012, 6, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, L.; Zheng, F.P.; Jia, C.F.; Ruan, Y.; Li, H. Correlation between the amplitude of glucose excursion and the oxidative/antioxidative system in subjects with different types of glucose regulation. Biomed. Environ. Sci. 2011, 24, 68–73. [Google Scholar] [PubMed]
- El-Hafidi, M.; Franco, M.; Ramírez, A.R.; Sosa, J.S.; Flores, J.A.P.; Acosta, O.L.; Salgado, M.C.; Cardoso-Saldaña, G. Glycine increases insulin sensitivity and glutathione biosynthesis and protects against oxidative stress in a model of sucrose-induced insulin resistance. Oxidative Med. Cell. Longev. 2018, 2018, 2101562. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Ramírez, A.; Ortiz-Balderas, E.; Cardozo-Saldaña, G.; Diaz-Diaz, E.; El-Hafidi, M. Glycine restores glutathione and protects against oxidative stress in vascular tissue from sucrose-fed rats. Clin. Sci. Lond. Engl. 2014, 126, 19–29. [Google Scholar] [CrossRef]
- Hua, H.W.; Xu, X.; Tian, W.; Li, P.; Zhu, H.L.; Wang, W.J.; Liu, Y.L.; Xiao, K. Glycine alleviated diquat-induced hepatic injury via inhibiting ferroptosis in weaned piglets. Anim. Biosci. 2022, 35, 938–947. [Google Scholar] [CrossRef]
- Xu, S.; He, Y.; Lin, L.H.; Chen, P.; Chen, M.H.; Zhang, S.H. The emerging role of ferroptosis in intestinal disease. Cell Death Dis. 2021, 12, 289. [Google Scholar] [CrossRef]
- Forcina, G.C.; Dixon, S.J. GPX4 at the crossroads of lipid homeostasis and ferroptosis. Proteomics 2019, 19, 1800311. [Google Scholar] [CrossRef]
- Gao, M.H.; Monian, P.; Jiang, X.J. Metabolism and iron signaling in ferroptotic cell death. Oncotarget 2015, 6, 35145–35146. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Yang, Y.Q.; Sheng, H.H.; Tang, Q.; Han, L.; Wang, S.M.; Wu, W.Y. GPX4 plays a crucial role in Fuzheng Kang’ai decoction-induced non-small cell lung cancer cell ferroptosis. Front. Pharmacol. 2022, 13, 851680. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Carlisle, A.E.; Peppers, A.; Park, S.J.; Doshi, M.B.; Spears, M.E.; Kim, D. XCT-driven expression of GPX4 determines sensitivity of breast cancer cells to ferroptosis inducers. Antioxidants 2021, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Bai, L.L.; Fang, X.; Liu, J.; Kang, R.; Zhou, D.; Tang, D.; Dai, E. SMG9 drives ferroptosis by directly inhibiting GPX4 degradation. Biochem. Biophys. Res. Commun. 2021, 567, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.C.; Wu, Y.; Chen, Y.T.; Xiong, X.L.; Li, P.; Peng, X.M.; Li, C.M.; Weng, W.J.; Zhu, Y.F.; Zhou, D.H.; et al. Arsenic trioxide increases apoptosis of SK-N-BE (2) cells partially by inducing GPX4-mediated ferroptosis. Mol. Biol. Rep. 2022, 49, 6573–6580. [Google Scholar] [CrossRef]
| Gene | Forward (5’-3’) | Reverse (5’-3’) | Annealing Temperature (℃) | Product Length (bp) | Accession Numbers |
|---|---|---|---|---|---|
| TFR1 | CGAAGTGGCTGGTCATCT | TGTCTCTTGTCTCTACATTCCT | 60 | 231 | NM_214001.1 |
| HSPB1 | CTCGGAGATCCAGCAGACT | TCGTGCTTGCCCGTGAT | 60 | 120 | NM_001007518 |
| SLC7A11 | GCCTTGTCCTATGCTGAGTTG | GTTCCAGAATGTAGCGTCCAA | 60 | 178 | XM_021101587.1 |
| GPX4 | CTGTTCCGCCTGCTGAA | ACCTCCGTCTTGCCTCAT | 60 | 218 | NM_214407.1 |
| GAPDH | CGTCCCTGAGACACGATGGT | GCCTTGACTGTGCCGTGGAAT | 60 | 194 | AF_017079.1 |
| Item | Saline | Diquat | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| Basal | Glycine | Basal | Glycine | Diet | Stress | Interaction | ||
| Jejunum | ||||||||
| VH (μm) | 255 | 284 | 216 | 268 | 9 | <0.001 | <0.001 | 0.052 |
| CD (μm) | 162 b | 167 b | 145 c | 176 a | 4 | <0.001 | 0.192 | <0.001 |
| VH/CD | 1.57 | 1.71 | 1.49 | 1.53 | 0.05 | 0.006 | <0.001 | 0.301 |
| Ileum | ||||||||
| VH (μm) | 272 a | 281 a | 242 b | 277 a | 7 | <0.001 | 0.004 | 0.034 |
| CD (μm) | 171 | 167 | 164 | 169 | 4 | 0.978 | 0.216 | 0.069 |
| VH/CD | 1.59 | 1.69 | 1.48 | 1.65 | 0.04 | <0.001 | 0.022 | 0.479 |
| Item | Saline | Diquat | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| Basal | Glycine | Basal | Glycine | Diet | Stress | Interaction | ||
| Jejunum | ||||||||
| Lactase | 25.8 | 32.5 | 27.9 | 31.6 | 2.4 | 0.003 | 0.854 | 0.680 |
| Sucrase | 36.4 a | 35.3 a | 26.6 b | 41.5 a | 4.0 | 0.312 | 0.412 | <0.001 |
| Maltase | 173 ab | 194 a | 158 b | 184 a | 16 | 0.188 | 0.067 | 0.041 |
| Ileum | ||||||||
| Lactase | 6.02 | 10.06 | 6.23 | 9.87 | 0.68 | <0.001 | 0.689 | 0.850 |
| Sucrase | 15.4 | 13.5 | 12.1 | 14.4 | 1.3 | 0.425 | 0.553 | 0.136 |
| Maltase | 107 a | 104 a | 74 b | 121 a | 12 | 0.215 | 0.430 | <0.001 |
| Item | Saline | Diquat | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| Basal | Glycine | Basal | Glycine | Diet | Stress | Interaction | ||
| Jejunum | ||||||||
| Protein | 5.48 a | 5.70 a | 4.65 b | 5.82 a | 0.26 | 0.006 | 0.135 | <0.001 |
| RNA/DNA | 6.48 | 7.14 | 5.08 | 6.21 | 0.47 | 0.014 | <0.001 | 0.215 |
| Protein/DNA | 0.12 | 0.15 | 0.10 | 0.12 | 0.01 | 0.034 | 0.038 | 0.674 |
| Ileum | ||||||||
| Protein | 5.69 a | 5.80 a | 4.43 c | 5.01 b | 0.24 | 0.245 | <0.001 | 0.014 |
| RNA/DNA | 4.14 a | 3.68 ab | 2.21 c | 3.32 b | 0.23 | 0.004 | <0.001 | <0.001 |
| Protein/DNA | 0.08 a | 0.07 a | 0.05 b | 0.07 a | 0.01 | 0.528 | 0.031 | 0.003 |
| Item | Saline | Diquat | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| Basal | Glycine | Basal | Glycine | Diet | Stress | Interaction | ||
| Jejunum | ||||||||
| T-AOC, U/mg protein | 0.510 | 0.554 | 0.404 | 0.463 | 0.042 | 0.046 | 0.011 | 0.753 |
| GSH-PX, U/mg protein | 16.3 b | 28.0 a | 12.3 c | 16.6 b | 1.9 | <0.001 | 0.022 | 0.014 |
| GSH, mg GSH/g protein | 27.9 a | 27.8 a | 18.2 b | 24.3 a | 2.0 | 0.025 | <0.001 | 0.032 |
| MDA, nmol/mg protein | 1.45 b | 1.32 b | 2.61 a | 1.68 b | 0.26 | <0.001 | <0.001 | 0.011 |
| Ileum | ||||||||
| T-AOC, U/mg protein | 0.304 | 0.298 | 0.225 | 0.285 | 0.035 | 0.216 | 0.031 | 0.386 |
| GSH-PX, U/mg protein | 24.5 | 53.6 | 20.4 | 42.5 | 3.1 | <0.001 | 0.017 | 0.524 |
| GSH, mg GSH/g protein | 13.8 bc | 15.4 b | 8.8 c | 22.4 a | 3.0 | <0.001 | 0.418 | <0.001 |
| MDA, nmol/mg protein | 1.80 | 1.63 | 1.91 | 1.67 | 0.16 | 0.421 | 0.753 | 0.535 |
| Item | Saline | Diquat | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| Basal | Glycine | Basal | Glycine | Diet | Stress | Interaction | ||
| Jejunum | ||||||||
| TFR1 | 1.00 b | 0.85 b | 1.63 a | 1.06 b | 0.14 | 0.010 | <0.001 | 0.020 |
| HSPB1 | 1.00 | 0.86 | 0.88 | 1.00 | 0.14 | 0.938 | 0.871 | 0.254 |
| SLC7A11 | 1.00 b | 1.46 a | 0.64 c | 1.44 a | 0.18 | 0.003 | 0.274 | 0.002 |
| GPX4 | 1.00 d | 4.69 c | 7.57 b | 16.02 a | 1.10 | <0.001 | <0.001 | 0.040 |
| Ileum | ||||||||
| TFR1 | 1.00 b | 0.99 b | 1.66 a | 1.20 b | 0.16 | 0.243 | 0.005 | 0.042 |
| HSPB1 | 1.00 | 0.89 | 1.37 | 1.04 | 0.13 | 0.025 | 0.003 | 0.354 |
| SLC7A11 | 1.00 a | 1.28 a | 0.35 b | 1.03 a | 0.15 | <0.001 | <0.001 | 0.028 |
| GPX4 | 1.00 b | 1.05 b | 0.80 b | 1.84 a | 0.13 | 0.024 | 0.421 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Wei, Y.; Hua, H.; Zhu, H.; Xiao, K.; Zhao, J.; Liu, Y. Glycine Alleviated Intestinal Injury by Inhibiting Ferroptosis in Piglets Challenged with Diquat. Animals 2022, 12, 3071. https://doi.org/10.3390/ani12223071
Xu X, Wei Y, Hua H, Zhu H, Xiao K, Zhao J, Liu Y. Glycine Alleviated Intestinal Injury by Inhibiting Ferroptosis in Piglets Challenged with Diquat. Animals. 2022; 12(22):3071. https://doi.org/10.3390/ani12223071
Chicago/Turabian StyleXu, Xiao, Yu Wei, Hongwei Hua, Huiling Zhu, Kan Xiao, Jiangchao Zhao, and Yulan Liu. 2022. "Glycine Alleviated Intestinal Injury by Inhibiting Ferroptosis in Piglets Challenged with Diquat" Animals 12, no. 22: 3071. https://doi.org/10.3390/ani12223071
APA StyleXu, X., Wei, Y., Hua, H., Zhu, H., Xiao, K., Zhao, J., & Liu, Y. (2022). Glycine Alleviated Intestinal Injury by Inhibiting Ferroptosis in Piglets Challenged with Diquat. Animals, 12(22), 3071. https://doi.org/10.3390/ani12223071







