Effects of Dietary Bioactive Lipid Compounds of Acacia nilotica Bark on Productive Performance, Antioxidant Status, and Antimicrobial Activities of Growing Rabbits under Hot Climatic Conditions
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Housing, Animals, and Design
2.2. Preparation of Acacia nilotica BLCs
2.3. Growth Performance
2.4. Carcass Measurements
2.5. Determination of the Total Antioxidant Capacity
2.6. Determination of the Total Phenolic Content of Acacia Extracts
2.7. Antibacterial Activity
2.8. Statistical Analysis
3. Results
3.1. Total Antioxidant Capacity, Total Phenols, and Total Flavonoids in Acacia Bark BLCs
3.2. Productive Performance
3.3. Carcass Criteria
3.4. Blood and Liver Tissue Total Antioxidant Capacity (TAC)
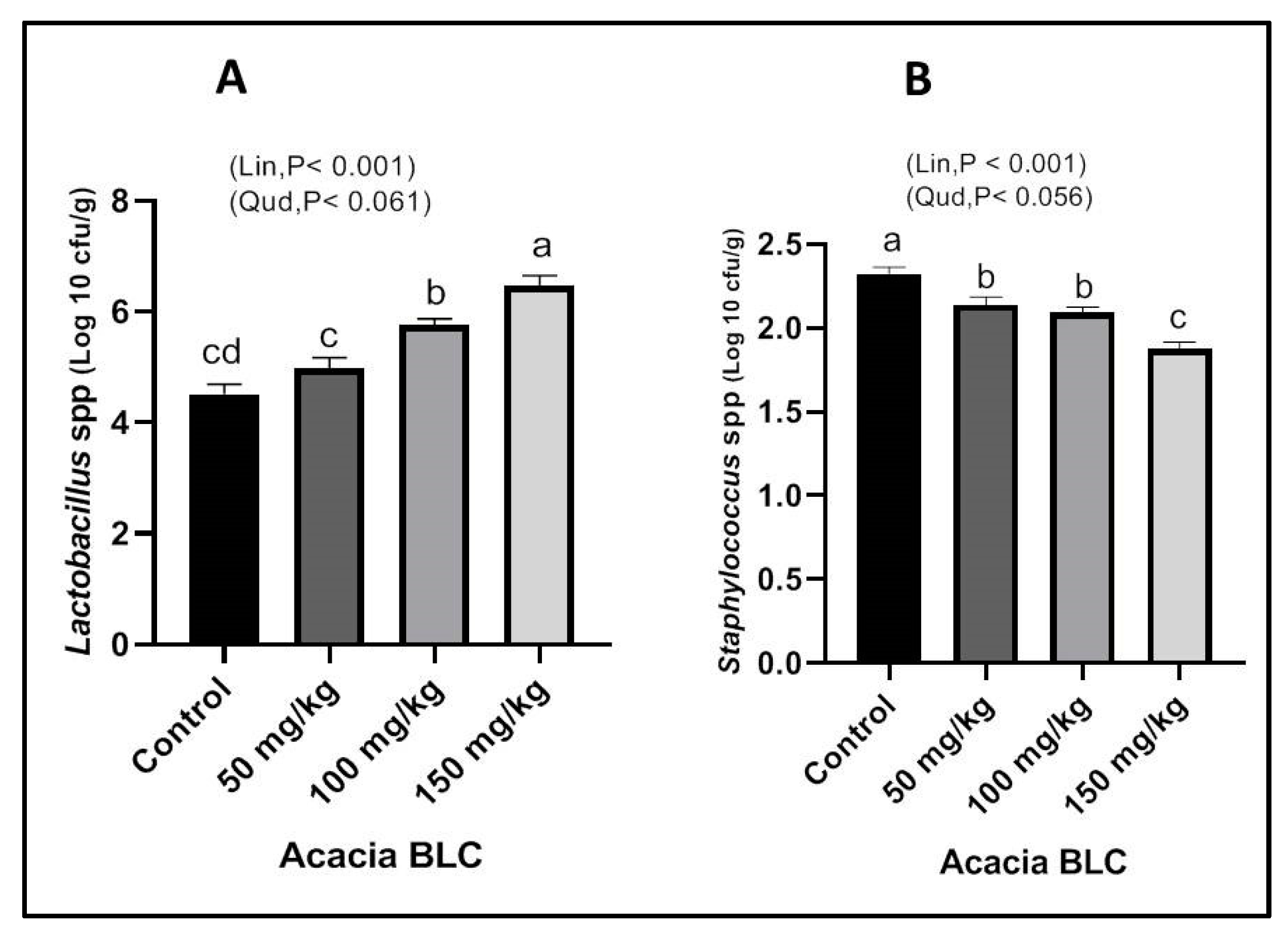
3.5. Cecum Microbial Counts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hausmann, J.; Deiner, C.; Immig, I.; Pieper, R.; Starke, A.; Aschenbach, J.R. Effects of combined supplementation with plant bioactive lipid compounds and biotin on ruminal fermentation, body condition and energy metabolism in transition dairy cows. Anim. Feed Sci. Technol. 2017, 225, 27–37. [Google Scholar] [CrossRef]
- Marai, I.F.M.; Habeeb, A.A.M.; Gad, A.E. Rabbits’ productive, reproductive and physiological performance traits as affected by heat stress: A review. Livest Prod Sci. 2022, 78, 71–90. [Google Scholar] [CrossRef]
- Zeferino, C.P.; Komiyama, C.M.; Fernandes, S.; Sartori, J.R.; Teixeira, P.S.S.; Moura, A.S.A.M.T. Carcass and meat quality traits of rabbits under heat stress. Animal 2013, 7, 518–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdalla, A.A.; Mustafa, M.I.; Makhawi, A.M. Phytochemical screening and antimicrobial activities studies of Acacia nilotica fruit cover. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Gaara, A.H.; El-Toumy, S.A.; Farrag, A.R.H.; Ahmed, N.M. Antidiabetic, antihyperlipidemic and antioxidant activities of acacia albida in streptozotoc in induced diabetes in rats and its metabolites. Egypt. J. Chem. 2020, 63, 337–348. [Google Scholar] [CrossRef]
- Aremu, O.; Olayemi, O.; Ajala, T.; Isimi, Y.; Oladosu, P.; Ekere, K.; John, J.; Emeje, M. Antibacterial evaluation of acacia nilotica lam (Mimosaceae) seed extract in dermatological preparations. J. Res. Pharm. 2020, 24, 170–181. [Google Scholar] [CrossRef] [Green Version]
- Ziani, B.E.C.; Carocho, M.; Abreu, R.M.V.; Bachari, K.; Alves, M.J.; Calhelha, R.C.; Talhi, O.; Barros, L.; Ferreira, I.C.F.R. Phenolic profiling, biological activities and in silico studies of Acacia tortilis (Forssk.) Hayne ssp. raddiana extracts. Food Biosci. 2020, 36, 100616. [Google Scholar] [CrossRef]
- El-Chaghaby, G.A.; Rashad, S.; Abdel-Kader, S.F.; Rawash, E.-S.A.; Moneem, M.A.; History, A. Assessment of phytochemical components, proximate composition and antioxidant properties of Scenedesmus obliquus, Chlorella vulgaris and Spirulina platensis algae extracts. J. Aquat. Biol. Fish. 2019, 23, 521–526. [Google Scholar] [CrossRef] [Green Version]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [Green Version]
- Adegoke, G.O.; Ojo, O.A. Phytochemical, Antioxidant and Antimicrobial Activities in the Leaf, Stem and Fruit Fractions of Basella Alba and Basella Rubra. Plant 2017, 5, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Čobanová, K.; Váradyová, Z.; Grešáková, Ľ.; Kucková, K.; Mravčáková, D.; Várady, M. Does herbal and/or zinc dietary supplementation improve the antioxidant and mineral status of lambs with parasite infection? Antioxidants 2020, 9, 1172. [Google Scholar] [CrossRef] [PubMed]
- Turkmen, N.; Sari, F.; Velioglu, Y.S. Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin–Ciocalteu methods. Food Chem. 2006, 99, 835–841. [Google Scholar] [CrossRef]
- Mohdaly, A.A.; Sarhan, M.A.; Smetanska, I.; Mahmoud, A. Antioxidant properties of various solvent extracts of potato peel, sugar beet pulp and sesame cake. J. Sci. Food Agric. 2010, 90, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Malabadi, R.B.; Mulgund, G.S.; Meti, N.T.; Nataraja, K. Antibacterial activity of silver nanoparticles synthesized by using whole plant extracts of Clitoria ternatea. Res. Pharm. 2012, 2, 10–21. [Google Scholar]
- SAS [SAS Institute]. SAS/STAT® 9.2User’s Guide, 2nd ed.; SAS Institute Inc.: Cary, NC, USA, 2009. [Google Scholar]
- El-Chaghaby, G.A.; Ahmad, A.F.; Ramis, E.S. Evaluation of the antioxidant and antibacterial properties of various solvents extracts of Annona squamosa L. leaves. Arab. J. Chem. 2014, 7, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Bangou, M.J.; Koama, K.B.; Coulidiati, T.H.; Meda, R.N.-T.; Thiombiano, A.M.E.; Traoré-Coulibaly, M.; Nacoulma, O.G. Antiplasmodial activities of Acacia gourmaensis A. RICH (Mimosaceae). Bio. Sci. Pharm. Res. 2019, 7, 46–57. [Google Scholar]
- Ali, R.; Tabrez, S.; Rahman, F.; Alouffi, A.S.; Alshehri, B.M.; Alshammari, F.A.; Alaidarous, M.A.; Banawas, S.; Dukhyil, A.A.; Rub, A. Antileishmanial evaluation of bark methanolic extract of Acacia nilotica: In vitro and in silico studies. ACS Omega 2021, 6, 8548–8560. [Google Scholar] [CrossRef]
- Abd-El-Hady, A.M. Performance, physiological parameters and slaughter characteristics in growing rabbits as affected by a herbal feed additives (digestarom). J. Agri. Food. 2014, 2, 353–365. [Google Scholar]
- Hills, J.M.; Aaronson, P.I. The mechanism of action of peppermint oil on gastrointestinal smooth muscle. An analysis using patch clamp electrophysiology and isolated tissue pharmacology in rabbit and guinea pig. Gastroenterology 1991, 101, 55–65. [Google Scholar] [CrossRef]
- Fran, K.; Donald, E.; James, G. Research trends in healthful foods. Food Technol. 2000, 54, 45–52. [Google Scholar]
- Saleem, A.; Ahotupa, M.; Pihlaja, K. Total phenolics concentration and antioxidant potential of extracts of medicinal plants of Pakistan. Z. Nat. C 2001, 56, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Dafallah, A.A.; Al-Mustafa, Z. Investigation of the antiinflammatory activity of Acacia nilotica and Hibiscus sabdariffa. Am. J. Chin. Med. 1996, 24, 263–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charan, J.; Riyad, P.; Ram, H.; Purohit, A.; Ambwani, S.; Kashyap, P.; Singh, G.; Hashem, A.; Abd_Allah, E.F.; Gupta, V.K.; et al. Ameliorations in dyslipidemia and atherosclerotic plaque by the inhibition of HMG-CoA reductase and antioxidant potential of phytoconstituents of an aqueous seed extract of Acacia senegal (L.) Willd in rabbits. PLoS ONE 2022, 17, e0264646. [Google Scholar] [CrossRef]
- Debalke, D.; Birhan, M.; Kinubeh, A.; Yayeh, M. Assessments of Antibacterial Effects of Aqueous-Ethanolic Extracts of Sida rhombifolia’s Aerial Part. Sci. World J. 2018, 2018, 8429809. [Google Scholar] [CrossRef] [Green Version]
- Ali, N.A.A.; Julich, W.D.; Kusnick, C.; Lindequist, U. Antimicrobial and phytochemical studies on 45 Indian medicinal plants against multi-drug resistant human pathogens. J. Ethnopharmacol. 2001, 74, 113–123. [Google Scholar]
- Chattopadhyay, D.; Arunachalam, G.; Mandal, A.; Sur, T.; Mandal, S.; Bhattacharya, S. Antimicrobial and antiinflam-matory activity of folklore: Mallotus peltatus leaf extract. J. Ethnopharmacol. 2002, 82, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Elamary, R.B.; Albarakaty, F.M.; Salem, W.M. Efficacy of Acacia nilotica aqueous extract in treating biofilm-forming and multidrug resistant uropathogens isolated from patients with UTI syndrome. Sci. Rep. 2020, 10, 11125. [Google Scholar] [CrossRef]
- Iheagwam, F.N.; Nsedu, E.I.; Kayode, K.O.; Emiloju, O.C.; Ogunlana, O.O.; Chinedu, S.N. Bioactive screening and in vitro antioxidant assessment of Nauclea latifolia leaf decoction. AIP Conf. Proc. 2018, 1954, 30015. [Google Scholar]
- Singh, R.; Singh, B.; Singh, S.; Kumar, N.; Kumar, S.; Arora, S. Antifree radical activities of kaempferol isolated from Acacia nilotica (L.) Willd Ex. Del. Toxicol. Vitr. 2008, 22, 1965–1970. [Google Scholar] [CrossRef]
- Chaubal, R.; Mujumdar, A.M.; Puranik, V.G.; Deshpande, V.H.; Deshpande, N.R. Isolation and X-ray study of an anti-inflammatory active androstene steroid from Acacia nilotica. Planta. Med. 2003, 69, 287–288. [Google Scholar] [CrossRef] [PubMed]
- Maldini, M.; Montoro, P.; Hamed, A.I.; Mahalel, U.A.; Oleszek, W.; Stochmal, A.; Piacente, S. Strong antioxidant phenolics from Acacia nilotica: Profiling by ESI-MS and qualitative–quantitative determination by LC–ESI-MS. J. Pharm. Biomed. Anal. 2011, 56, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Kalaivani, T.; Rajasekaran, C.; Mathew, L. Free radical scavenging, cytotoxic, and hemolytic activities of an active antioxidant compound ethyl gallate from leaves of Acacia nilotica (L.) wild. Ex. Delile subsp. Indica (Benth.) Brenan. J. Food. Sci. 2011, 76, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Kannan, N.; Sakthivel, K.M.; Guruvayoorappan, C. Protective effect of Acacia nilotica (L.) against acetaminophen-induced hepatocellular damage in Wistar rats. Adv. Pharmacol. Sci. 2013, 2013, 987692. [Google Scholar] [PubMed] [Green Version]
- Hanafy, Z.E.M.; El-Gendy, A.M.; Zaky, A.A.; Mansour, A.M.; Mostafa, N. Protective role of Acacia nilotica extracs and silymarin against mutagenic and hepatic injuries induced by 2-butoxyethanol in male mice. Egypt J. Hosp. Med. 2017, 69, 2876–2889. [Google Scholar] [CrossRef]
- Akpoyowvare, E.S.; Nonso, I.F.; Olakunle, O.A. Potassium dichromate-induced hepato- and hematotoxicity in rats: Nutritive composition and ameliorative role of Acacia nilotica L. leaf. Jundishapur J. Nat. Pharm. Prod. 2021, 16, e104346. [Google Scholar] [CrossRef]
- Ahmed, Z.S.O.; Tahon, M.A.; Hasan, R.S.; El-Sayed, H.G.M.; AbuBaker, H.O.; Ismaiel, M.; Ahmed, I.M.; Ahmed, Y.O. Histopathological, immunohistochemical, and molecular investigation of atrazine toxic effect on some organs of adult male albino rats with a screening of Acacia nilotica as a protective trial. Environ. Sci. Pollut. Res. 2022, 29, 83797–83809. [Google Scholar] [CrossRef]
- Abdel-Wareth, A.A.A.; Taha, E.M.M.; Südekum, K.-H.; Lohakare, J. Thyme oil inclusion levels in a rabbit ration: Evaluation of productive performance, carcass criteria and meat quality under hot environmental conditions. Anim. Nutr. 2018, 4, 410–416. [Google Scholar] [CrossRef]
- Dalle Zotte, A.; Celiaa, C.; Cullere, M.; Szendrő, Z.; Kovács, M.; Gerencsér, Z.; Dal Bosco, A.; Giaccone, V.; Matics, Z. Effect of an in-vivo and/or in-meat application of a liquorice (Glycyrrhiza glabra L.) extract on fattening rabbits live performance, carcass traits and meat quality. Anim. Feed Sci. Technol. 2020, 260, 114333. [Google Scholar] [CrossRef]


| Ingredients | g/kg | Chemical Composition Analyzed | (g/kg, as Fed) |
|---|---|---|---|
| Corn | 310 | Dry matter | 936 |
| Wheat bran | 200 | Gross Energy (MJ/kg DM) | 17.3 |
| Soybean meal (440 g/kg CP) | 190 | Crude protein | 178 |
| Wheat straw | 120 | Ether extract (fat) | 39.7 |
| Lucerne hay | 60 | aNDFom | 392 |
| Rice bran | 40 | ADFom | 232 |
| Linseed straw | 28 | ADL | 69.5 |
| Sunflower meal | 25 | Ash | 92.82 |
| Limestone | 20 | Calcium | 15.80 |
| Sodium chloride | 3 | Phosphorus | 7.73 |
| Vitamin-mineral premix 1 | 3 | Zinc | 45 |
| Dl-Methionine | 1 |
| Properties | Ethanol Extract |
|---|---|
| Total antioxidant capacity (mg AAE/100 g) | 1321 ± 56 |
| Total phenols (mg GAE/100 g) | 15,376 ± 85 |
| Total flavonoids (mg QE/Kg) | 513 ± 6 |
| Items | Acacia BLC Levels (mg/kg) | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| 0 | 50 | 100 | 150 | Lin | Quad | ||
| Body weight | |||||||
| 35 days | 675 | 664 | 643 | 673 | 19.08 | 0.768 | 0.296 |
| 63 days | 1470 b | 1594 a | 1600 a | 1650 a | 26.63 | 0.001 | 0.179 |
| 91 days | 2426 c | 2620 b | 2712 b | 2852 a | 53.04 | <0.001 | 0.618 |
| Body weight gain | |||||||
| 35–63 days | 28.39 b | 33.23 a | 34.18 a | 34.87 a | 0.988 | 0.001 | 0.052 |
| 64–91 days | 34.14 c | 36.61 b | 39.71 a | 42.93 a | 1.856 | 0.003 | 0.844 |
| 35–91 days | 62.54 b | 69.84 a | 73.89 a | 77.8 a | 1.923 | <0.001 | 0.389 |
| Feed intake | |||||||
| 35–63 days | 86.36 b | 91.07 a | 90.86 a | 89.32 a | 1.021 | 0.075 | 0.007 |
| 64–91 days | 98.39 | 97.28 | 99.98 | 99.21 | 1.333 | 0.398 | 0.899 |
| 35–91 days | 184.7 | 188.4 | 190.8 | 188.5 | 1.663 | 0.081 | 0.095 |
| Feed conversion ratio | |||||||
| 35–63 days | 3.072 a | 2.743 b | 2.659 b | 2.572 b | 0.094 | 0.002 | 0.221 |
| 64–91 days | 2.949 a | 2.695 b | 2.520 b | 2.328 c | 0.161 | 0.013 | 0.849 |
| 35–91 days | 2.972 a | 2.708 b | 2.584 c | 2.426 c | 0.076 | <0.001 | 0.499 |
| Items | Acacia BLC Levels (mg/kg) | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| 0 | 50 | 100 | 150 | Lin | Quad | ||
| LBW, g | 2660 | 2720 | 2732 | 2652 | 43.93 | 0.945 | 0.132 |
| Dress,% | 55.08 b | 58.45 a | 57.63 a | 59.88 a | 1.04 | 0.010 | 0.599 |
| Liver,% | 2.58 | 2.83 | 2.82 | 2.59 | 0.20 | 0.971 | 0.248 |
| Heart,% | 0.367 | 0.323 | 0.272 | 0.297 | 0.02 | 0.741 | 0.541 |
| Head,% | 4.240 | 5.274 | 5.152 | 5.501 | 0.58 | 0.175 | 0.562 |
| Spleen,% | 0.033 | 0.047 | 0.046 | 0.047 | 0.007 | 0.190 | 0.379 |
| Kidney,% | 0.636 | 0.653 | 0.695 | 0.578 | 0.039 | 0.468 | 0.111 |
| Abdominal fat,% | 1.636 a | 1.158 b | 1.131 b | 1.069 b | 0.066 | <0.001 | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Wareth, A.A.A.; El-Sayed, H.G.M.; Hassan, H.A.; El-Chaghaby, G.A.; Abdel-Warith, A.-W.A.; Younis, E.M.; Amer, S.A.; Rashad, S.; Lohakare, J. Effects of Dietary Bioactive Lipid Compounds of Acacia nilotica Bark on Productive Performance, Antioxidant Status, and Antimicrobial Activities of Growing Rabbits under Hot Climatic Conditions. Animals 2023, 13, 1933. https://doi.org/10.3390/ani13121933
Abdel-Wareth AAA, El-Sayed HGM, Hassan HA, El-Chaghaby GA, Abdel-Warith A-WA, Younis EM, Amer SA, Rashad S, Lohakare J. Effects of Dietary Bioactive Lipid Compounds of Acacia nilotica Bark on Productive Performance, Antioxidant Status, and Antimicrobial Activities of Growing Rabbits under Hot Climatic Conditions. Animals. 2023; 13(12):1933. https://doi.org/10.3390/ani13121933
Chicago/Turabian StyleAbdel-Wareth, Ahmed A. A., Hazem G. M. El-Sayed, Hamdy A. Hassan, Ghadir A. El-Chaghaby, Abdel-Wahab A. Abdel-Warith, Elsayed M. Younis, Shimaa A. Amer, Sayed Rashad, and Jayant Lohakare. 2023. "Effects of Dietary Bioactive Lipid Compounds of Acacia nilotica Bark on Productive Performance, Antioxidant Status, and Antimicrobial Activities of Growing Rabbits under Hot Climatic Conditions" Animals 13, no. 12: 1933. https://doi.org/10.3390/ani13121933
APA StyleAbdel-Wareth, A. A. A., El-Sayed, H. G. M., Hassan, H. A., El-Chaghaby, G. A., Abdel-Warith, A.-W. A., Younis, E. M., Amer, S. A., Rashad, S., & Lohakare, J. (2023). Effects of Dietary Bioactive Lipid Compounds of Acacia nilotica Bark on Productive Performance, Antioxidant Status, and Antimicrobial Activities of Growing Rabbits under Hot Climatic Conditions. Animals, 13(12), 1933. https://doi.org/10.3390/ani13121933







