On-field Gross Morphology Evaluation of Dromedary Camel (Camelus dromedarius) Fetal Membranes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Inclusion Criteria
2.2. Data Collection
Placental Measurements
2.3. Statistical Analysis
3. Results
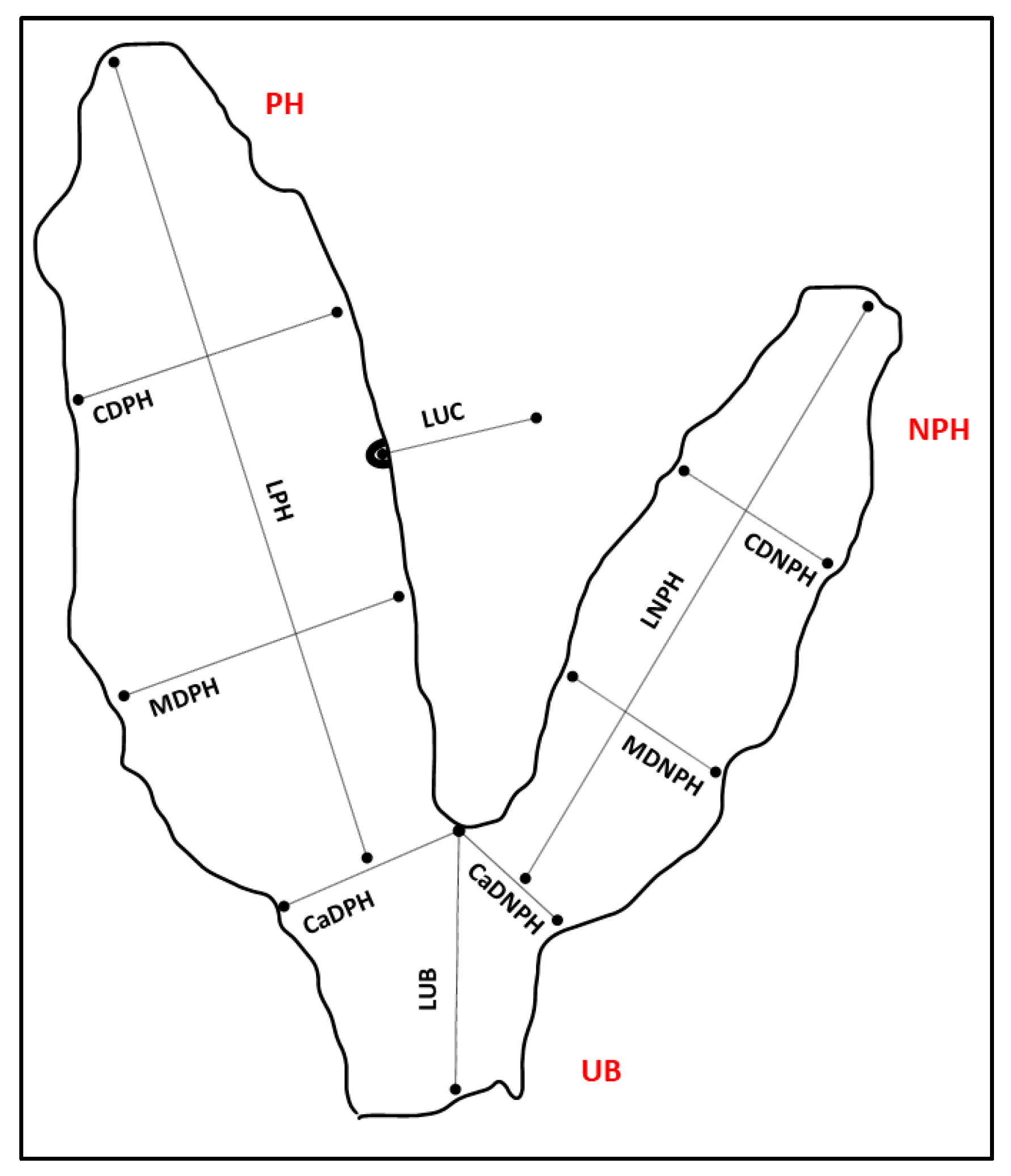
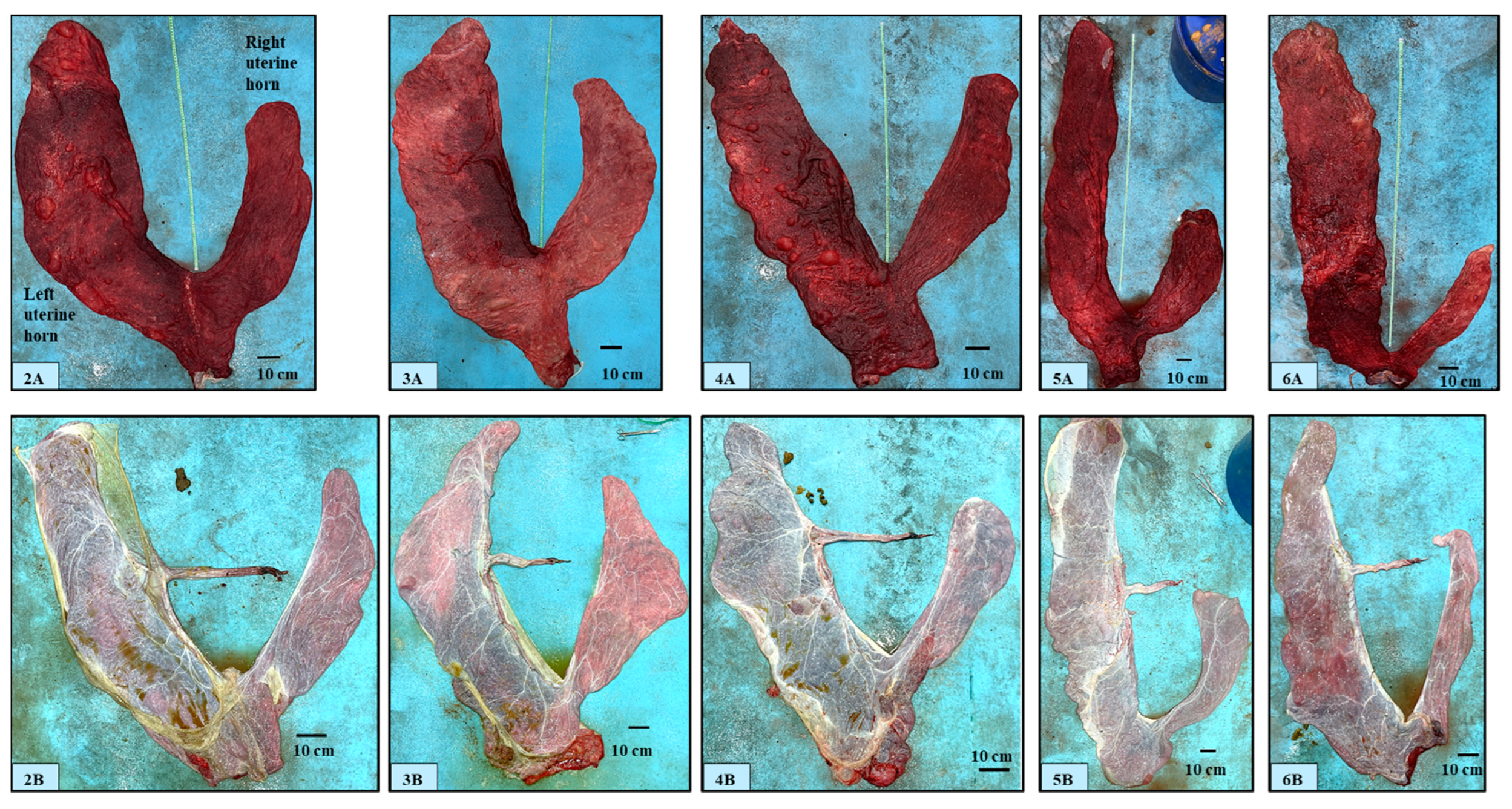
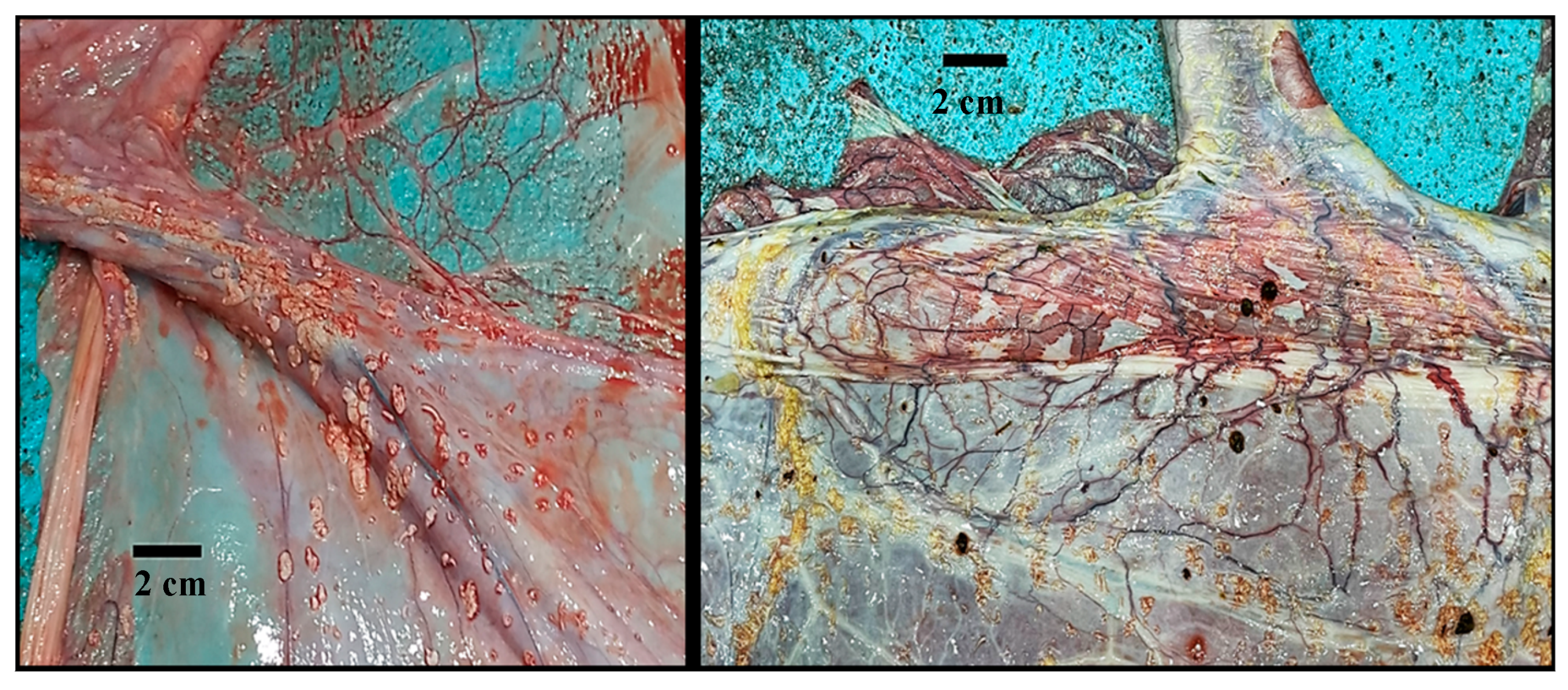
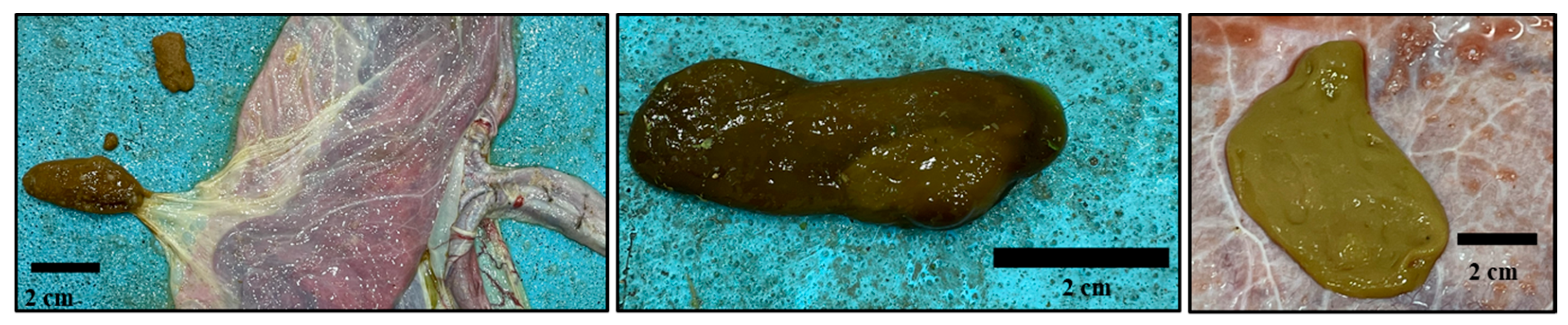
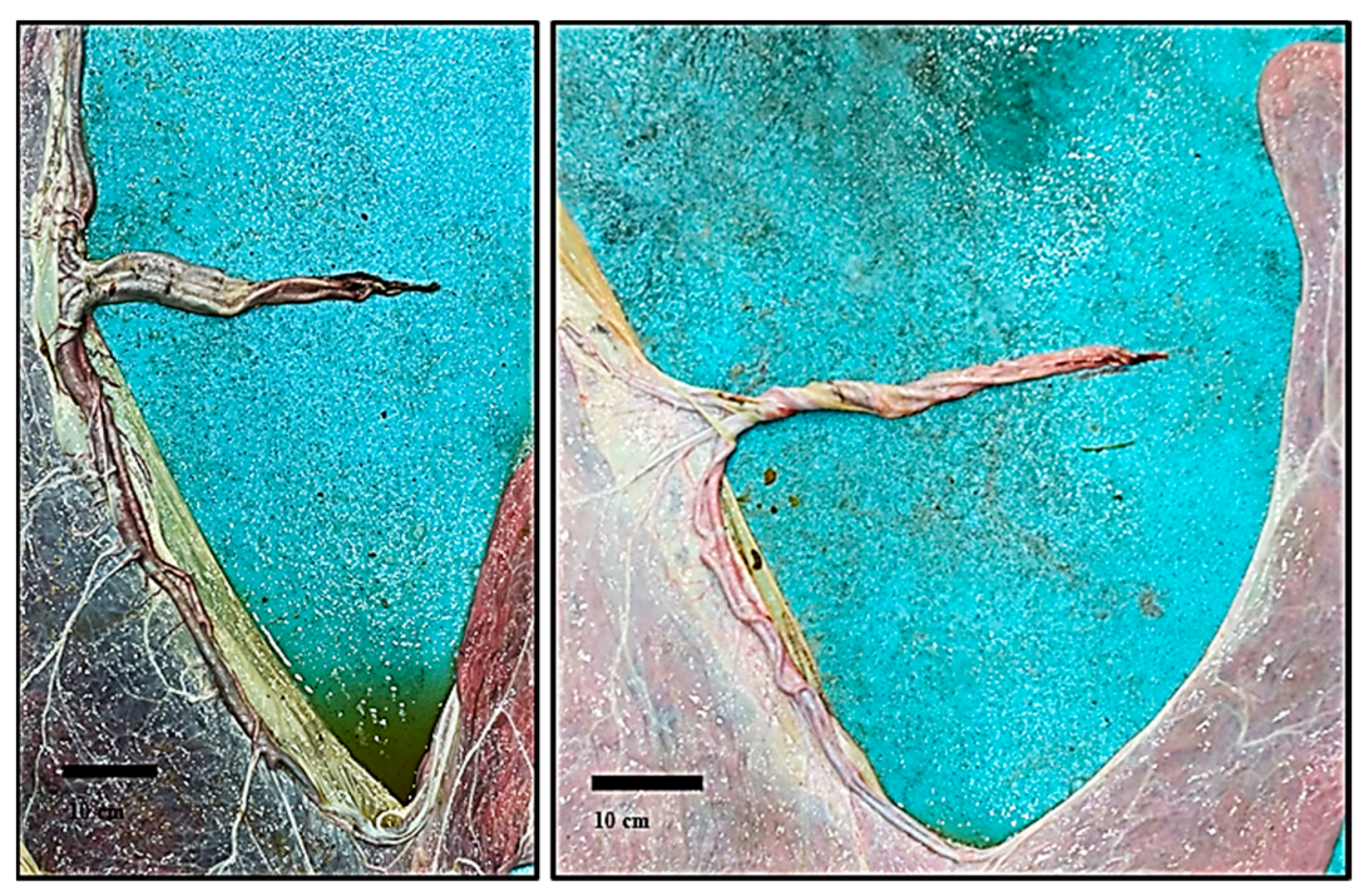
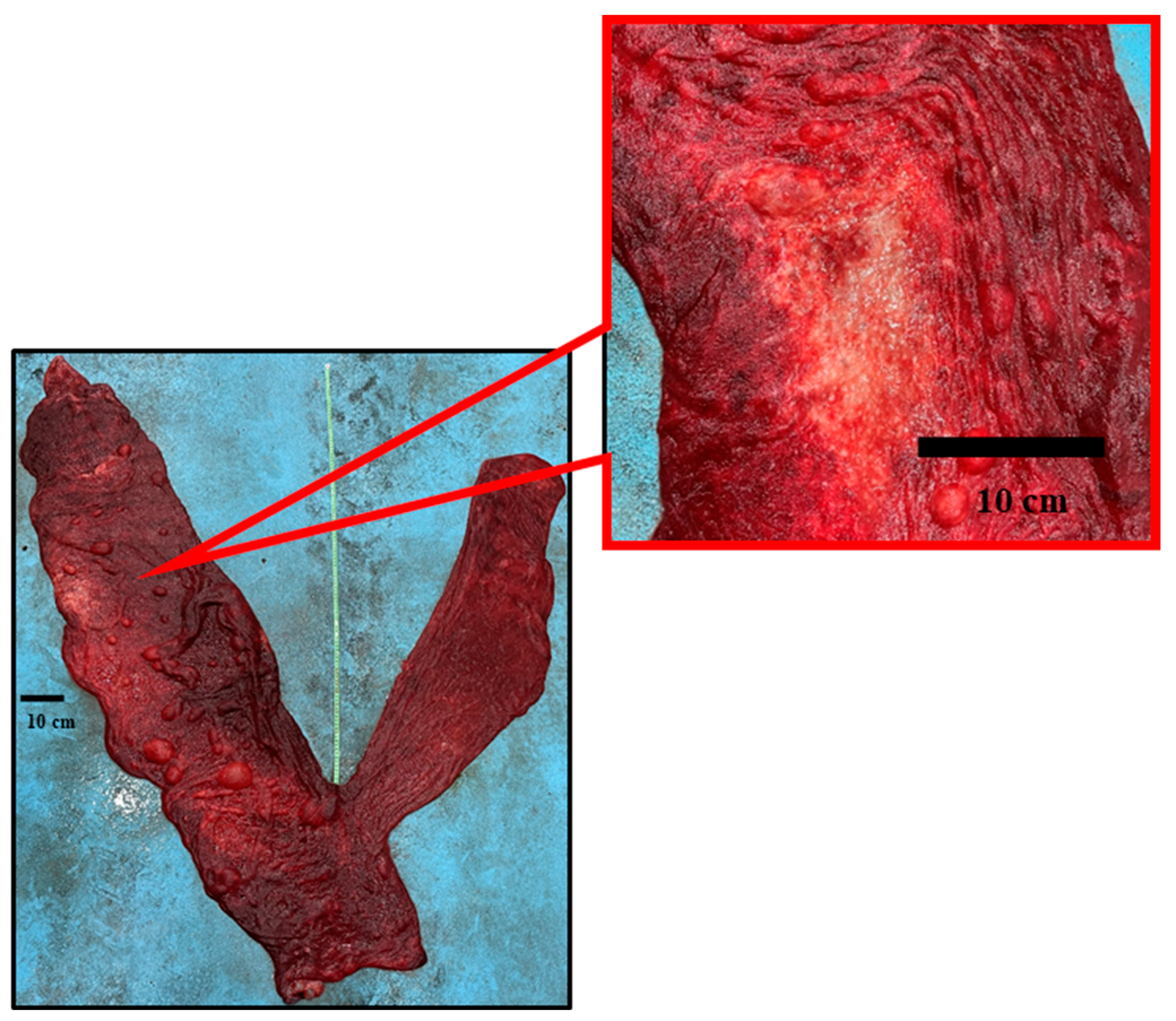
3.1. Gross Description of Different Placental Components
3.2. Placental Measurements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wooding, F.B.; Ozturk, M.; Skidmore, J.A.; Allen, W.R. Developmental changes in localization of steroid synthesis enzymes in camelid placenta. Reproduction 2003, 126, 239–247. [Google Scholar] [CrossRef]
- Skidmore, J.A.; Wooding, F.B.; Allen, W.R. Implantation and early placentation in the one-humped camel (Camelus dromedarius). Placenta 1996, 17, 253–262. [Google Scholar] [CrossRef]
- Pozor, M. Equine placenta—A clinician’s perspective. Part 1: Normal placenta—Physiology and evaluation. Equine Vet. Educ. 2016, 28, 327–334. [Google Scholar] [CrossRef]
- Knight, J.W. Aspects of placental estrogen synthesis in the pig. Exp. Clin. Endocrinol. 1994, 102, 175–184. [Google Scholar] [CrossRef]
- Braun, B.C.; Zschockelt, L.; Dehnhard, M.; Jewgenow, K. Progesterone and estradiol in cat placenta-biosynthesis and tissue concentration. J. Steroid Biochem. Mol. Biol. 2012, 132, 295–302. [Google Scholar] [CrossRef]
- Veronesi, M.C.; Riccaboni, P.; Faustini, M.; Battocchio, M.; Cairoli, F.; Villani, M. Potential Association between Placental Features and Apgar Scores after Normal Parturition in the Thoroughbred Horse. J. Anim. Vet. Adv. 2005, 4, 965–970. [Google Scholar]
- Laudadio, V.; Tufarelli, V.; Dario, M.; Hammadi, M.; Seddik, M.M.; Lacalandra, G.M.; Dario, C. A survey of chemical and nutritional characteristics of halophytes plants used by camels in Southern Tunisia. Trop. Anim. Health Prod. 2009, 41, 209–215. [Google Scholar] [CrossRef]
- Faye, B. The camel, new challenges for a sustainable development. Trop. Anim. Health Prod. 2016, 48, 689–692. [Google Scholar] [CrossRef]
- Cottrill, C.M.; Jeffers-Lo, J.; Ousey, J.C.; McGladdery, A.J.; Ricketts, S.W.; Silver, M.; Rossdale, P.D. The placenta as a determinant of fetal well-being in normal and abnormal equine pregnancies. J. Reprod. Fertil. Suppl. 1991, 44, 591–601. [Google Scholar]
- Carluccio, A.; Panzani, S.; Tosi, U.; Riccaboni, P.; Contri, A.; Veronesi, M.C. Morphological features of the placenta at term in the Martina Franca donkey. Theriogenology 2008, 69, 918–924. [Google Scholar] [CrossRef]
- Pirrone, A.; Antonelli, C.; Mariella, J.; Castagnetti, C. Gross placental morphology and foal serum biochemistry as predictors of foal health. Theriogenology 2014, 81, 1293–1299. [Google Scholar] [CrossRef]
- Hecka, L.; Clauss, M.; Sánchez-Villagra, M.R. Gestation length variation in domesticated horses and its relation to breed and body size diversity. Mamm. Biol. 2017, 84, 44–51. [Google Scholar] [CrossRef]
- Morton, W.R.M. Observations on the full-term foetal membranes of three members of the Camelidae (Camelus dromedarius L., Camelus bactrianus L. and Lama glama L. J. Anat. 1961, 25, 200–209. [Google Scholar]
- Arthur, G.H.; Al-Rahim, A.T.; Al-Hindi, A.S. Reproduction and genital diseases of the camel. Brit Vet. J. 1985, 141, 650–659. [Google Scholar] [CrossRef]
- Whitwell, K.E.; Jeffcott, L.B. Morphological studies on the fetal membranes of the normal singleton foal at term. Res. Vet. Sci. 1975, 19, 44–55. [Google Scholar]
- Meesters, M.; Opsomer, G.; Govaere, J. Macroscopic evaluation of the placenta of the alpaca (Vicugna pacos). Reprod. Domest. Anim. 2018, 54, 996–1002. [Google Scholar] [CrossRef]
- Schlafer, D.H. Postmortem examination of the equine placenta, fetus and neonate, methods and interpretation of findings. Proc. Am. Assoc. Equine Pract. 2004, 50, 144–161. [Google Scholar]
- Schlafer, D.H. Examination of the placenta. In Equine Reproduction, 2nd ed.; McKinnon, A.O., Squires, E.L., Vaala, W.E., Varner, D.D., Eds.; Wiley-Blackwell: Ames, Iowa, 2011; pp. 99–110. [Google Scholar]
- Monaco, D.; Padalino, B.; Lacalandra, G.M. Distinctive features of female reproductive physiology and artificial insemination in the dromedary camel species. Emir. J. Food Agric. 2015, 27, 328–337. [Google Scholar] [CrossRef]
- Nagy, P.; Juhasz, J. Pregnancy and parturition in dromedary camels I. Factors affecting gestation length, calf birth weight and timing of delivery. Theriogenology 2019, 134, 24–33. [Google Scholar] [CrossRef]
- Atieh, M.A.; Zeitoun, M.M.; Abdelsalam, M.M.; Al-Sobayil, K.A. Breed differences in the placenta morphometric parameters of the normally pregnant dromedary she camels relative to neonatal traits in Saudi Arabia. J. Vet. Sci. 2014, 115, 350–355. [Google Scholar]
- Al Mutairi, S.E. Evaluation of Saudi camel calves’ performance under an improved management system. Rev. Élev. Méd. Vét. Pays Trop. 2000, 53, 219–222. [Google Scholar] [CrossRef][Green Version]
- Allen, W.R.; Wilsher, S.; Turnbull, C.; Stewart, F.; Ousey, J.; Rossdale, P.D.; Fowden, A.L. Influence of maternal size on placental, fetal and postnatal growth in the horse. I. Development in utero. Reproduction 2002, 123, 445–453. [Google Scholar] [CrossRef]
- Sharma, S.S.; Vyas, K.K. Parturition in the camel (Camelus dromedarius). Ceylon Vet. J. 1970, 18, 7–9. [Google Scholar]
- Musa, B.E. Normal Parturition in the camel (Camelus dromedarius). Vlaams Diergeneeskd. Tijdschr. 1983, 52, 255–258. [Google Scholar]
- Tibary, A.; Anouassi, A. Reproductive physiology in the female Camelidae. In Theriogenology in Camelidae: Anatomy, Physiology, BSE, Pathology and Artificial Breeding, Actes Editions; Tibary, A., Ed.; Institut Agronomique et Vétérinaire Hassan II: Rabat, Morocco, 1997; pp. 169–229. [Google Scholar]
- Bravo, P.W.; Garnica, J.; Puma, G. Cria alpaca body weight and perinatal survival in relation to age of the dam. Anim. Reprod. Sci. 2009, 111, 214–219. [Google Scholar] [CrossRef]
- Whitehead, A.E.; Chenier, T.S.; Foster, R.A. Placental Characteristics of Standardbred Mares. In AAEP Annual Convention—Seattle; American Association of Equine Practitioners: Lexington, KY, USA, 2005. [Google Scholar]
- Benaissa, M.H.; Faye, B.; Youngs, C.R.; Kaidi, R. Slaughterhouse survey of culled female camels (Camelus dromedarius) in southeast Algeria: Foetal wastage and pregnancy characteristics. Emir. J. Food Agric. 2016, 28, 805–812. [Google Scholar] [CrossRef]
- Mariella, J.; Iacono, E.; Lanci, A.; Merlo, B.; Palermo, C.; Morris, L.; Castagnetti, C. Macroscopic characteristics of the umbilical cord in Standardbred, Thoroughbred and Warmblood horses. Theriogenology 2018, 113, 166–170. [Google Scholar] [CrossRef]
- Carluccio, A.; Veronesi, M.C.; Ippedico, G.; Contri, A. Is Pregnancy Length in Martina Franca Jennies Influenced by Lunar Cycle? Arch. Vet. Sci. Med. 2021, 4, 34–42. [Google Scholar] [CrossRef]
- Veronesi, M.C.; Villani, M.; Wilsher, S.; Contri, A.; Carluccio, A. A comparative stereological study of the term placenta in the donkey, pony and Thoroughbred. Theriogenology 2010, 74, 627–631. [Google Scholar] [CrossRef]
- Risnes, K.R.; Romundstad, P.R.; Nilsen, T.I.; Eskild, A.; Vatten, L.J. Placental weight relative to birth weight and long-term cardiovascular mortality: Findings from a cohort of 31,307 men and women. Am. J. Epidemiol. 2009, 5, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Wilsher, S.; Allen, W.R. The effects of maternal age and parity on placental and fetal development in the mare. Equine Vet. J. 2003, 35, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Gloria, A.; Veronesi, M.C.; Contri, A. Does Placental Efficiency and Vascularization Affect Puppy Health? A Study in Boxer and Dobermann Dogs. Animals 2024, 28, 423. [Google Scholar] [CrossRef] [PubMed]
| Pregnancy Length (Days) n = 11 | Calf Expulsion Time (min) n = 11 | Placenta Expulsion Time (min) n = 11 | Male Calves BW (kg) n = 5 | Female Calves BW (kg) n = 6 |
|---|---|---|---|---|
| 372.0 ± 8.9 | 14.83 ± 1.18 | 71.20 ± 36.67 | 44.4 ± 1.97 | 41.2 ± 4.38 |
| (357–381) | (13–16.5) | (20–150) | (41.8–46.6) | (35.6–47.4) |
| Mean (cm) ± SD | Median (Min–Max) (cm) | |
|---|---|---|
| LPH | 159 ± 21.2 | 160 (119–190) |
| LNPH | 105.2 ± 16.83 | 109 (81–136) |
| LUB | 40.5 ± 6.61 | 41 (28–51) |
| CDPH | 36.1 ± 5.38 | 35 (26–45) |
| MDPH | 42.5 ± 6.21 | 41 (34–52) |
| CaDPH | 48.8 ± 4.49 | 48 (42–57) |
| CDNPH | 24.5 ± 6.22 | 23 (15–37) |
| MDNPH | 25.6 ± 4.41 | 25 (18–33) |
| CaDNPH | 29.8 ± 5.59 | 29 (21–43) |
| LUC | 42.9 ± 7.02 | 44 (33–53) |
| Mean (mL) ±SD | Median (Min–Max) (mL) | |
|---|---|---|
| VPH | 3832.7 ± 1114.94 | 3500 (2450–6300) |
| VNPH | 1475 ± 533.98 | 1250 (900–2500) |
| VUB | 975 ± 313.80 | 1000 (600–1600) |
| VUC | 295.5 ± 85.01 | 300 (200–500) |
| TV | 6877.3 ± 1623.63 | 6550 (4700–10,200) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monaco, D.; Castagnetti, C.; Lanci, A.; Osman, T.K.; Lacalandra, G.M.; Fusi, J. On-field Gross Morphology Evaluation of Dromedary Camel (Camelus dromedarius) Fetal Membranes. Animals 2024, 14, 1553. https://doi.org/10.3390/ani14111553
Monaco D, Castagnetti C, Lanci A, Osman TK, Lacalandra GM, Fusi J. On-field Gross Morphology Evaluation of Dromedary Camel (Camelus dromedarius) Fetal Membranes. Animals. 2024; 14(11):1553. https://doi.org/10.3390/ani14111553
Chicago/Turabian StyleMonaco, Davide, Carolina Castagnetti, Aliai Lanci, Taher Kamal Osman, Giovanni Michele Lacalandra, and Jasmine Fusi. 2024. "On-field Gross Morphology Evaluation of Dromedary Camel (Camelus dromedarius) Fetal Membranes" Animals 14, no. 11: 1553. https://doi.org/10.3390/ani14111553
APA StyleMonaco, D., Castagnetti, C., Lanci, A., Osman, T. K., Lacalandra, G. M., & Fusi, J. (2024). On-field Gross Morphology Evaluation of Dromedary Camel (Camelus dromedarius) Fetal Membranes. Animals, 14(11), 1553. https://doi.org/10.3390/ani14111553





