Insect Bite Hypersensitivity in Horses: Causes, Diagnosis, Scoring and New Therapies
Abstract
:Simple Summary
Abstract
1. Implicated Insects
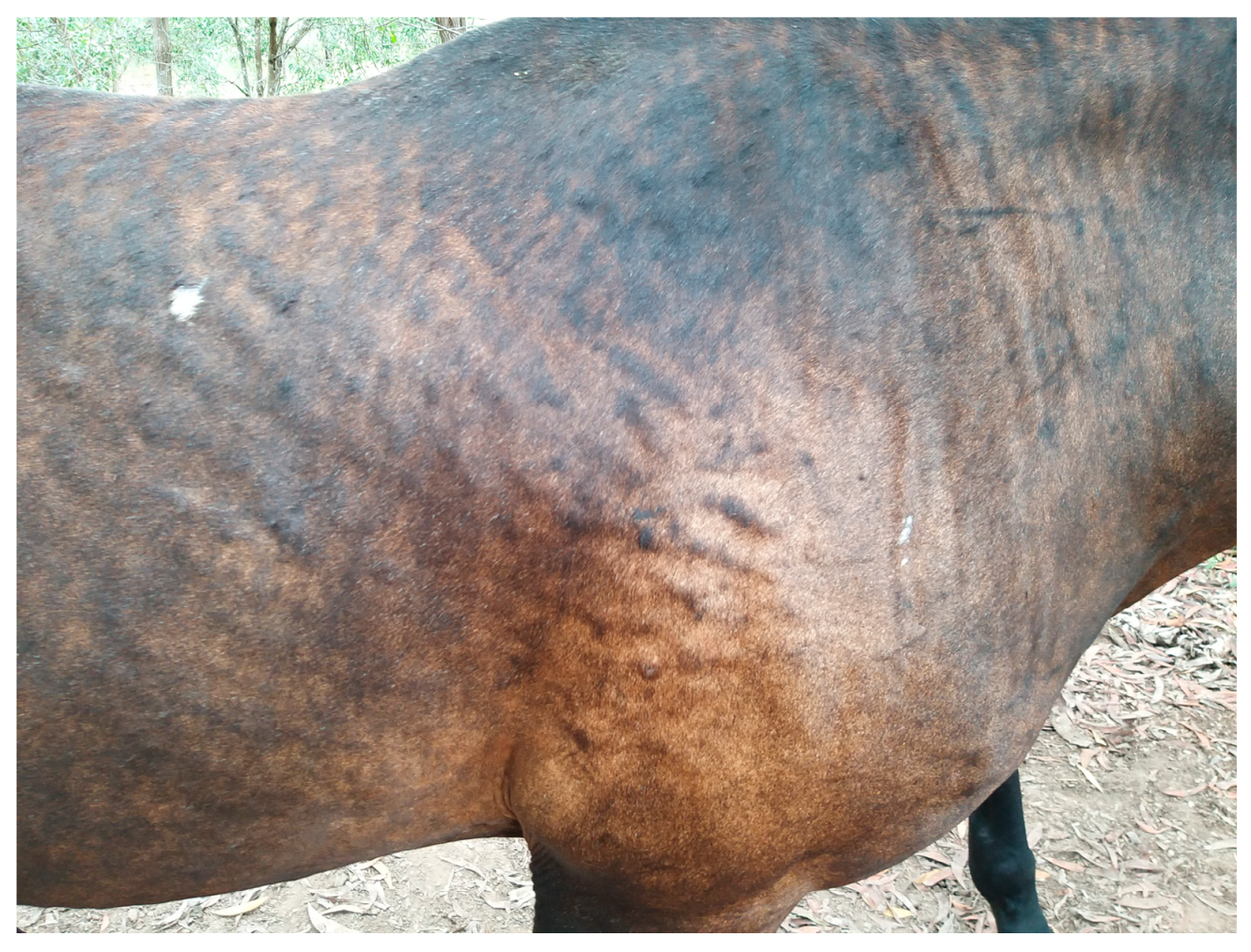
2. Distribution and Type of Lesions
3. Pathophysiology
4. Diagnosis
4.1. Intradermal Skin and Serum Allergy Testing
4.2. Genetic Testing of Horses
4.3. Scoring the Severity of IBH
5. Management
5.1. Traditional Treatments
- Minimising exposure to the Culicoides midge—this is considered the most important control measure and includes rugging, stabling at dawn and dusk, and the use of fans. It has been shown that horses affected by IBH show more itching behaviours (when compared with unaffected controls) in the evening when midges are most likely to be feeding [41].
- Reduction in disease severity through the use of corticosteroids. Due to potential complications with the long-term use of corticosteroids in horses, they should only be used as a short-term treatment tool [7,33,43,44] and ideally after testing for insulin dysregulation [45].
- ○
- The authors have used the following protocol with success: dexamethasone at 0.04 mg/kg IM initially, then 0.03 mg/kg IM for 3 days, then 0.02 mg/kg IM for 14 days, then 0.02 mg/kg IM every other day as required. If there is no initial reduction in pruritus after the initial 5 days (and no evidence of insulin dysregulation), then higher dosages can be trialled. The authors recommend avoiding dosages greater than 0.1 mg/kg IM for more than 5 days.
- ○
- The injectable dexamethasone solution can be given orally with a bioavailability of between 28–66% [46]. An intramuscular dosage of 20 mg for a 500 kg horse would be approximately 42 mg orally, assuming an average bioavailability of 47%.
5.2. Whole-Pathogen Immunotherapy
5.3. Allergen-Specific Immunotherapy
5.4. Cytokine Vaccination
5.4.1. Virus-like Particle (VLP)-Based Therapeutic Vaccines Targeting IL-5
5.4.2. Virus-like Particle (VLP)-Based Therapeutic Vaccines Targeting IL-31
5.5. Topical Treatments
5.6. Other Treatments
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fadok, V.A. Update on Equine Allergies. Vet. Clin. N. Am. Equine Pract. 2013, 29, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Björnsdóttir, S.; Sigvaldadóttir, J.; Broström, H.; Langvad, B.; Sigurðsson, A. Summer eczema in exported Icelandic horses: Influence of environmental and genetic factors. Acta Vet. Scand. 2006, 48, 3. [Google Scholar] [CrossRef] [Green Version]
- Grevenhof, E.M.; Ducro, B.; Heuven, H.C.M.; Bijma, P. Identification of environmental factors affecting prevalence of insect bite hypersensitivity in Shetland ponies and Friesian horses in the Netherlands. Equine Vet. J. 2007, 39, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Lomas, H.R.; Robinson, P.A. A Pilot Qualitative Investigation of Stakeholders’ Experiences and Opinions of Equine Insect Bite Hypersensitivity in England. Vet. Sci. 2018, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Rashmir-Raven, A.M. Disorders of the Skin. In Equine Internal Medicine; Reed, S.M., Bayly, W.M., Sellon, D.C., Eds.; Elsevier: Philadelphia, PA, USA, 2017; pp. 1159–1216. [Google Scholar]
- Rashmir-Raven, A.M. A review of physical urticarias in the horse. Equine Vet. Educ. 2019, 31, 195–197. [Google Scholar] [CrossRef]
- Knottenbelt, D.C. Pascoe’s Principles & Practice of Equine Dermatology; Saunders: London, UK, 2014. [Google Scholar]
- McAuliffe, S.B. Knottenbelt and Pascoe’s Color Atlas of Diseases and Disorders of the Horse E-Book; Elsevier–Health Sciences Division: Saint Louis, MO, USA, 2014. [Google Scholar]
- Riek, R.F. Studies On Allergic Dermatitis (“Queensland Itch”) Of The Horse: I-Description, Distribution, Symptoms And Pathology. Aust. Vet. J. 1953, 29, 177–184. [Google Scholar] [CrossRef]
- Torsteinsdottir, S.; Scheidegger, S.; Baselgia, S.; Jonsdottir, S.; Svansson, V.; Björnsdottir, S.; Marti, E. A prospective study on insect bite hypersensitivity in horses exported from Iceland into Switzerland. Acta Vet. Scand. 2018, 60, 69. [Google Scholar] [CrossRef] [Green Version]
- Sommer-Locher, B.V.; Endriss, V.; Fromm, E. Various Circumstances Regarding Initial Allergen Exposure and Their Influence on Development of Insect Bite Hypersensitivity in Horses. J. Equine Vet. Sci. 2012, 32, 158–163. [Google Scholar] [CrossRef]
- Schaffartzik, A.; Hamza, E.; Janda, J.; Crameri, R.; Marti, E.; Rhyner, C. Equine insect bite hypersensitivity: What do we know? Vet. Immunol. Immunopathol. 2012, 147, 113–126. [Google Scholar] [CrossRef]
- Miller, J.E.; Mann, S.; Fettelschoss-Gabriel, A.; Wagner, B. Comparison of three clinical scoring systems for Culicoides hypersensitivity in a herd of Icelandic horses. Vet. Dermatol. 2019, 30, 536-e163. [Google Scholar] [CrossRef]
- Jonsdottir, S.; Svansson, V.; Stefansdottir, S.B.; Mäntylä, E.; Marti, E.; Torsteinsdottir, S. Oral administration of transgenic barley expressing a Culicoides allergen induces specific antibody response. Equine Vet. J. 2017, 49, 512–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fettelschoss-Gabriel, A.; Fettelschoss, V.; Thoms, F.; Giese, C.; Daniel, M.; Olomski, F.; Kamarachev, J.; Birkmann, K.; Bühler, M.; Kummer, M.; et al. Treating insect-bite hypersensitivity in horses with active vaccination against IL-5. J. Allergy Clin. Immunol. 2018, 142, 1194–1205.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fettelschoss-Gabriel, A.; Fettelschoss, V.; Olomski, F.; Birkmann, K.; Thoms, F.; Bühler, M.; Kummer, M.; Zeltins, A.; Kündig, T.M.; Bachmann, M.F. Active vaccination against interleukin-5 as long-term treatment for insect-bite hypersensitivity in horses. Allergy 2019, 74, 572–582. [Google Scholar] [CrossRef]
- Olomski, F.; Fettelschoss, V.; Jonsdottir, S.; Birkmann, K.; Thoms, F.; Marti, E.; Bachmann, M.F.; Kündig, T.M.; Fettelschoss-Gabriel, A. Interleukin 31 in insect bite hypersensitivity—Alleviating clinical symptoms by active vaccination against itch. Allergy 2020, 75, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Fettelschoss-Gabriel, A.; Birkmann, K.; Pantelyushin, S.; Kündig, T.M. Molecular mechanisms and treatment modalities in equine Culicoides hypersensitivity. Vet. J. 2021, 276, 105741. [Google Scholar] [CrossRef] [PubMed]
- Gleich, G.J.; Frigas, E.; Loegering, D.A.; Wassom, D.L.; Steinmuller, D. Cytotoxic Properties of the Eosinophil Major Basic Protein. J. Immunol. 1979, 123, 2925–2927. [Google Scholar] [CrossRef]
- Tai, P.C.; Hayes, D.J.; Clark, J.B.; Spry, C.J.F. Toxic effects of human eosinophil products on isolated rat heart cells in vitro. Biochem. J. 1982, 204, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Olsén, L.; Bondesson, U.; Broström, H.; Olsson, U.; Mazogi, B.; Sundqvist, M.; Tjälve, H.; Ingvast-Larsson, C. Pharmacokinetics and effects of cetirizine in horses with insect bite hypersensitivity. Vet. J. 2011, 187, 347–351. [Google Scholar] [CrossRef]
- Mitson-Salazar, A.; Prussin, C. Pathogenic Effector Th2 Cells in Allergic Eosinophilic Inflammatory Disease. Front. Med. 2017, 4, 165. [Google Scholar] [CrossRef] [Green Version]
- Jonsdottir, S.; Cvitas, I.; Svansson, V.; Fettelschloss-Gabriel, A.; Torsteinsdottir, S.; Marti, E. New Strategies for Prevention and Treatment of Insect Bite Hypersensitivity in Horses. Curr. Dermatol. Rep. 2019, 8, 303–312. [Google Scholar] [CrossRef] [Green Version]
- Heimann, M.; Janda, J.; Sigurdardottir, O.G.; Svansson, V.; Klukowska, J.; von Tscharner, C.; Doherr, M.; Broström, H.; Andersson, L.S.; Einarsson, S.; et al. Skin-infiltrating T cells and cytokine expression in Icelandic horses affected with insect bite hypersensitivity: A possible role for regulatory T cells. Vet. Immunol. Immunopathol. 2011, 140, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.D.; Harwood, L.J.; Björnsdottir, S.; Marti, E.; Day, M.J. Detection of IgG and IgE serum antibodies to Culicoides salivary gland antigens in horses with insect dermal hypersensitivity (sweet itch). Equine Vet. J. 2001, 33, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Cvitas, I.; Oberhänsli, S.; Leeb, T.; Dettwiler, M.; Müller, E.; Bruggman, R.; Marti, E.I. Investigating the epithelial barrier and immune signatures in the pathogenesis of equine insect bite hypersensitivity. PLoS ONE 2020, 15, e0232189. [Google Scholar] [CrossRef] [PubMed]
- Cvitas, I.; Oberhaensli, S.; Leeb, T.; Marti, E. Equine keratinocytes in the pathogenesis of insect bite hypersensitivity: Just another brick in the wall? PLoS ONE 2022, 17, e0266263. [Google Scholar] [CrossRef] [PubMed]
- van der Meide, N.M.A.; Meulenbroeks, C.; van Altena, C.; Schurink, A.; Ducro, B.J.; Wagner, B.; Leibold, W.; Rohwer, J.; Jacobs, F.; van Oldruitenborgh-Oosterbaan, M.M.S.; et al. Culicoides obsoletus extract relevant for diagnostics of insect bite hypersensitivity in horses. Vet. Immunol. Immunop. 2012, 149, 245–254. [Google Scholar] [CrossRef] [PubMed]
- van der Meide, N.M.A.; Savelkoul, H.F.J.; Meulenbroeks, C.; Ducro, B.J.; Tijhaar, E. Evaluation of a diagnostic ELISA for insect bite hypersensitivity in horses using recombinant Obsoletus complex allergens. Vet. J. 2014, 200, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Novotny, E.N.; White, S.J.; Wilson, A.D.; Stefánsdóttir, S.B.; Tijhaar, E.; Jonsdóttir, S.; Frey, R.; Reiche, D.; Rose, H.; Rhyner, C.; et al. Component-resolved microarray analysis of IgE sensitization profiles to Culicoides recombinant allergens in horses with insect bite hypersensitivity. Allergy 2020, 76, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, M.; Solé, M.; Ducro, B.J.; Sundquist, M.; Thomas, R.; Schurink, A.; Eriksson, S.; Lindgren, G. Genome-wide association study for insect bite hypersensitivity susceptibility in horses revealed novel associated loci on chromosome 1. J. Anim. Breed Genet. 2019, 137, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Schurink, A.; Wolc, A.; Ducro, B.J.; Frankena, K.; Garrick, D.J.; Dekkers, J.C.; van Arendonk, J.A. Genome-wide association study of insect bite hypersensitivity in two horse populations in the Netherlands. Genet. Sel. Evol. 2012, 44, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, D.W.; Miller, W.H. Equine Dermatology, 2nd ed.; Elsevier: Amsterdam, The Netherlands; Saunders: Maryland Heights, MO, USA, 2011. [Google Scholar]
- Elman, S.; Hynan, L.S.; Gabriel, V.; Mayo, M.J. The 5-D itch scale: A new measure of pruritus: The 5-D itch scale. Br. J. Dermatol. 2010, 162, 587–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, S.A.; Brown, T.M.; Fehnel, S.; Deal, L.S.; Katz, E.G.; Chiou, C.-F. The atopic dermatitis itch scale: Development of a new measure to assess pruritus in patients with atopic dermatitis. J. Dermatolog. Treat. 2020, 31, 484–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivry, T.; Marsella, R.; Iwasaki, T.; Mueller, R.; International Task Force On Canine Atopic Dermatitis. Validation of CADESI-03, a severity scale for clinical trials enrolling dogs with atopic dermatitis. Vet. Dermatol. 2007, 18, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Olivry, T.; Mueller, R.; Nuttall, T.; Favrot, C.; Prélaud, P.; International Task Force on Canine Atopic Dermatitis. Determination of CADESI-03 thresholds for increasing severity levels of canine atopic dermatitis. Vet. Dermatol. 2008, 19, 115–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivry, T.; Saridomichelakis, M.; Nuttall, T.; Bensignor, E.; Griffin, C.E.; Hill, P.B. Validation of the Canine Atopic Dermatitis Extent and Severity Index (CADESI)-4, a simplified severity scale for assessing skin lesions of atopic dermatitis in dogs. Vet. Dermatol. 2014, 25, 77–85.e25. [Google Scholar] [CrossRef] [PubMed]
- Geiben, T. Untersuchungen zum Sommerekzem Sowie zum Einfluss des Immunmodulators Baypamun N® auf die Typ I-Allergie der Pferde, in the University of Veterinary Medicine Hanover; The University of Veterinary Medicine Hanover: Hanover, Germany, 2003. [Google Scholar]
- Cox, A.; Wood, K.; Randhawa, I.; Kamphuis, E.; Medina-Torres, C.E.; Stewart, A.J. Analysis of Scoring Systems for the Diagnosis of Insect Bite Hypersensitivity in Horses [Abstract]. In Proceedings of the Australian and New Zealand College of Veterinary Scientists Conference Week, Gold Coast, Australia, 23–25 June 2022. [Google Scholar]
- Söderroos, D.; Ignell, R.; Andersen, P.H.; Bergvall, K.; Riihimäki, M. The Effect of Insect Bite Hypersensitivity on Movement Activity and Behaviour of the Horse. Animals 2023, 13, 1283. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.; Wood, K.; Coleman, G.; Stewart, A.; Bertin, F.; Owen, H.; Suen, W.; Medina-Torres, C. Essential oil spray reduces clinical signs of insect bite hypersensitivity in horses. Aust. Vet. J. 2020, 98, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Pilsworth, R.C.; Knottenbelt, D.C. Equine insect hypersensitivity. Equine Vet. Ed. 2004, 16, 324–325. [Google Scholar] [CrossRef]
- Smith, B.P. Large Animal Internal Medicine; Elsevier Mosby: Saint Louis, MO, USA, 2014. [Google Scholar]
- Clark, B.L.; Stewart, A.J.; Kemp, K.L.; Bamford, N.J.; Bertin, F.R. Evaluation of field-testing protocols to diagnose insulin dysregulation in ponies using a Baysesian approach. Vet. J. 2023, 30, 885–891. [Google Scholar]
- Grady, J.A.; Davis, E.G.; KuKanich, B.; Sherck, A.B. Pharmacokinetics and pharmacodynamics of dexamethasone after oral administration in apparently healthy horses. Am. J. Vet. Res. 2010, 71, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Ginel, P.J.; Hernández, E.; Lucena, R.; Blanco, B.; Novales, M.; Mozos, E. Allergen-specific immunotherapy in horses with insect bite hypersensitivity: A double-blind, randomized, placebo-controlled study. Vet. Dermatol. 2014, 25, 29-e10. [Google Scholar] [CrossRef] [PubMed]
- Barbet, J.L.; Bevier, D.; Greiner, E.C. Specific immunotherapy in the treatment of Culicoides hypersensitive horses: A double-blind study. Equine Vet. J. 1990, 22, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.S.; Belton, P.; Jahren, E.; Lange, H.; Kleider, N. Immunotherapy Trial for Horses in British Columbia with Culicoides (Diptera: Ceratopogonidae) Hypersensitivity. J. Med. Entomol. 1996, 33, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Birras, J.; White, S.J.; Jonsdottir, S.; Novotny, E.N.; Ziegler, A.; Wilson, A.D.; Frey, R.; Torsteinsdottir, S.; Alcocer, M.; Marti, E. First clinical expression of equine insect bite hypersensitivity is associated with co-sensitization to multiple Culicoides allergens. PLoS ONE 2021, 16, e0257819. [Google Scholar] [CrossRef] [PubMed]
- Jonsdottir, S.; Svansson, V.; Stefansdottir, S.B.; Schüpbach, G.; Rhyner, C.; Marti, E.; Torsteinsdottir, S. A preventive immunization approach against insect bite hypersensitivity: Intralymphatic injection with recombinant allergens in Alum or Alum and monophosphoryl lipid A. Vet. Immunol. Immunopathol. 2016, 172, 14–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefansdottir, S.B.; Jonsdottir, S.; Kristjansdottir, H.; Svansson, V.; Marti, E.; Torsteinsdottir, S. Establishment of a protocol for preventive vaccination against equine insect bite hypersensitivity. Vet. Immunol. Immunop. 2022, 253, 110502. [Google Scholar] [CrossRef] [PubMed]
- Jonsdottir, S.; Stefansdottir, S.B.; Kristinarson, S.B.; Svansson, V.; Bjornsson, J.M.; Runarsdottir, A.; Wagner, B.; Marti, E.; Torsteinsdottir, S. Barley produced Culicoides allergens are suitable for monitoring the immune response of horses immunized with E. coli expressed allergens. Vet. Immunol. Immunopathol. 2018, 201, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Rhiner, T.; Fettelschoss, V.; Schoster, A.; Birkmann, K.; Fettelschoss-Gabriel, A. Targeting eosinophils by active vaccination against interleukin-5 reduces basophil counts in horses with insect bite hypersensitivity in the 2nd year of vaccination. Vet. J. 2022, 288, 105896. [Google Scholar] [CrossRef] [PubMed]
- Jonsdottir, S.; Fettelschoss, V.; Olomski, F.; Talker, S.C.; Mirkovitch, J.; Rhiner, T.; Birkmann, K.; Thoms, F.; Wagner, B.; Bachmann, M.F.; et al. Safety Profile of a Virus-Like Particle-Based Vaccine Targeting Self-Protein Interleukin-5 in Horses. Vaccines 2020, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Langreder, N.; Schäckermann, D.; Meier, D.; Becker, M.; Schubert, M.; Dübel, S.; Reinard, T.; Figge-Wegener, S.; Roßbach, K.; Bäumer, W.; et al. Development of an inhibiting antibody against equine interleukin 5 to treat insect bite hypersensitivity of horses. Sci. Rep. 2023, 13, 4029. [Google Scholar] [CrossRef] [PubMed]
- Huhmann, R.; Mueller, R.S. A cream containing omega-3-fatty acids, humectants and emollients as an aid in the treatment of equine Culicoides hypersensitivity. Vet. Dermatol. 2019, 30, 155-e46. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-C.; Chang, W.-T.; Hseu, Y.-C.; Chen, H.-Y.; Chuang, C.H.; Lin, C.-C.; Lee, M.-S.; Lin, M.-K. Immunosuppressive Effect of Litsea cubeba L. Essential Oil on Dendritic Cell and Contact Hypersensitivity Responses. Int. J. Mol. Sci. 2016, 17, 1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, D.E.; Moore, G.P.; Pfaff, J.A. Camphor ingestion. Am. J. Emerg. Med. 1989, 7, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Parker, T.L. Lemongrass (Cymbopogon flexuosus) essential oil demonstrated anti-inflammatory effect in pre-inflamed human dermal fibroblasts. Biochim. Open 2017, 4, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.; Vasudevan, P.; Tandon, M.; Razdan, R. Larvicidal and mosquito repellent action of peppermint (Mentha piperita) oil. Bioresour. Technol. 2000, 71, 267–271. [Google Scholar] [CrossRef]
- Baldacchino, F.; Tramut, C.; Salem, A.; Liénard, E.; Delétré, E.; Franc, M.; Martin, T.; Duvallet, G.; Jay-Robert, P. The repellency of lemongrass oil against stable flies, tested using video tracking. Parasite 2013, 20, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsaie, L.T.; El Mohsen, A.M.; Ibrahim, I.M.; Mohey-Eddin, M.H.; Elsaie, M.L. Effectiveness of topical peppermint oil on symptomatic treatment of chronic pruritus. Clin. Cosmet. Investig. Dermatol. 2016, 9, 333–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.-J.; Chen, H.-M.; Li, C.-W.; Wu, D.-W.; Wu, X.-L.; Shi, S.-J.; Li, Y.-C.; Chen, J.-N.; Su, Z.-R.; Lai, X.-P. Experimental study on antinociceptive and anti-allergy effects of patchouli oil. J. Essent. Oil Res. 2013, 25, 488–496. [Google Scholar] [CrossRef]
- Yoon, S.C.; Je, I.-G.; Cui, X.; Park, H.R.; Khang, D.; Park, J.-S.; Kim, S.-H.; Shin, T.-Y. Anti-allergic and anti-inflammatory effects of aqueous extract of Pogostemon cablin. Int. J. Mol. Med. 2016, 37, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, A.J.; Bowman, J.W.; Fici, G.J.; Zhang, M.; Mann, D.W.; Mitton-Fry, M. Oclacitinib (APOQUEL®) is a novel Janus kinase inhibitor with activity against cytokines involved in allergy. J. Vet. Pharmacol. Ther. 2014, 37, 317–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosgrove, S.B.; Wren, J.A.; Cleaver, D.M.; Walsh, K.F.; Follis, S.I.; King, V.I.; Tena, J.-K.S.; Stegemann, M.R. A blinded, randomized, placebo-controlled trial of the efficacy and safety of the Janus kinase inhibitor oclacitinib (Apoquel®) in client-owned dogs with atopic dermatitis. Vet. Dermatol. 2013, 24, 587-e142. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cox, A.; Stewart, A.J. Insect Bite Hypersensitivity in Horses: Causes, Diagnosis, Scoring and New Therapies. Animals 2023, 13, 2514. https://doi.org/10.3390/ani13152514
Cox A, Stewart AJ. Insect Bite Hypersensitivity in Horses: Causes, Diagnosis, Scoring and New Therapies. Animals. 2023; 13(15):2514. https://doi.org/10.3390/ani13152514
Chicago/Turabian StyleCox, Abbey, and Allison J. Stewart. 2023. "Insect Bite Hypersensitivity in Horses: Causes, Diagnosis, Scoring and New Therapies" Animals 13, no. 15: 2514. https://doi.org/10.3390/ani13152514





