Amino Acid Digestibility of Different Formulations of Torula Yeast in an In Vitro Porcine Gastrointestinal Digestion Model and Their Protective Effects on Barrier Function and Inflammation in a Caco-2/THP1Co-Culture Model
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Product Description
2.2. Testing Conditions
2.3. Upper Gastrointestinal Simulation
2.4. Hydrolysis Procedures
2.5. High-Performance Liquid Chromatography with Diode-Array Detection
2.6. Cell Culture
2.7. Statistical Methods
3. Results
3.1. Total Amino Acid Content and Amino Acid Profile of Torula Yeast Products
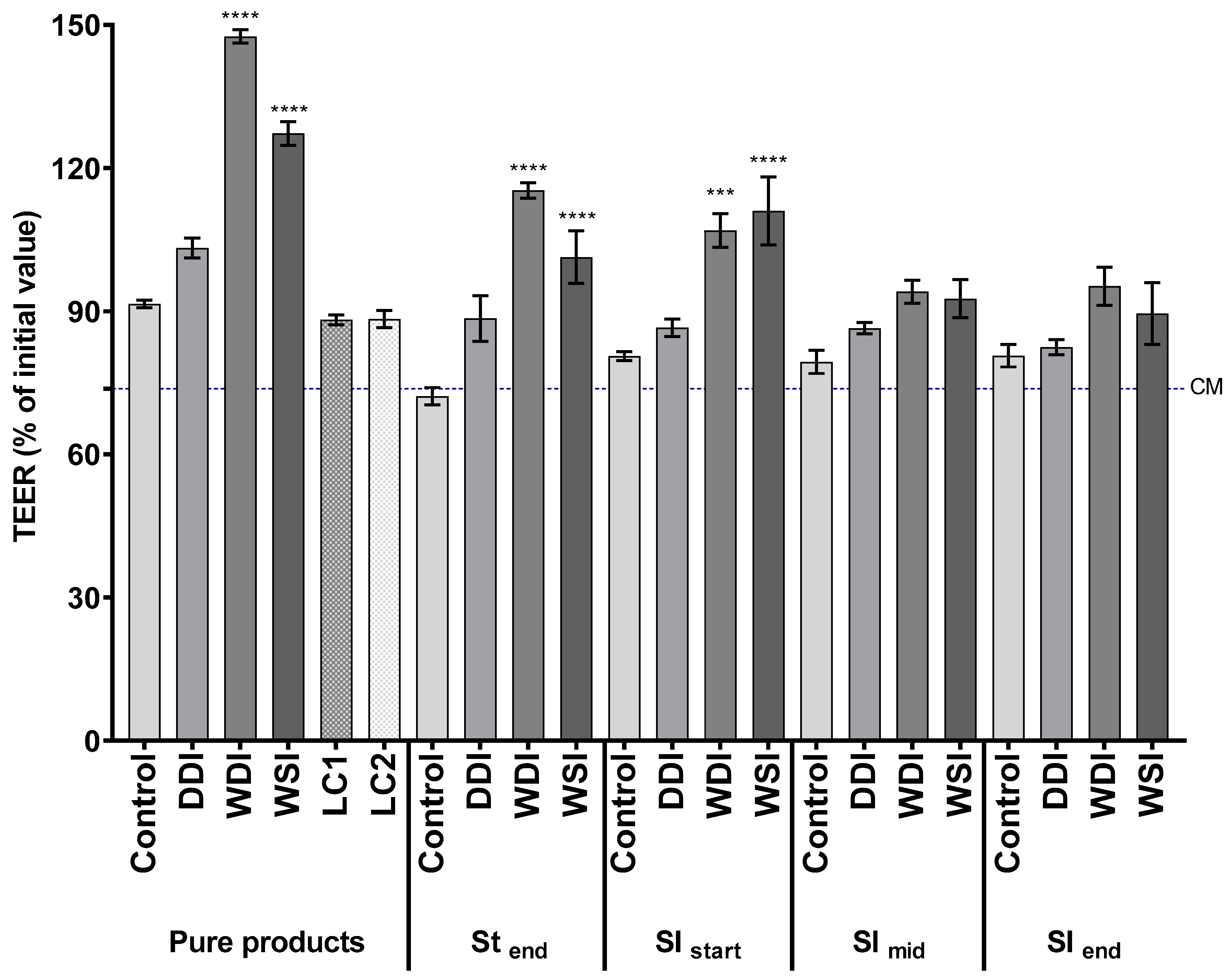
3.2. Pure Products and Proximal Gastrointestinal Digests Are More Effective in Protecting the Epithelial Barrier Integrity
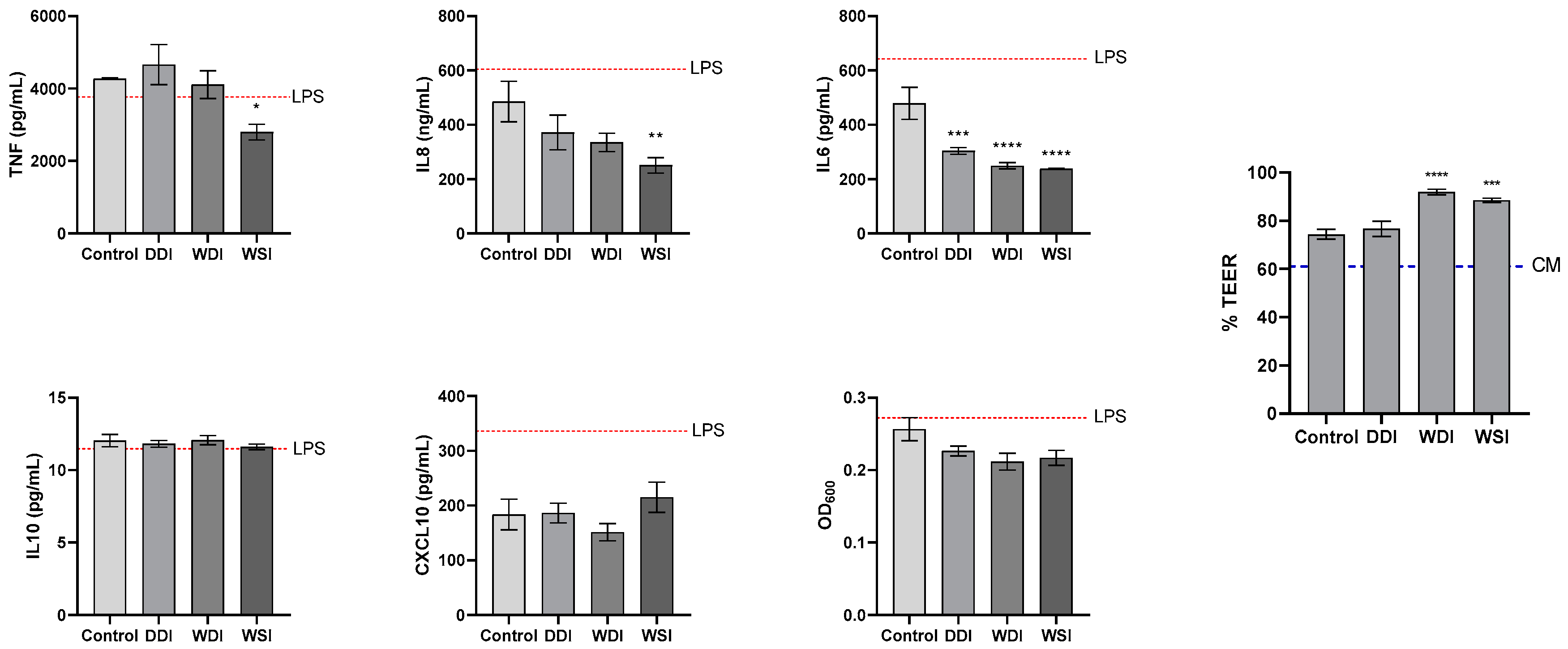
3.3. Effect of Ingredients Digests on Intestinal Inflammatory Response
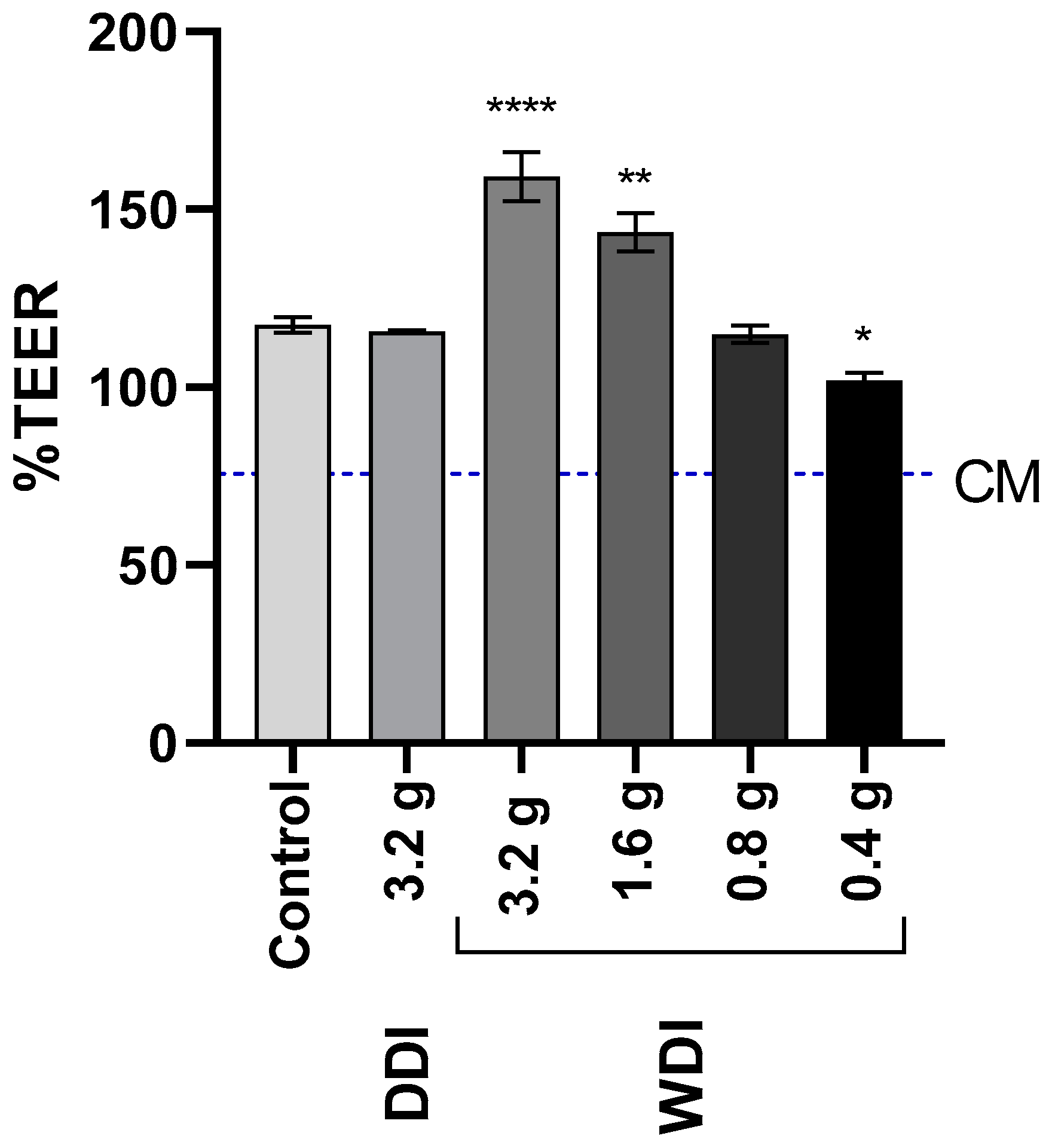
3.4. WDI Confers a Dose-Dependent Protection of the Epithelial Barrier
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Z.; Liao, S.F. Physiological Effects of Dietary Amino Acids on Gut Health and Functions of Swine. Front. Vet. Sci. 2019, 6, 169. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Sharma, P.; Shu, S.; Lin, T.-S.; Ciais, P.; Tubiello, F.N.; Smith, P.; Campbell, N.; Jain, A.K. Global Greenhouse Gas Emissions from Animal-Based Foods Are Twice Those of Plant-Based Foods. Nat. Food 2021, 2, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Andretta, I.; Hickmann, F.M.W.; Remus, A.; Franceschi, C.H.; Mariani, A.B.; Orso, C.; Kipper, M.; Létourneau-Montminy, M.-P.; Pomar, C. Environmental Impacts of Pig and Poultry Production: Insights from a Systematic Review. Front. Vet. Sci. 2021, 8, 750733. [Google Scholar] [CrossRef] [PubMed]
- Soare, E.; Chiurciu, I.-A. Study on the Pork Market Worldwide. Sci. Pap. Ser. Manag. Econ. Eng. Agric. Rural Dev. 2017, 17, 321–326. [Google Scholar]
- Stødkilde, L.; Mogensen, L.; Bache, J.K.; Ambye-Jensen, M.; Vinther, J.; Jensen, S.K. Local Protein Sources for Growing-Finishing Pigs and Their Effects on Pig Performance, Sensory Quality and Climate Impact of the Produced Pork. Livest. Sci. 2023, 267, 105128. [Google Scholar] [CrossRef]
- Cappelaere, L.; Le Cour Grandmaison, J.; Martin, N.; Lambert, W. Amino Acid Supplementation to Reduce Environmental Impacts of Broiler and Pig Production: A Review. Front. Vet. Sci. 2021, 8, 689259. [Google Scholar] [CrossRef]
- van Milgen, J.; Dourmad, J.-Y. Concept and Application of Ideal Protein for Pigs. J. Anim. Sci. Biotechnol. 2015, 6, 15. [Google Scholar] [CrossRef]
- Rodrigues, D.B.; Marques, M.C.; Hacke, A.; Filho, P.S.L.; Cazarin, C.B.B.; Mariutti, L.R.B. Trust Your Gut: Bioavailability and Bioaccessibility of Dietary Compounds. Curr. Res. Food Sci. 2022, 5, 228–233. [Google Scholar] [CrossRef]
- Gaudichon, C.; Calvez, J. Determinants of Amino Acid Bioavailability from Ingested Protein in Relation to Gut Health. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 55–61. [Google Scholar] [CrossRef]
- Nørgaard, J.V.; Florescu, I.C.; Krogh, U.; Nielsen, T.S. Amino Acid Absorption Profiles in Growing Pigs Fed Different Protein Sources. Animals 2021, 11, 1740. [Google Scholar] [CrossRef]
- Zhang, Q.; Hou, Y.; Bazer, F.W.; He, W.; Posey, E.A.; Wu, G. Amino Acids in Swine Nutrition and Production. In Amino Acids in Nutrition and Health: Amino Acids in the Nutrition of Companion, Zoo and Farm Animals; Wu, G., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 81–107. ISBN 978-3-030-54462-1. [Google Scholar]
- Tang, X.; Xiong, K.; Fang, R.; Li, M. Weaning Stress and Intestinal Health of Piglets: A Review. Front. Immunol. 2022, 13, 1042778. [Google Scholar] [CrossRef] [PubMed]
- Bratosin, B.C.; Darjan, S.; Vodnar, D.C. Single Cell Protein: A Potential Substitute in Human and Animal Nutrition. Sustainability 2021, 13, 9284. [Google Scholar] [CrossRef]
- Brenne, T.; Naess, B.; Farstad, L. The Nutritive Value, for Growing Pigs, of Single Cell Protein (Candida utilis) Produced from Sulphite Spent Liquor. Acta Agric. Scand. 1974, 24, 3–6. [Google Scholar] [CrossRef]
- Espinosa, C.D.; Lagos, L.V.; Stein, H.H. Effect of Torula Yeast on Growth Performance, Diarrhea Incidence, and Blood Characteristics in Weanling Pigs. J. Anim. Sci. 2020, 98, skaa307. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.; Håkenåsen, I.M.; Skugor, A.; Mydland, L.T.; Åkesson, C.P.; Hellestveit, S.S.; Sørby, R.; Press, C.M.; Øverland, M. Candida utilis Yeast as a Protein Source for Weaned Piglets: Effects on Growth Performance and Digestive Function. Livest. Sci. 2019, 226, 31–39. [Google Scholar] [CrossRef]
- Holt, D.A.; Aldrich, C.G. Evaluation of Torula Yeast as a Protein Source in Extruded Feline Diets. J. Anim. Sci. 2022, 100, skac327. [Google Scholar] [CrossRef]
- Cruz, A.; Sterten, H.; Steinhoff, F.S.; Mydland, L.T.; Øverland, M. Cyberlindnera Jadinii Yeast as a Protein Source for Broiler Chickens: Effects on Growth Performance and Digestive Function from Hatching to 30 Days of Age. Poult. Sci. 2020, 99, 3168–3178. [Google Scholar] [CrossRef]
- Lagos, L.V.; Stein, H.H. Torula Yeast Has Greater Digestibility of Amino Acids and Phosphorus, but Not Energy, Compared with a Commercial Source of Fish Meal Fed to Weanling Pigs. J. Anim. Sci. 2020, 98, skz375. [Google Scholar] [CrossRef]
- Leeper, A.; Ekmay, R.; Knobloch, S.; Skírnisdóttir, S.; Varunjikar, M.; Dubois, M.; Smárason, B.Ö.; Árnason, J.; Koppe, W.; Benhaïm, D. Torula Yeast in the Diet of Atlantic Salmon Salmo salar and the Impact on Growth Performance and Gut Microbiome. Sci. Rep. 2022, 12, 567. [Google Scholar] [CrossRef]
- Øverland, M.; Skrede, A. Yeast Derived from Lignocellulosic Biomass as a Sustainable Feed Resource for Use in Aquaculture. J. Sci. Food Agric. 2017, 97, 733–742. [Google Scholar] [CrossRef]
- Agboola, J.O.; Schiavone, M.; Øverland, M.; Morales-Lange, B.; Lagos, L.; Arntzen, M.Ø.; Lapeña, D.; Eijsink, V.G.H.; Horn, S.J.; Mydland, L.T.; et al. Impact of Down-Stream Processing on Functional Properties of Yeasts and the Implications on Gut Health of Atlantic Salmon (Salmo salar). Sci. Rep. 2021, 11, 4496. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-C. Use of Microorganisms and Their Products as Feedstuffs and Functional Feed Additives in Nursery Pigs; North Carolina State University: Raleigh, NC, USA, 2022. [Google Scholar]
- Jach, M.E.; Serefko, A.; Ziaja, M.; Kieliszek, M. Yeast Protein as an Easily Accessible Food Source. Metabolites 2022, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Shurson, G. Yeast and Yeast Derivatives in Feed Additives and Ingredients: Sources, Characteristics, Animal Responses, and Quantification Methods. Anim. Feed Sci. Technol. 2018, 235, 60–76. [Google Scholar] [CrossRef]
- Halas, V.; Nochta, I. Mannan Oligosaccharides in Nursery Pig Nutrition and Their Potential Mode of Action. Animals 2012, 2, 261–274. [Google Scholar] [CrossRef]
- Tiwari, U.P.; Fleming, S.A.; Rasheed, M.S.A.; Jha, R.; Dilger, R.N. The Role of Oligosaccharides and Polysaccharides of Xylan and Mannan in Gut Health of Monogastric Animals. J. Nutr. Sci. 2020, 9, e21. [Google Scholar] [CrossRef]
- Atanasov, J.; Schloermann, W.; Trautvetter, U.; Glei, M. The Effects of β-Glucans on Intestinal Health. Ernahr. Umsch. 2020, 67, 52–59. [Google Scholar]
- Yang, Z.; Wang, Y.; He, T.; Ziema Bumbie, G.; Wu, L.; Sun, Z.; Sun, W.; Tang, Z. Effects of Dietary Yucca Schidigera Extract and Oral Candida utilis on Growth Performance and Intestinal Health of Weaned Piglets. Front. Nutr. 2021, 8, 685540. [Google Scholar] [CrossRef]
- Håkenåsen, I.M. Novel Protein Sources in Diets for Weaned Piglets–Effect on Growth Performance, Gut Function, and Health; Norwegian University of Life Sciences: Ås, Norway, 2022. [Google Scholar]
- Zhao, D.; Liu, H.; Zhang, H.; Liu, K.; Zhang, X.; Liu, Q.; Wu, Y.; Zhang, T.; Zhang, Q. Dietary Supplementation with Cyberlindnera Jadinii Improved Growth Performance, Serum Biochemical Indices, Antioxidant Status, and Intestinal Health in Growing Raccoon Dogs (Nyctereutes procyonoides). Front. Microbiol. 2022, 13, 973384. [Google Scholar] [CrossRef]
- Håkenåsen, I.M.; Øverland, M.; Ånestad, R.; Åkesson, C.P.; Sundaram, A.Y.; Press, C.M.; Mydland, L.T. Gene Expression and Gastrointestinal Function Is Altered in Piglet Small Intestine by Weaning and Inclusion of Cyberlindnera Jadinii Yeast as a Protein Source. J. Funct. Foods 2020, 73, 104118. [Google Scholar] [CrossRef]
- Daguet, D.; Pinheiro, I.; Verhelst, A.; Possemiers, S.; Marzorati, M. Arabinogalactan and Fructooligosaccharides Improve the Gut Barrier Function in Distinct Areas of the Colon in the Simulator of the Human Intestinal Microbial Ecosystem. J. Funct. Foods 2016, 20, 369–379. [Google Scholar] [CrossRef]
- Desobry, S.A.; Netto, F.M.; Labuza, T.P. Comparison of Spray-drying, Drum-drying and Freeze-drying for Β-carotene Encapsulation and Preservation. J. Food Sci. 1997, 62, 1158–1162. [Google Scholar] [CrossRef]
- Yang, Z.; Htoo, J.K.; Liao, S.F. Methionine Nutrition in Swine and Related Monogastric Animals: Beyond Protein Biosynthesis. Anim. Feed Sci. Technol. 2020, 268, 114608. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Scientific Opinion on DL-methionine, DL-methionine Sodium Salt, the Hydroxy Analogue of Methionine and the Calcium Salt of Methionine Hydroxy Analogue in All Animal Species; on the Isopropyl Ester of Methionine Hydroxy Analogue and DL-methionine Technically Pure Protected with Copolymer Vinylpyridine/Styrene in Dairy Cows; and on DL-methionine Technically Pure Protected with Ethylcellulose in Ruminants. EFSA J. 2012, 10, 2623. [Google Scholar]
- Liu, Y.; Wang, X.; Hou, Y.; Yin, Y.; Qiu, Y.; Wu, G.; Hu, C.-A.A. Roles of Amino Acids in Preventing and Treating Intestinal Diseases: Recent Studies with Pig Models. Amino Acids 2017, 49, 1277–1291. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, R.; Wang, W.; Wu, Z.; Dai, Z.; Wang, J.; Wu, G. Biochemical and Physiological Bases for Utilization of Dietary Amino Acids by Young Pigs. J. Anim. Sci. Biotechnol. 2013, 4, 7. [Google Scholar] [CrossRef]
- Li, P.; Yin, Y.-L.; Li, D.; Kim, S.W.; Wu, G. Amino Acids and Immune Function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef]
- Wu, S.; Liu, M.; Chen, H.; Song, Q.; Wu, Z.; Dai, Z. Tryptophan Regulates Bile and Nitrogen Metabolism in Two Pig Gut Lactobacilli Species in vitro Based on Metabolomics Study. Amino Acids 2022, 54, 1421–1435. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, L.; Li, P.; Li, X.; Zhou, H.; Wang, F.; Li, D.; Yin, Y.; Wu, G. Gene Expression Is Altered in Piglet Small Intestine by Weaning and Dietary Glutamine Supplementation. J. Nutr. 2008, 138, 1025–1032. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Yang, G.; Zhang, Q.; Meng, L.; Xin, Y.; Jiang, X. The Role of Short-Chain Fatty Acids in Intestinal Barrier Function, Inflammation, Oxidative Stress, and Colonic Carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef]
- Judkins, T.C.; Archer, D.L.; Kramer, D.C.; Solch, R.J. Probiotics, Nutrition, and the Small Intestine. Curr. Gastroenterol. Rep. 2020, 22, 2. [Google Scholar] [CrossRef]
- Wijtten, P.J.; van der Meulen, J.; Verstegen, M.W. Intestinal Barrier Function and Absorption in Pigs after Weaning: A Review. Br. J. Nutr. 2011, 105, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Lærke, H.; Hedemann, M. Chapter 5, The Digestive System of the Pig. In Nutritional Physiology of Pigs—Online Publication; Videncenter for Svineproduktion: Foulum, Denmark, 2012. [Google Scholar]
- Maroilley, T.; Berri, M.; Lemonnier, G.; Esquerré, D.; Chevaleyre, C.; Mélo, S.; Meurens, F.; Coville, J.L.; Leplat, J.J.; Rau, A.; et al. Immunome Differences between Porcine Ileal and Jejunal Peyer’s Patches Revealed by Global Transcriptome Sequencing of Gut-Associated Lymphoid Tissues. Sci. Rep. 2018, 8, 9077. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Song, M.; Liu, Y.; Ji, P. Enterotoxigenic Escherichia Coli Infection of Weaned Pigs: Intestinal Challenges and Nutritional Intervention to Enhance Disease Resistance. Front. Immunol. 2022, 13, 885253. [Google Scholar] [CrossRef] [PubMed]
- Penney, J.; Lu, Y.; Pan, B.; Feng, Y.; Walk, C.; Li, J. Pure Yeast Beta-Glucan and Two Types of Yeast Cell Wall Extracts Enhance Cell Migration in Porcine Intestine Model. J. Funct. Foods 2019, 59, 129–137. [Google Scholar] [CrossRef]
- Hromádková, Z.; Ebringerová, A.; Sasinková, V.; Šandula, J.; Hříbalová, V.; Omelková, J. Influence of the Drying Method on the Physical Properties and Immunomodulatory Activity of the Particulate (1→3)-β-d-Glucan from Saccharomyces Cerevisiae. Carbohydr. Polym. 2003, 51, 9–15. [Google Scholar] [CrossRef]

| g/100 g | ||||
|---|---|---|---|---|
| Moisture | Crude Protein | Crude Fat | Ash | |
| DDI | 5.3 | 54.8 | 1.0 | 10 |
| WDI | 5.0 | 51.0 | 5.4 | 9.8 |
| WSI | 5.0 | 46.1 | 5.2 | 9.8 |
| mg AA/Incubation (3.2 g) | % AA | |||||
|---|---|---|---|---|---|---|
| Amino Acid | DDI | WDI | WSI | DDI | WDI | WSI |
| Alanine (Ala) | 94.08 ± 0.29 | 116.51 ± 0.07 | 69.54 ± 0.07 | 6.9 ± 0.02 | 8.1 ± 0.01 | 6.5 ± 0.01 |
| Arginine (Arg) | 58.85 ± 0.10 | 61.02 ± 0.13 | 38.26 ± 0.08 | 4.3 ± 0.01 | 4.3 ± 0.01 | 3.6 ± 0.01 |
| Aspartic acid (Asp) | 128.70 ± 0.13 | 136.32 ± 0.06 | 107.35 ± 0.07 | 9.4 ± 0.02 | 9.5 ± 0.01 | 10.0 ± 0.01 |
| Cysteine (Cys) | 71.50 ± 0.25 | 50.82 ± 0.18 | 38.52 ± 0.17 | 5.2 ± 0.02 | 3.5 ± 0.01 | 3.6 ± 0.02 |
| Glutamic acid (Glu) | 238.38 ± 0.24 | 233.76 ± 0.09 | 153.43 ± 0.15 | 17.5 ± 0.04 | 16.3 ± 0.01 | 14.3 ± 0.01 |
| Glycine (Gly) | 52.75 ± 0.08 | 55.31 ± 0.10 | 42.40 ± 0.08 | 3.9 ± 0.01 | 3.9 ± 0.01 | 3.9 ± 0.01 |
| Histidine (His) | 21.13 ± 0.03 | 21.95 ± 0.06 | 15.79 ± 0.04 | 1.5 ± 0.00 | 1.5 ± 0.00 | 1.5 ± 0.00 |
| Isoleucine (Ile) | 39.86 ± 0.07 | 41.29 ± 0.06 | 32.56 ± 0.02 | 2.9 ± 0.01 | 2.9 ± 0.00 | 3.0 ± 0.00 |
| Leucine (Leu) | 63.08 ± 0.10 | 68.34 ± 0.06 | 53.01 ± 0.03 | 4.6 ± 0.01 | 4.8 ± 0.01 | 4.9 ± 0.00 |
| Lysine (Lys) | 23.40 ± 0.04 | 25.35 ± 0.02 | 19.66 ± 0.01 | 1.7 ± 0.00 | 1.8 ± 0.00 | 1.8 ± 0.00 |
| Methionine (Met) | 192.00 ± 0.40 | 228.00 ± 0.67 | 199.26 ± 0.43 | 14.1 ± 0.04 | 15.9 ± 0.05 | 18.5 ± 0.04 |
| Phenylalanine (Phe) | 45.60 ± 0.09 | 46.01 ± 0.15 | 32.87 ± 0.08 | 3.3 ± 0.01 | 3.2 ± 0.01 | 3.1 ± 0.01 |
| Proline (Pro) | 40.28 ± 0.15 | 47.85 ± 0.03 | 30.02 ± 0.00 | 3.0 ± 0.01 | 3.3 ± 0.00 | 2.8 ± 0.00 |
| Serine (Ser) | 63.98 ± 0.12 | 64.75 ± 0.20 | 48.68 ± 0.15 | 4.7 ± 0.01 | 4.5 ± 0.01 | 4.5 ± 0.01 |
| Threonine (Thr) | 61.98 ± 0.26 | 71.14 ± 0.05 | 46.89 ± 0.06 | 4.5 ± 0.02 | 5.0 ± 0.00 | 4.4 ± 0.01 |
| Tryptophan (Trp) | 104.36 ± 0.90 | 61.91 ± 0.75 | 69.03 ± 0.57 | 7.6 ± 0.07 | 4.3 ± 0.05 | 6.4 ± 0.05 |
| Tyrosine (Tyr) | 48.70 ± 0.07 | 48.46 ± 0.15 | 35.74 ± 0.11 | 3.6 ± 0.01 | 3.4 ± 0.01 | 3.3 ± 0.01 |
| Valine (Val) | 51.26 ± 0.09 | 52.94 ± 0.07 | 42.44 ± 0.01 | 3.8 ± 0.01 | 3.7 ± 0.01 | 3.9 ± 0.00 |
| Total AA | 1364.91 ± 2.46 | 1431.74 ± 0.98 | 1075.43 ± 0.41 | 100 ± 0.25 | 100 ± 0.10 | 100 ± 0.05 |
| Absorbed AA (mg/Reactor; 3.2 g Product) | % AA Absorbed (to Total Protein Absorbed) | % AA Absorbed (to Individual AA in Raw Product) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| DDI | WDI | WSI | DDI | WDI | WSI | DDI | WDI | WSI | |
| Alanine (Ala) | 55.3 ± 1.1 | 69.9 ± 5.5 | 26.5 ± 2.0 | 7.0 ± 0.3 | 9.3 ± 0.8 | 6.0 ± 0.8 | 58.7 ± 1.2 | 60.0 ± 4.7 | 38.1 ± 2.8 |
| Arginine (Arg) | 36.2 ± 1.3 | 34.2 ± 0.3 | 14.9 ± 0.4 | 4.6 ± 0.2 | 4.6 ± 0.2 | 3.4 ± 0.4 | 61.6 ± 2.2 | 56.0 ± 0.5 | 39.0 ± 1.1 |
| Aspartic acid (Asp) | 53.9 ± 1.0 | 47.7 ± 11.6 | 27.3 ± 2.4 | 6.8 ± 0.2 | 6.4 ± 1.6 | 6.1 ± 0.9 | 41.9 ± 0.8 | 35.0 ± 8.5 | 25.4 ± 2.2 |
| Cysteine (Cys) | 50.4 ± 6.9 | 39.6 ± 4.5 | 29.9 ± 5.0 | 6.4 ± 0.9 | 5.3 ± 0.6 | 6.7 ± 1.4 | 70.5 ± 9.7 | 78.0 ± 8.9 | 77.7 ± 13.1 |
| Glutamic acid (Glu) | 135.5 ± 2.3 | 114.2 ± 13.2 | 50.6 ± 4.1 | 17.2 ± 0.6 | 15.2 ± 1.9 | 11.4 ± 1.5 | 56.8 ± 1.0 | 48.8 ± 5.6 | 33.0 ± 2.7 |
| Glycine (Gly) | 25.2 ± 1.3 | 21.3 ± 0.8 | 12.9 ± 0.6 | 3.2 ± 0.2 | 2.8 ± 0.2 | 2.9 ± 0.3 | 47.7 ± 2.4 | 38.5 ± 1.5 | 30.3 ± 1.5 |
| Histidine (His) | 9.9 ± 0.7 | 8.2 ± 0.8 | 3.6 ± 0.1 | 1.3 ± 0.1 | 1.1 ± 0.1 | 0.8 ± 0.1 | 47.1 ± 3.4 | 37.5 ± 3.8 | 23.0 ± 0.9 |
| Isoleucine (Ile) | 13.1 ± 0.4 | 9.4 ± 2.5 | 4.0 ± 1.1 | 1.7 ± 0.1 | 1.3 ± 0.3 | 0.9 ± 0.3 | 33.1 ± 1.1 | 22.7 ± 6.0 | 12.3 ± 3.2 |
| Leucine (Leu) | 29.1 ± 1.1 | 27.7 ± 3.3 | 15.7 ± 1.5 | 3.7 ± 0.2 | 3.7 ± 0.5 | 3.5 ± 0.5 | 46.2 ± 1.7 | 40.5 ± 4.8 | 29.6 ± 2.9 |
| Lysine (Lys) | 10.3 ± 0.4 | 9.9 ± 2.0 | 5.7 ± 1.0 | 1.3 ± 0.1 | 1.3 ± 0.3 | 1.3 ± 0.3 | 44.2 ± 1.5 | 39.1 ± 7.7 | 28.9 ± 4.8 |
| Methionine (Met) | 148.7 ± 4.1 | 176.7 ± 7.7 | 155.4 ± 2.1 | 18.9 ± 0.8 | 23.6 ± 1.4 | 34.9 ± 3.8 | 77.5 ± 2.1 | 77.5 ± 3.4 | 78.0 ± 1.0 |
| Phenylalanine (Phe) | 24.9 ± 1.8 | 21.2 ± 1.0 | 9.0 ± 0.3 | 3.2 ± 0.2 | 2.8 ± 0.2 | 2.0 ± 0.2 | 54.6 ± 3.9 | 46.0 ± 2.1 | 27.5 ± 0.9 |
| Proline (Pro) | 17.2 ± 0.7 | 19.9 ± 2.1 | 5.7 ± 0.4 | 2.2 ± 0.1 | 2.7 ± 0.3 | 1.3 ± 0.2 | 42.8 ± 1.8 | 41.7 ± 4.5 | 19.1 ± 1.3 |
| Serine (Ser) | 32.7 ± 1.8 | 25.3 ± 3.1 | 13.5 ± 1.4 | 4.1 ± 0.3 | 3.4 ± 0.4 | 3.0 ± 0.5 | 51.1 ± 2.8 | 39.1 ± 4.9 | 27.6 ± 2.9 |
| Threonine (Thr) | 24.2 ± 1.2 | 24.2 ± 0.2 | 3.5 ± 3.6 | 3.1 ± 0.2 | 3.2 ± 0.1 | 0.8 ± 0.8 | 39.0 ± 2.0 | 34.0 ± 0.3 | 7.4 ± 7.7 |
| Tryptophan (Trp) | 104.4 ± 0.9 | 61.9 ± 0.7 | 25.8 ± 0.6 | 13.2 ± 0.4 | 8.3 ± 0.3 | 5.8 ± 0.6 | 100.0 ± 0.9 | 100.0 ± 1.2 | 37.4 ± 0.8 |
| Tyrosine (Tyr) | 28.8 ± 1.7 | 25.7 ± 1.5 | 13.7 ± 0.1 | 3.7 ± 0.2 | 3.4 ± 0.2 | 3.1 ± 0.3 | 59.2 ± 3.5 | 53.1 ± 3.2 | 38.4 ± 0.4 |
| Valine (Val) | 17.8 ± 0.7 | 12.4 ± 3.2 | 5.9 ± 1.7 | 2.3 ± 0.1 | 1.7 ± 0.4 | 1.3 ± 0.4 | 34.8 ± 1.3 | 23.5 ± 6.0 | 14.0 ± 4.0 |
| Total AA | 787.9 ± 24.2 | 749.3 ± 28.3 | 445.2 ± 48.7 | 100.0 ± 4.3 | 100.0 ± 5.3 | 100.0 ± 15.5 | 57.7 ± 1.8 | 52.3 ± 2.0 | 41.4 ± 4.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verstrepen, L.; Calatayud-Arroyo, M.; Duysburgh, C.; De Medts, J.; Ekmay, R.D.; Marzorati, M. Amino Acid Digestibility of Different Formulations of Torula Yeast in an In Vitro Porcine Gastrointestinal Digestion Model and Their Protective Effects on Barrier Function and Inflammation in a Caco-2/THP1Co-Culture Model. Animals 2023, 13, 2812. https://doi.org/10.3390/ani13182812
Verstrepen L, Calatayud-Arroyo M, Duysburgh C, De Medts J, Ekmay RD, Marzorati M. Amino Acid Digestibility of Different Formulations of Torula Yeast in an In Vitro Porcine Gastrointestinal Digestion Model and Their Protective Effects on Barrier Function and Inflammation in a Caco-2/THP1Co-Culture Model. Animals. 2023; 13(18):2812. https://doi.org/10.3390/ani13182812
Chicago/Turabian StyleVerstrepen, Lynn, Marta Calatayud-Arroyo, Cindy Duysburgh, Jelle De Medts, Ricardo D. Ekmay, and Massimo Marzorati. 2023. "Amino Acid Digestibility of Different Formulations of Torula Yeast in an In Vitro Porcine Gastrointestinal Digestion Model and Their Protective Effects on Barrier Function and Inflammation in a Caco-2/THP1Co-Culture Model" Animals 13, no. 18: 2812. https://doi.org/10.3390/ani13182812





