Gas/Liquid Chromatography–Mass Spectrometry Analysis of Key Functional Substances Regulating Poll Gland Secretion in Male Camels during Seasonal Estrus
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Use
2.2. Collection and Processing of Samples
2.3. Metabolite Detection
2.4. Metabolome Data Collection
2.5. Metabolome Data Analysis
2.6. Metabolic Pathway Analysis
3. Results
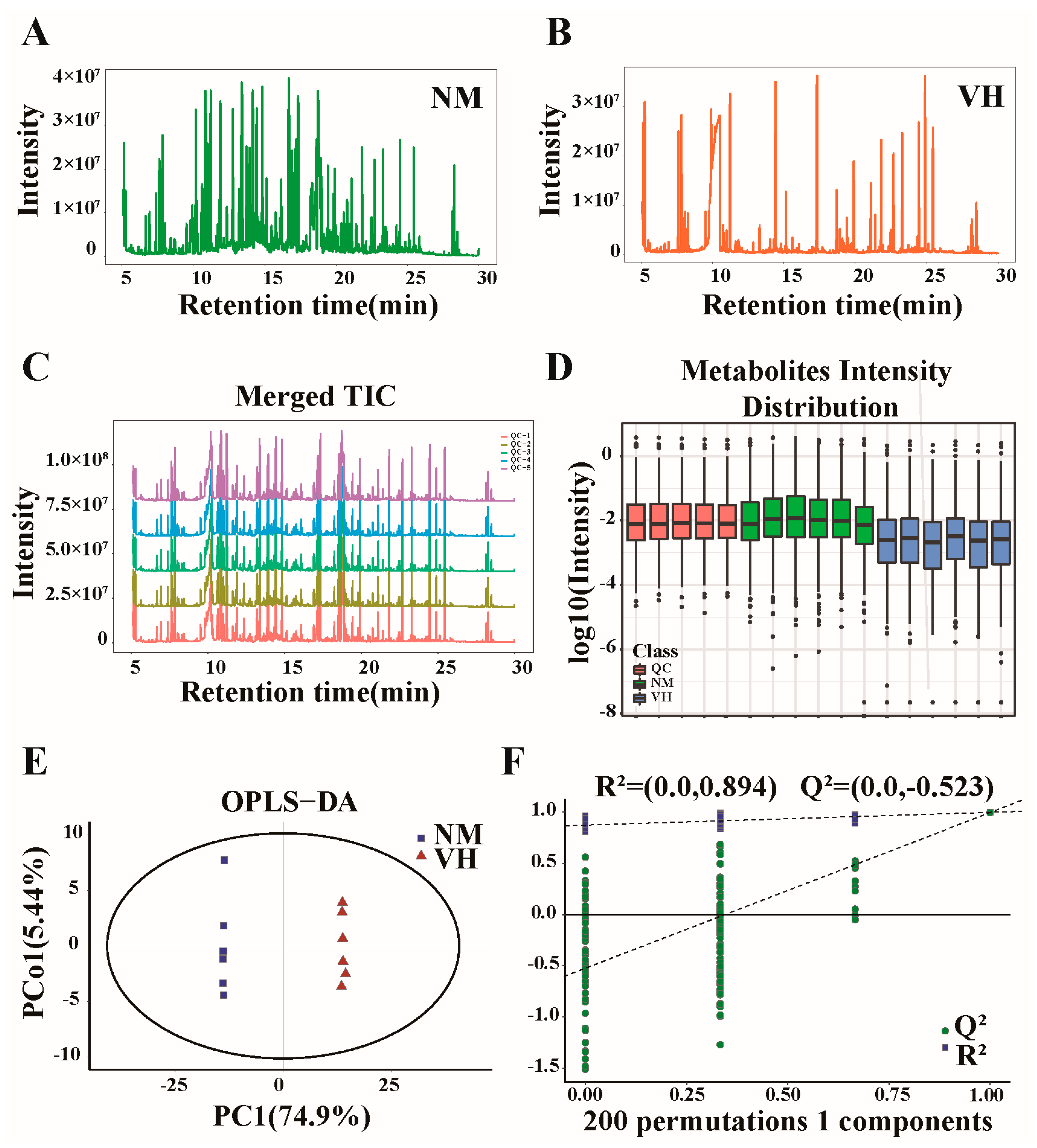
3.1. Overview of GC-MS Detection
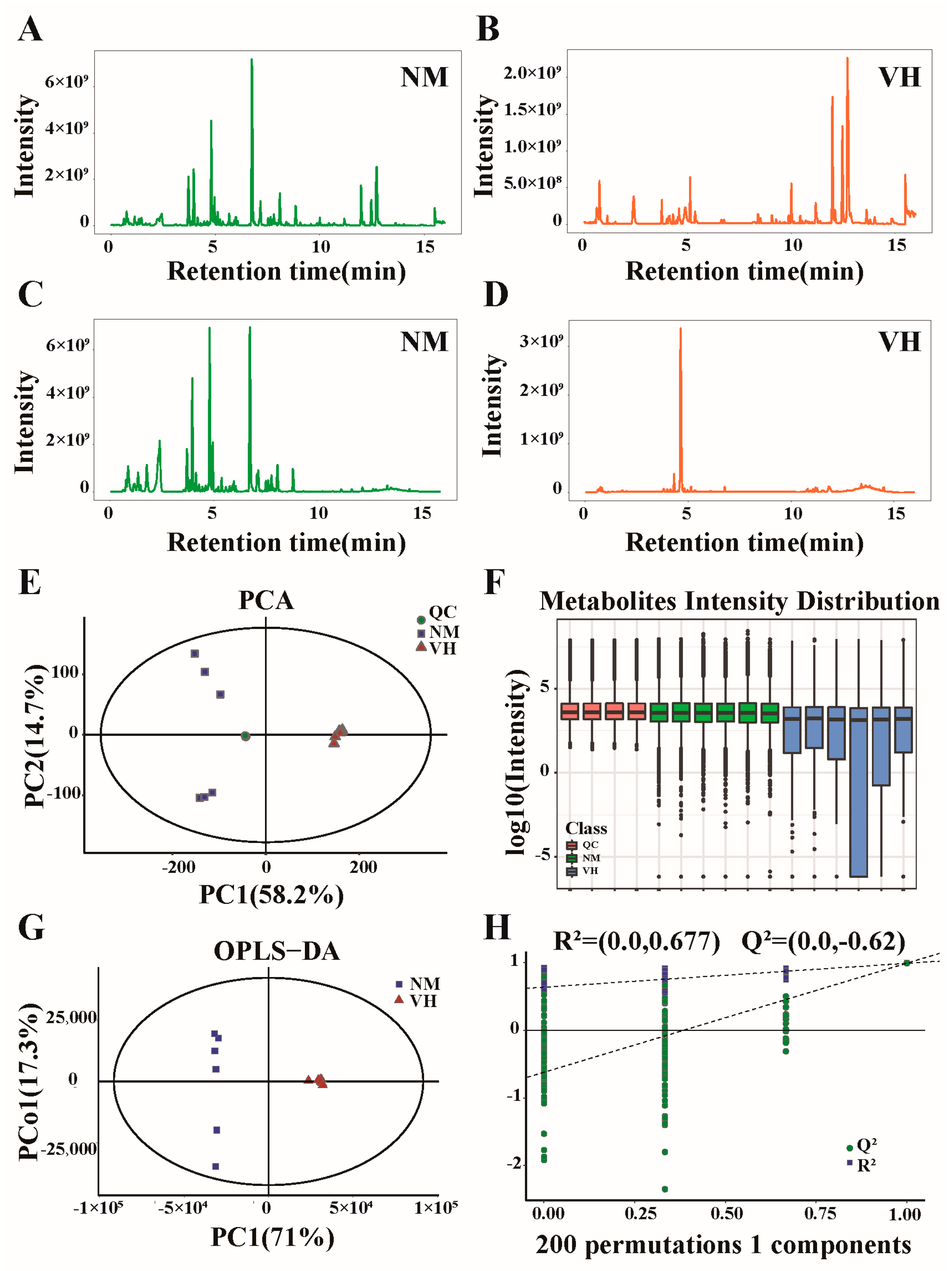
3.2. Overview of LC-MS Detection
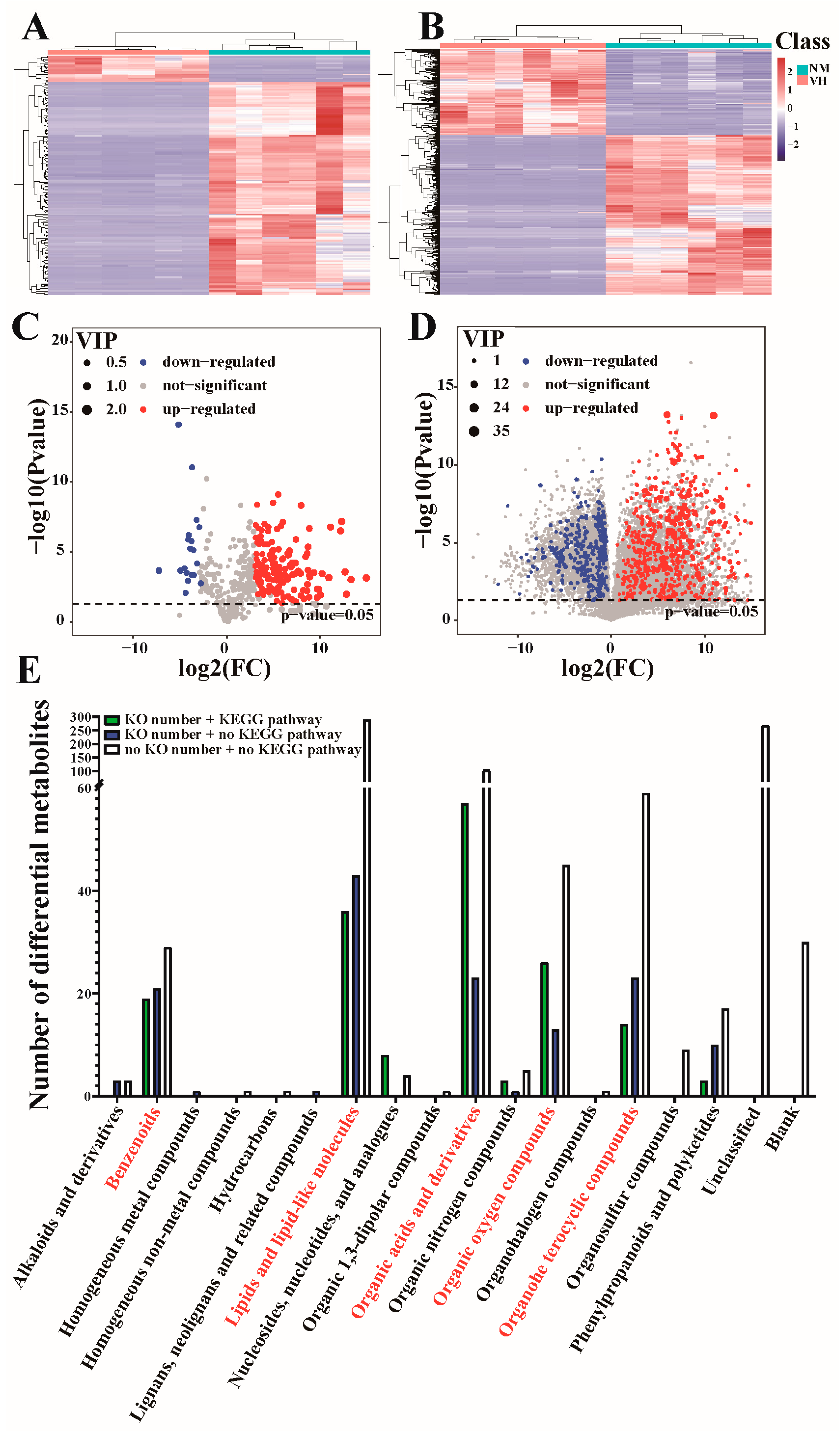
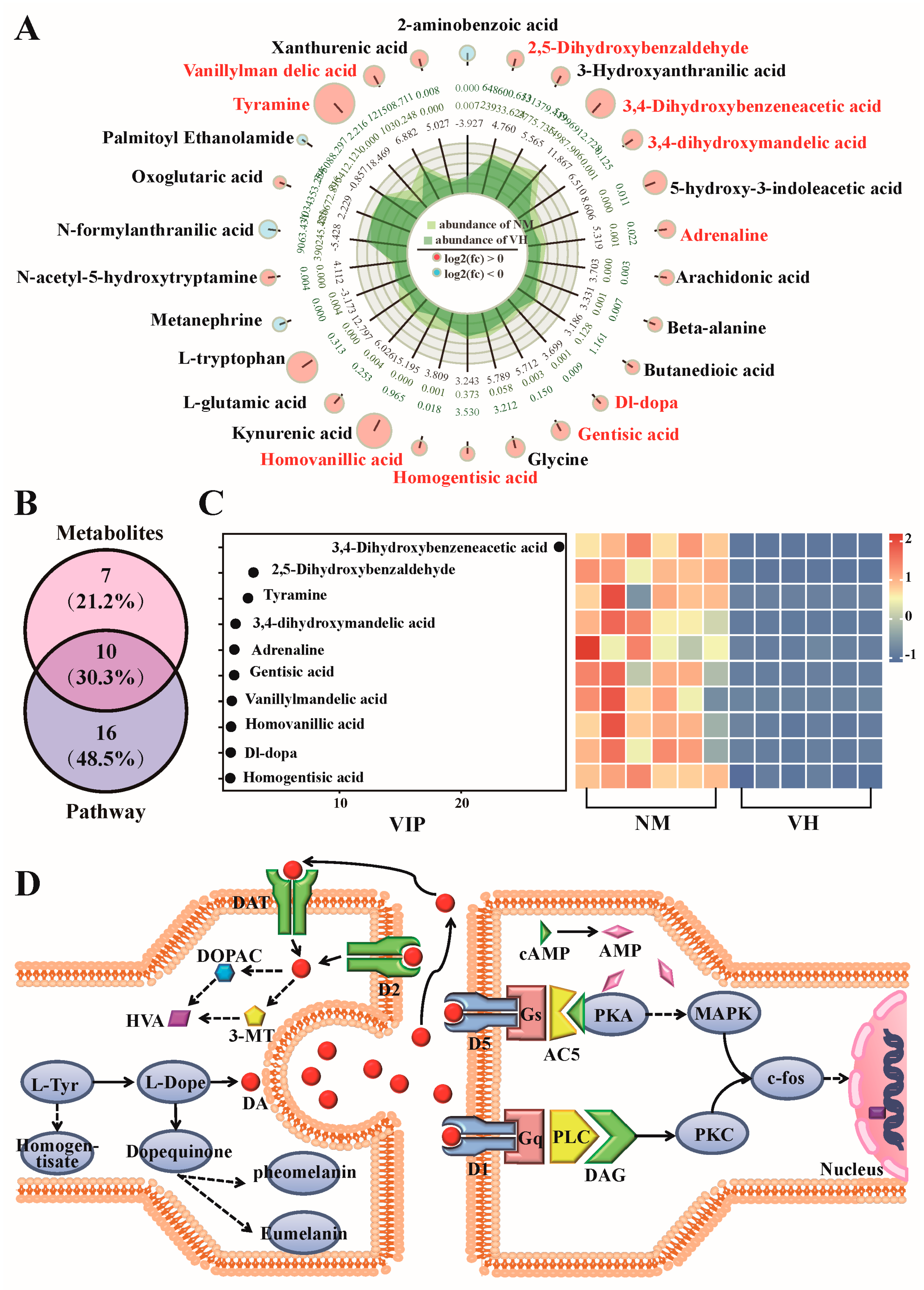
3.3. DEMs Identified by GC/LC-MS
3.4. Analysis of DEMs
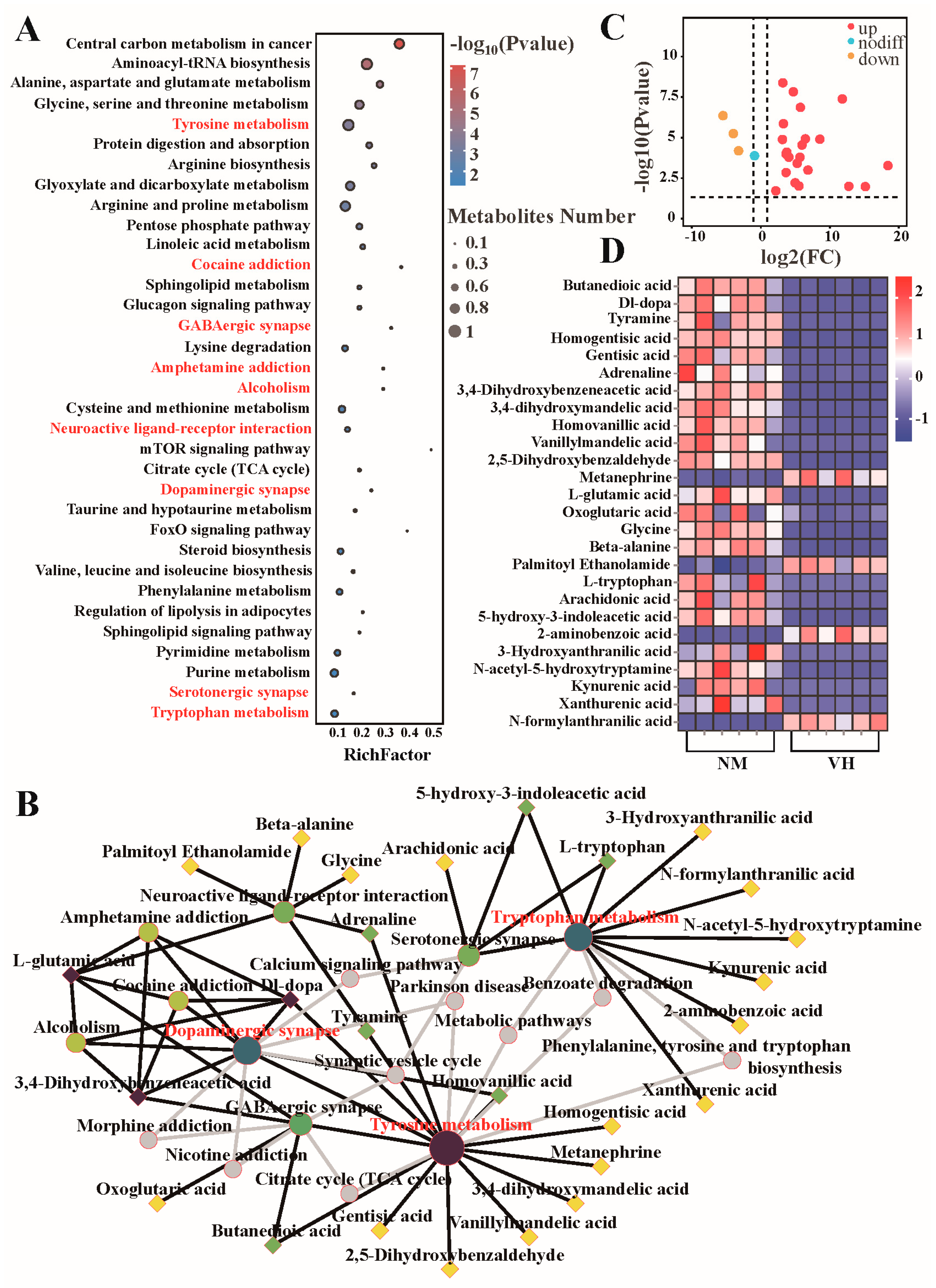
3.5. Analysis of Differential Metabolic Pathways
3.6. Determine the Functional Prediction of Metabolites
4. Discussion
4.1. Metabolites Relating to Steroid Synthesis and Metabolism in Poll Gland Secretions
4.2. Metabolites Relating to Neural Metabolism in Poll Gland Secretions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, L.; Wang, H.; Yang, H.; Chen, S.; Yang, D.; Zhang, Y. Expression of the NSE, SP, NFH and DβH in normal and cryptorchid testes of Bactrian camel. Anim. Reprod. 2022, 19, e20210087. [Google Scholar] [CrossRef]
- Johnston, C.H.; Tolesa, Z. Lepus fagani. The IUCN Red List of Threatened Species. 2019: e.T11798A45178437. Available online: https://www.iucnredlist.org/species/11798/45178437 (accessed on 1 May 2023).
- Chen, B.X.; Yuen, Z.X.; Pan, G.W. Semen-induced ovulation in the bactrian camel (Camelus bactrianus). J. Reprod. Fertil. 1985, 74, 335–339. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Q.; Gan, Z.; Li, H.; Yang, Y.; Zhang, Y.; Zhao, X. Screening for reproductive biomarkers in Bactrian camel via iTRAQ analysis of proteomes. Reprod. Domest. Anim. 2020, 55, 189–199. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, P. A preliminary study on the main chemical constituents and physiological functions of the secretion of the male camel’s poll glands. Anim. Husb. Vet. Med. 1987, 11. [Google Scholar]
- Ebada, S.; Helal, A.; Alkafafy, M. Immunohistochemical studies on the poll gland of the dromedary camel (Camelus dromedarius) during the rutting season. Acta Histochem. 2012, 114, 363–369. [Google Scholar] [CrossRef]
- Ibrahim, Z.H.; Al-Kheraije, K.A. Seasonal morphology and immunoreactivity of cytokeratin and atrial natriuretic peptide in dromedary camel poll glands. Anat. Histol. Embryol. 2020, 50, 307–315. [Google Scholar] [CrossRef]
- Liang, L.; Zhang, Q.; Jing, H.; Li, L.; Wei, Q.; Niu, H.; Xue, G.; Yu, H. Study on the histological structure of skin in camel. In Proceedings of the 16th Academic Symposium of Animal Anatomy and Histology and Embryology Branch of Chinese Society of Animal Husbandry and Veterinary Medicine; Chinese Society of Animal Husbandry and Veterinary Medicine (Chinese Journal): Beijing, China, 2010; pp. 490–493. [Google Scholar]
- Luo, Y.; Li, H.; Lv, Y.; Xu, S.; Liu, Y.; Zhang, N.; Wang, D.; Shao, B.; Wang, J. Anatomical and histochemical features of the bulbourethral glands in bactrian camel (Camelus bactrianus). J. Camel Pract. Res. 2016, 23, 121–125. [Google Scholar] [CrossRef]
- Orgodol, K.; Guo, C.; Zhang, M.; Liu, W.; Ming, L.; Yili, H.; Jiri, M. Study on the acute toxicity of camel poll gland secretion. Improve the scientific and technological content of the camel industry and create a well-known brand in the camel industry. China Camel Conf. 2013, 4, 71–76. [Google Scholar]
- Chen, B.; Kang, C.; Yuan, Z.; Ge, X. The second report of camel reproductive physiology and camel’s sexual activity. J. Anim. Husb. Vet. Med. 1980, 11, 65–76. [Google Scholar]
- Guo, C.; Ming, L.; Wei, L.; Min, Z.; Jun, H.; Ha-Si-su-rong; Ji-Ri-mu-tu. Research Progress on the Bokhi of Camel. China Anim. Husb. Vet. Med. 2013, 40, 235–238. [Google Scholar]
- Ayorinde, F.; Wheeler, J.W.; Wemmer, C.; Murtaugh, J. Volatile components of the occipital gland secretion of the bactrian camel (Camelus bactrianus). J. Chem. Ecol. 1982, 8, 177–183. [Google Scholar] [CrossRef]
- Yagil, R.; Etzion, Z. Hormonal and behavioural patterns in the male camel (Camelus dromedarius). J. Reprod. Fertil. 1980, 58, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Tingari, M.D.; Rahma, B.A.; Saad, A. Studies on the poll glands of the one-humped camel in relation to reproductive activity. I. Seasonal morphological and histochemical changes. J. Anat. 1984, 138, 193–205. [Google Scholar] [PubMed]
- Peng, Z.-X.; Wang, Y.; Gu, X.; Xue, Y.; Wu, Q.; Zhou, J.-Y.; Yan, C. Metabolic transformation of breast cancer in a MCF-7 xenograft mouse model and inhibitory effect of volatile oil from Saussurea lappa Decne treatment. Metabolomics 2015, 11, 636–656. [Google Scholar] [CrossRef]
- Clancy, M.V.; Zytynska, S.E.; Moritz, F.; Witting, M.; Schmitt-Kopplin, P.; Weisser, W.W.; Schnitzler, J.-P. Metabotype variation in a field population of tansy plants influences aphid host selection. Plant Cell Environ. 2018, 41, 2791–2805. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Xie, G.; Chen, T.; Qiu, Y.; Zou, X.; Zheng, M.; Tan, B.; Feng, B.; Dong, T.; He, P.; et al. Distinct Urinary Metabolic Profile of Human Colorectal Cancer. J. Proteome Res. 2012, 11, 1354–1363. [Google Scholar] [CrossRef]
- Kind, T.; Wohlgemuth, G.; Lee, D.Y.; Lu, Y.; Palazoglu, M.; Shahbaz, S.; Fiehn, O. FiehnLib: Mass Spectral and Retention Index Libraries for Metabolomics Based on Quadrupole and Time-of-Flight Gas Chromatography/Mass Spectrometry. Anal. Chem. 2009, 81, 10038–10048. [Google Scholar] [CrossRef]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef]
- Fujisaka, S.; Avila-Pacheco, J.; Soto, M.; Kostic, A.; Dreyfuss, J.M.; Pan, H.; Ussar, S.; Altindis, E.; Li, N.; Bry, L.; et al. Diet, Genetics, and the Gut Microbiome Drive Dynamic Changes in Plasma Metabolites. Cell Rep. 2018, 22, 3072–3086. [Google Scholar] [CrossRef]
- Zhai, X.; Li, X.-Y.; Wang, Y.-J.; Qin, K.-R.; Hu, J.-R.; Li, M.-N.; Wang, H.-L.; Guo, R. Fancd2os Reduces Testosterone Production by Inhibiting Steroidogenic Enzymes and Promoting Cellular Apoptosis in Murine Testicular Leydig Cells. Endocrinol. Metab. 2022, 37, 533–546. [Google Scholar] [CrossRef]
- Yang, Y.J.; Lee, J.; Choi, M.H.; Chung, B.C. Direct determination of estriol 3- and 16-glucuronides in pregnancy urine by column-switching liquid chromatography with electrospray tandem mass spectrometry. Biomed. Chromatogr. 2003, 17, 219–225. [Google Scholar] [CrossRef]
- Tingari, M.D.; George, M.A. Studies on the poll glands of the one-humped camel in relation to reproductive activity. II. Ultrastructural observations. J. Anat. 1984, 139 Pt 3, 463–474. [Google Scholar] [PubMed]
- Tingari, M.D.; Rahma, B.A. Morphological and histochemical changes in the camel poll glands as related to reproductive activity. Sixth Eur. Anat. Congr. Acta Anat. 1981, 111, 151–152. [Google Scholar]
- Taha, A.A.M.; Abdel-Magied, E.M.; Abdalla, M.A.; Abdalla, A.B. The Poll Glands of the Dromedary (Camelus dromedarius): Ultrastructural Characteristics. Anat. Histol. Embryol. 1994, 23, 269–274. [Google Scholar] [CrossRef]
- Zhao, X.X. Ecological Physiology and Reproduction of Camels; Gansu Science and Technology Press: Lanzhou, China, 1995. [Google Scholar]
- Jiao, X.; Zhu, W.; Zhang, W.; Ba, L.; Sa, R. Effects of exogenous hormones on estrus and fertility of camels. China Anim. Husb. Commun. 2008, 11, 35–36. [Google Scholar]
- McBride, C.S.; Baier, F.; Omondi, A.B.; Spitzer, S.A.; Lutomiah, J.; Sang, R.; Ignell, R.; Vosshall, L.B. Evolution of mosquito preference for humans linked to an odorant receptor. Nature 2014, 515, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Brennan, A.P.; Kendrick, K.M. Mammalian social odours: Attraction and individual recognition. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 2061–2078. [Google Scholar] [CrossRef] [PubMed]
- Snowdon, C.T.; Ziegler, E.T.; Schultz-Darken, N.J.; Ferris, C.F. Social odours, sexual arousal and pairbonding in primates. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 2079–2089. [Google Scholar] [CrossRef]
- Ba, L.; Hong, G.; Ming, L.; Li, Y.; Mu, T. Research on the structure of the poll glands of Mongolian male camels. In Proceedings of the China Camel Conference and the Founding Meeting of the Camel Branch of the China Animal Husbandry, Alxa, China, 24–25 November 2012; pp. 104–107. [Google Scholar]
- Zhang, Y.; Zeng, Q. The estrus season and behavior of Bactrian male camels. Spec. Econ. Anim. Plants 2000, 3, 8. [Google Scholar]
- Goldstein, D.S.; Eisenhofer, G.; Kopin, I.J. Sources and Significance of Plasma Levels of Catechols and Their Metabolites in Humans. J. Pharmacol. Exp. Ther. 2003, 305, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, F.; Allard, J. Dopamine and Male Sexual Function. Eur. Urol. 2001, 40, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Ghashghayi, E.; Zendehdel, M.; Khodadadi, M.; Rahmani, B. Central dopaminergic, serotoninergic, as well as GABAergic systems mediate NMU-induced hypophagia in newborn chicken. Int. J. Neurosci. 2022, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pravikova, P.D.; Ivanova, L.N. Analysis of Dopamine D1- and D2-Receptors Effect on Renal Osmoregulatory Function in Rats with Different Blood Vasopressin Level. J. Evol. Biochem. Physiol. 2022, 58, 922–929. [Google Scholar] [CrossRef]
- Moreau, C.; El Habnouni, C.; Lecron, J.-C.; Morel, F.; Delwail, A.M.; Le Gall-Ianotto, C.; Le Garrec, R.; Misery, L.; Piver, E.; Vaillant, L.; et al. Salivary metabolome indicates a shift in tyrosine metabolism in patients with burning mouth syndrome: A prospective case–control study. Pain 2022, 164, e144–e156. [Google Scholar] [CrossRef] [PubMed]
- Booth, S.; Ramadan, A.; Zhang, D.; Lu, L.; Kirouac, G.; Jackson, M.F.; Anderson, C.; Ko, J.H. The Vasomotor Response to Dopamine Is Altered in the Rat Model of l -dopa-Induced Dyskinesia. Mov. Disord. 2020, 36, 938–947. [Google Scholar] [CrossRef]
- Giuliano, F.; Allard, J. Dopamine and sexual function. Int. J. Impot. Res. 2001, 13 (Suppl. S3), S18–S28. [Google Scholar] [CrossRef]
- Melis, M.R.; Argiolas, A. Dopamine and sexual behavior. Neurosci. Biobehav. Rev. 1995, 19, 19–38. [Google Scholar] [CrossRef]
- Soldatkin, V.A.; Shkurat, T.P.; Bobkov, A.S.; Mashkina, E.V.; Tretyakov, A.V.; Perekhov, A.Y.; Mrykhin, V.V.; Kovalev, A.I.; Bukhanovskaya, O.A.; Kryuchkova, M.N. The MAOA and COMT Gene Polymorphisms in Patients with Schizophrenia Committed Homicide. Int. J. Biomed. 2014, 4, 213–217. [Google Scholar]
- Volavka, J.; Bilder, R.; Nolan, K. Catecholamines and Aggression: The Role of COMT and MAO Polymorphisms. Ann. N. Y. Acad. Sci. 2004, 1036, 393–398. [Google Scholar] [CrossRef]
- Shahveisi, K.; Abdoli, N.; Farnia, V.; Khazaie, H.; Hosseini, M.; Ghazvini, H.; Khodamoradi, M. Maternal sleep deprivation affects extinction and reinstatement of methamphetamine reward memory in male offspring: Role of the D1-like and D2-like dopamine receptors. Brain Res. 2022, 1792, 148033. [Google Scholar] [CrossRef]
- Shetty, M.S.; Sajikumar, S. Differential involvement of Ca2+/calmodulin-dependent protein kinases and mitogen-activated protein kinases in the dopamine D1/D5 receptor-mediated potentiation in hippocampal CA1 pyramidal neurons. Neurobiol. Learn. Mem. 2017, 138, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Yang, D.; Han, Y.; Wang, W.; Wang, N.; Yang, J.; Zeng, C. Dopamine D1-Like Receptors Suppress the Proliferation of Macrophages Induced by Ox-LDL. Cell. Physiol. Biochem. 2016, 38, 415–426. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, L.; Yuan, B.; Zhang, B.; Chen, W.; Yuan, X.; Liu, X.; Gao, Y.; Zhang, Y.; Zhang, Q.; Zhao, X. Gas/Liquid Chromatography–Mass Spectrometry Analysis of Key Functional Substances Regulating Poll Gland Secretion in Male Camels during Seasonal Estrus. Animals 2023, 13, 2024. https://doi.org/10.3390/ani13122024
Dai L, Yuan B, Zhang B, Chen W, Yuan X, Liu X, Gao Y, Zhang Y, Zhang Q, Zhao X. Gas/Liquid Chromatography–Mass Spectrometry Analysis of Key Functional Substances Regulating Poll Gland Secretion in Male Camels during Seasonal Estrus. Animals. 2023; 13(12):2024. https://doi.org/10.3390/ani13122024
Chicago/Turabian StyleDai, Lijun, Bao Yuan, Bohao Zhang, Wenli Chen, Xixue Yuan, Xinhong Liu, Yuan Gao, Yong Zhang, Quanwei Zhang, and Xingxu Zhao. 2023. "Gas/Liquid Chromatography–Mass Spectrometry Analysis of Key Functional Substances Regulating Poll Gland Secretion in Male Camels during Seasonal Estrus" Animals 13, no. 12: 2024. https://doi.org/10.3390/ani13122024
APA StyleDai, L., Yuan, B., Zhang, B., Chen, W., Yuan, X., Liu, X., Gao, Y., Zhang, Y., Zhang, Q., & Zhao, X. (2023). Gas/Liquid Chromatography–Mass Spectrometry Analysis of Key Functional Substances Regulating Poll Gland Secretion in Male Camels during Seasonal Estrus. Animals, 13(12), 2024. https://doi.org/10.3390/ani13122024






