The Molecular and Function Characterization of Porcine MID2
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Tissues
2.2. Sequence, Structure, and Phylogenetic Analysis
2.3. RNA Isolation and RT-qPCRs Analysis
2.4. Plasmids Construction
2.5. Cell Transfection and RNA Interference
2.6. Immunofluorescence Microscopy
2.7. Dual-Luciferase Reporter Assay
2.8. Statistical Analysis
3. Results
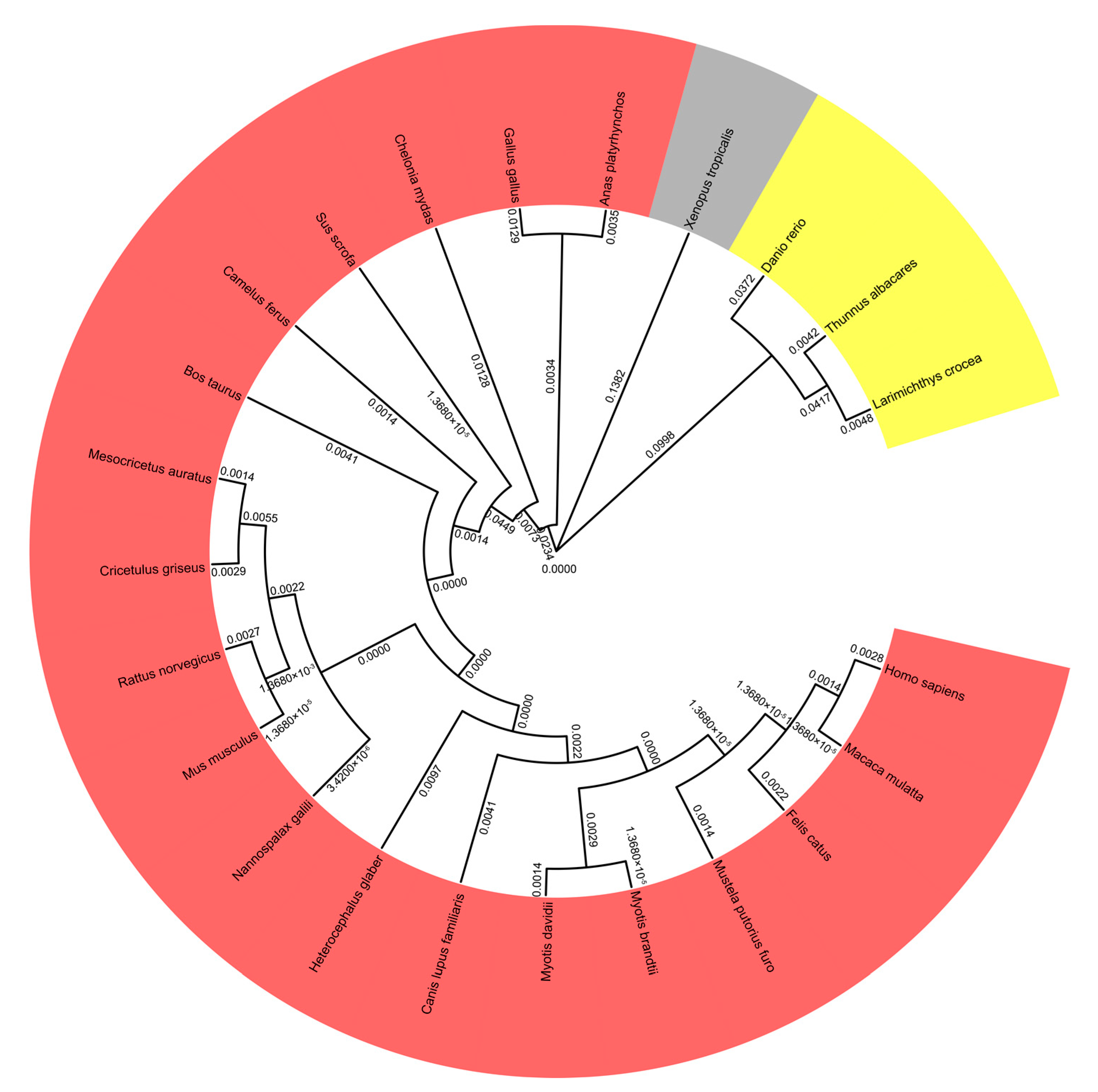
3.1. Phylogenetic and Sequence Analysis of pMID2
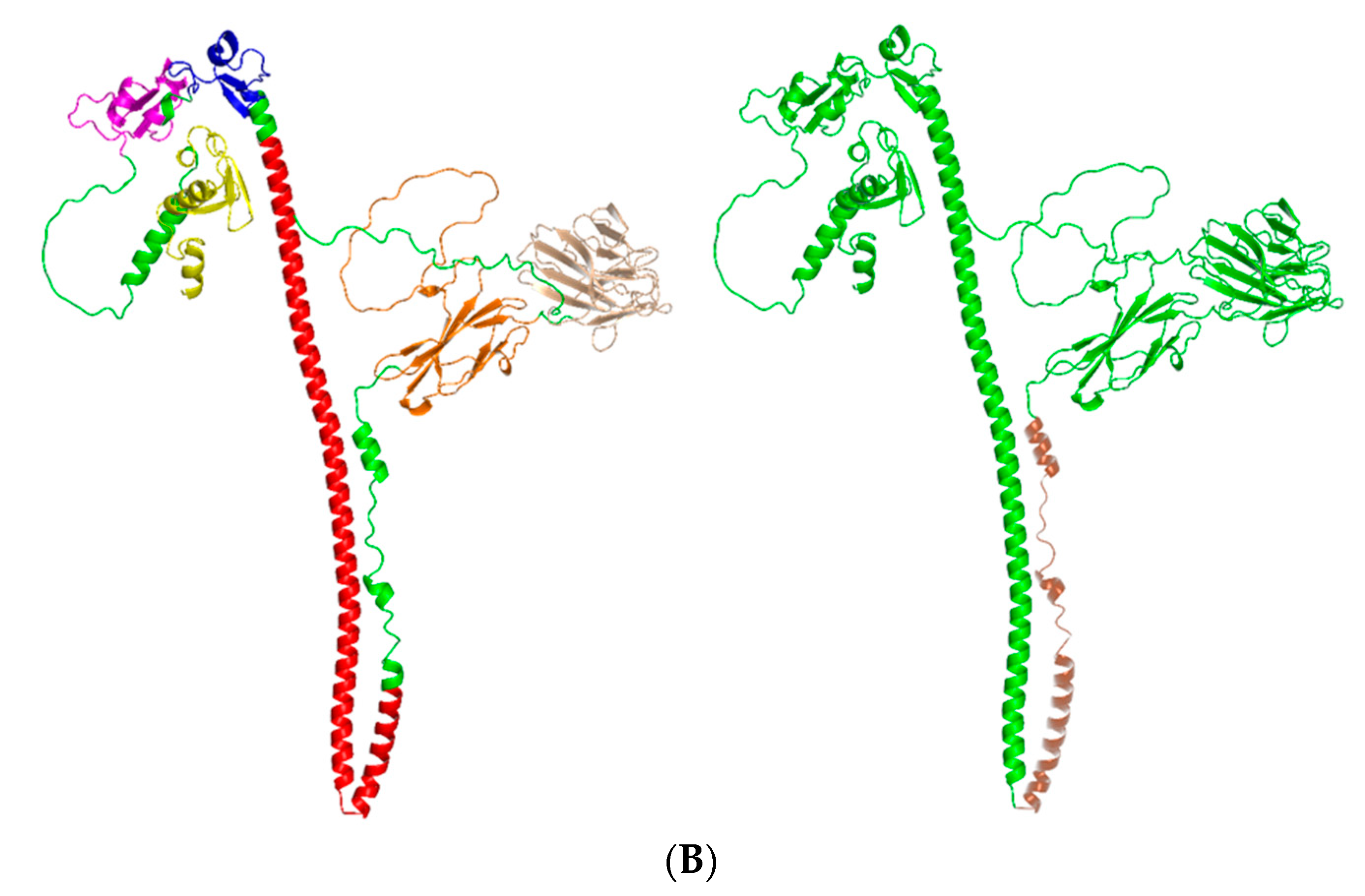
3.2. Structural Analysis of pMID2
3.3. Tissue Distribution and Expression Analysis of pMID2
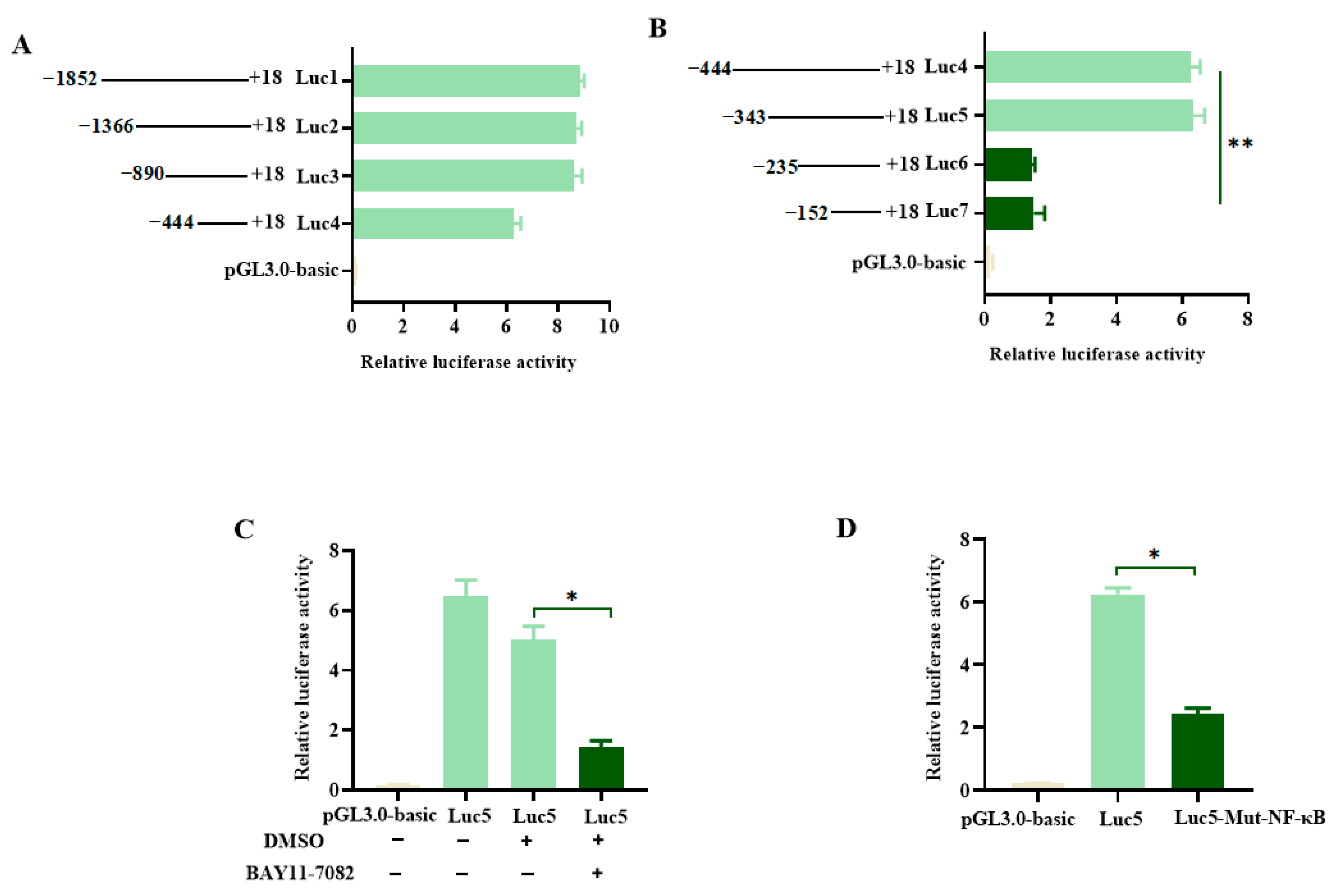
3.4. Functional Promoter Analysis of pMID2
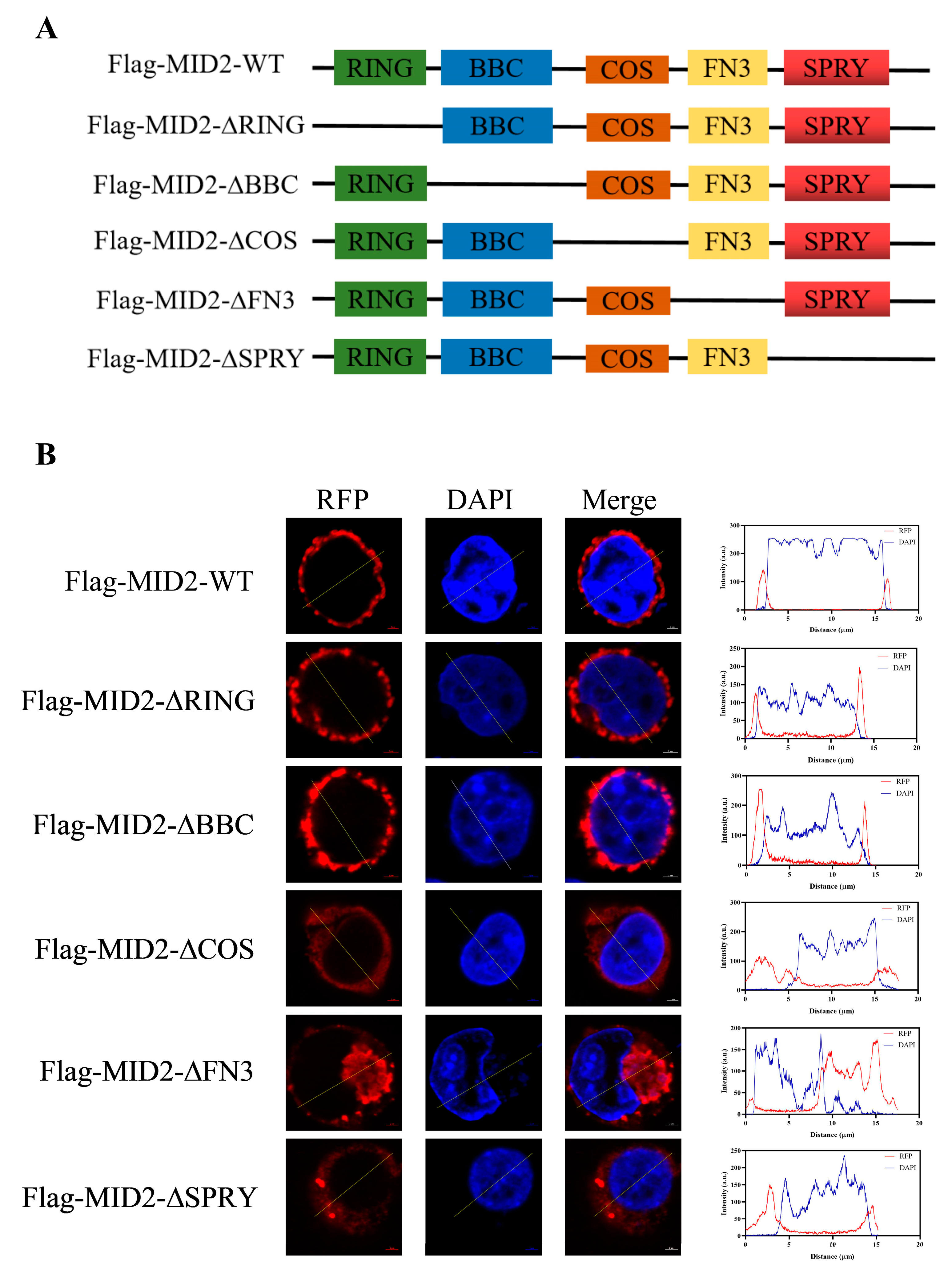
3.5. Cellular Localization of pMID2
3.6. Positive Regulatory of JAK-STAT Signaling Pathway by pMID2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, N.; Sun, X.; Li, P.; Liu, X.; Zhang, X.; Chen, Q.; Xin, H. TRIM family contribute to tumorigenesis, cancer development, and drug resistance. Exp. Hematol. Oncol. 2022, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Ozato, K.; Shin, D.M.; Chang, T.H.; Morse, H.C., 3rd. TRIM family proteins and their emerging roles in innate immunity. Nat. Rev. Immunol. 2008, 8, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Meroni, G.; Diez-Roux, G. TRIM/RBCC, a novel class of ‘single protein RING finger’ E3 ubiquitin ligases. Bioessays 2005, 27, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Marin, I. Origin and diversification of TRIM ubiquitin ligases. PLoS ONE 2012, 7, e50030. [Google Scholar] [CrossRef]
- Short, K.M.; Cox, T.C. Subclassification of the RBCC/TRIM superfamily reveals a novel motif necessary for microtubule binding. J. Biol. Chem. 2006, 281, 8970–8980. [Google Scholar] [CrossRef]
- Short, K.M.; Hopwood, B.; Yi, Z.; Cox, T.C. MID1 and MID2 homo- and heterodimerise to tether the rapamycin-sensitive PP2A regulatory subunit, alpha 4, to microtubules: Implications for the clinical variability of X-linked Opitz GBBB syndrome and other developmental disorders. BMC Cell Biol. 2002, 3, 1. [Google Scholar] [CrossRef]
- Gholkar, A.A.; Senese, S.; Lo, Y.C.; Vides, E.; Contreras, E.; Hodara, E.; Capri, J.; Whitelegge, J.P.; Torres, J.Z. The X-Linked-Intellectual-Disability-Associated Ubiquitin Ligase Mid2 Interacts with Astrin and Regulates Astrin Levels to Promote Cell Division. Cell Rep. 2016, 14, 180–188. [Google Scholar] [CrossRef]
- Corominas, R.; Yang, X.; Lin, G.N.; Kang, S.; Shen, Y.; Ghamsari, L.; Broly, M.; Rodriguez, M.; Tam, S.; Wanamaker, S.A.; et al. Protein interaction network of alternatively spliced isoforms from brain links genetic risk factors for autism. Nat. Commun. 2014, 5, 3650. [Google Scholar] [CrossRef]
- Wang, L.; Wu, J.; Yuan, J.; Zhu, X.; Wu, H.; Li, M. Midline2 is overexpressed and a prognostic indicator in human breast cancer and promotes breast cancer cell proliferation in vitro and in vivo. Front. Med. 2016, 10, 41–51. [Google Scholar] [CrossRef]
- Luo, J.; Zeng, S.; Tian, C. MORC4 Promotes Chemoresistance of Luminal A/B Breast Cancer via STAT3-Mediated MID2 Upregulation. Onco Targets Ther. 2020, 13, 6795–6803. [Google Scholar] [CrossRef]
- Geetha, T.S.; Michealraj, K.A.; Kabra, M.; Kaur, G.; Juyal, R.C.; Thelma, B.K. Targeted deep resequencing identifies MID2 mutation for X-linked intellectual disability with varied disease severity in a large kindred from India. Hum. Mutat. 2014, 35, 41–44. [Google Scholar] [CrossRef]
- Uchil, P.D.; Hinz, A.; Siegel, S.; Coenen-Stass, A.; Pertel, T.; Luban, J.; Mothes, W. TRIM protein-mediated regulation of inflammatory and innate immune signaling and its association with antiretroviral activity. J. Virol. 2013, 87, 257–272. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.W.; Kim, D.G.; Nam, B.H.; Kim, Y.O.; Park, J.Y.; Kong, H.J. Molecular characterization of Rhodeus uyekii tripartite motif protein 1 (TRIM1) involved in IFN-gamma/LPS-induced NF-kappaB signaling. Fish. Shellfish. Immunol. 2018, 79, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Buchner, G.; Montini, E.; Andolfi, G.; Quaderi, N.; Cainarca, S.; Messali, S.; Bassi, M.T.; Ballabio, A.; Meroni, G.; Franco, B. MID2, a homologue of the Opitz syndrome gene MID1: Similarities in subcellular localization and differences in expression during development. Hum. Mol. Genet. 1999, 8, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Richman, J.M.; Fu, K.K.; Cox, L.L.; Sibbons, J.P.; Cox, T.C. Isolation and characterisation of the chick orthologue of the Opitz syndrome gene, Mid1, supports a conserved role in vertebrate development. Int. J. Dev. Biol. 2002, 46, 441–448. [Google Scholar]
- Dal Zotto, L.; Quaderi, N.A.; Elliott, R.; Lingerfelter, P.A.; Carrel, L.; Valsecchi, V.; Montini, E.; Yen, C.H.; Chapman, V.; Kalcheva, I.; et al. The mouse Mid1 gene: Implications for the pathogenesis of Opitz syndrome and the evolution of the mammalian pseudoautosomal region. Hum. Mol. Genet. 1998, 7, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, Y.; Zhao, S.; Cui, Z.; Chen, Y.; Xu, P.; Chen, J.; Zhang, Y.; Xia, P. Differences in Humoral Immune Response against the Type 2 Porcine Reproductive and Respiratory Syndrome Virus via Different Immune Pathways. Viruses 2022, 14, 1435. [Google Scholar] [CrossRef]
- Cui, Z.; Zhou, L.; Zhao, S.; Li, W.; Li, J.; Chen, J.; Zhang, Y.; Xia, P. The Host E3-Ubiquitin Ligase TRIM28 Impedes Viral Protein GP4 Ubiquitination and Promotes PRRSV Replication. Int. J. Mol. Sci. 2023, 24, 10965. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Suzuki, M.; Hara, Y.; Takagi, C.; Yamamoto, T.S.; Ueno, N. MID1 and MID2 are required for Xenopus neural tube closure through the regulation of microtubule organization. Development 2010, 137, 2329–2339. [Google Scholar] [CrossRef] [PubMed]
- Reymond, A.; Meroni, G.; Fantozzi, A.; Merla, G.; Cairo, S.; Luzi, L.; Riganelli, D.; Zanaria, E.; Messali, S.; Cainarca, S.; et al. The tripartite motif family identifies cell compartments. EMBO J. 2001, 20, 2140–2151. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.; Short, K.M.; Romer, J.T.; Swift, S.; Cox, T.C.; Ashworth, A. FXY2/MID2, a gene related to the X-linked Opitz syndrome gene FXY/MID1, maps to Xq22 and encodes a FNIII domain-containing protein that associates with microtubules. Genomics 1999, 62, 385–394. [Google Scholar] [CrossRef]
- Rajsbaum, R.; Versteeg, G.A.; Schmid, S.; Maestre, A.M.; Belicha-Villanueva, A.; Martinez-Romero, C.; Patel, J.R.; Morrison, J.; Pisanelli, G.; Miorin, L.; et al. Unanchored K48-linked polyubiquitin synthesized by the E3-ubiquitin ligase TRIM6 stimulates the interferon-IKKepsilon kinase-mediated antiviral response. Immunity 2014, 40, 880–895. [Google Scholar] [CrossRef]
- van Tol, S.; Atkins, C.; Bharaj, P.; Johnson, K.N.; Hage, A.; Freiberg, A.N.; Rajsbaum, R. VAMP8 Contributes to the TRIM6-Mediated Type I Interferon Antiviral Response during West Nile Virus Infection. J. Virol. 2020, 94, e01454-19. [Google Scholar] [CrossRef]
- Su, X.; Zhang, Q.; Yue, J.; Wang, Y.; Zhang, Y.; Yang, R. TRIM59 suppresses NO production by promoting the binding of PIAS1 and STAT1 in macrophages. Int. Immunopharmacol. 2020, 89, 107030. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Ni, G.; Zhang, W.; Hua, J.; Sun, L.; Zheng, M.; Dong, Q.; Huang, W. TRIM59 attenuates IL-1beta-driven cartilage matrix degradation in osteoarthritis via direct suppression of NF-kappaB and JAK2/STAT3 signaling pathway. Biochem. Biophys. Res. Commun. 2020, 529, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Okumura, F.; Matsunaga, Y.; Katayama, Y.; Nakayama, K.I.; Hatakeyama, S. TRIM8 modulates STAT3 activity through negative regulation of PIAS3. J. Cell Sci. 2010, 123, 2238–2245. [Google Scholar] [CrossRef]
- Okumura, F.; Okumura, A.J.; Matsumoto, M.; Nakayama, K.I.; Hatakeyama, S. TRIM8 regulates Nanog via Hsp90beta-mediated nuclear translocation of STAT3 in embryonic stem cells. Biochim. Biophys. Acta 2011, 1813, 1784–1792. [Google Scholar] [CrossRef]
- Toniato, E.; Chen, X.P.; Losman, J.; Flati, V.; Donahue, L.; Rothman, P. TRIM8/GERP RING finger protein interacts with SOCS-1. J. Biol. Chem. 2002, 277, 37315–37322. [Google Scholar] [CrossRef]
- Hatakeyama, S. TRIM proteins and cancer. Nat. Rev. Cancer 2011, 11, 792–804. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lee, H.; Herrmann, A.; Buettner, R.; Jove, R. Revisiting STAT3 signalling in cancer: New and unexpected biological functions. Nat. Rev. Cancer 2014, 14, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Li, H.; Hong, X.; Ying, G.; Lu, X.; Zhuang, L.; Wu, S. TRIM14 promotes colorectal cancer cell migration and invasion through the SPHK1/STAT3 pathway. Cancer Cell Int. 2018, 18, 202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.X.; Xu, Z.S.; Lin, H.; Li, M.; Xia, T.; Cui, K.; Wang, S.Y.; Li, Y.; Shu, H.B.; Wang, Y.Y. TRIM27 mediates STAT3 activation at retromer-positive structures to promote colitis and colitis-associated carcinogenesis. Nat. Commun. 2018, 9, 3441. [Google Scholar] [CrossRef]
- Xu, W.; Xu, B.; Yao, Y.; Yu, X.; Cao, H.; Zhang, J.; Liu, J.; Sheng, H. RNA interference against TRIM29 inhibits migration and invasion of colorectal cancer cells. Oncol. Rep. 2016, 36, 1411–1418. [Google Scholar] [CrossRef]
- Pan, S.; Deng, Y.; Fu, J.; Zhang, Y.; Zhang, Z.; Ru, X.; Qin, X. TRIM52 promotes colorectal cancer cell proliferation through the STAT3 signaling. Cancer Cell Int. 2019, 19, 57. [Google Scholar] [CrossRef]
- Jagdale, A.; Iwase, H.; Klein, E.C.; Cooper, D.K. Incidence of Neoplasia in Pigs and Its Relevance to Clinical Organ Xenotransplantation. Comp. Med. 2019, 69, 86–94. [Google Scholar] [CrossRef]
- Quaderi, N.A.; Schweiger, S.; Gaudenz, K.; Franco, B.; Rugarli, E.I.; Berger, W.; Feldman, G.J.; Volta, M.; Andolfi, G.; Gilgenkrantz, S.; et al. Opitz G/BBB syndrome, a defect of midline development, is due to mutations in a new RING finger gene on Xp22. Nat. Genet. 1997, 17, 285–291. [Google Scholar] [CrossRef]



| Primer Name | Sequence (5′-3′) | Purpose |
|---|---|---|
| Flag-pMID2-For | AAGCTTATGGGTGAAAGCCCAGCC | expression |
| Flag-pMID2-Rev | GTCGACCTAGAGAGCCGGTGAATTATATGCT | |
| Flag-pMID2-∆RING-For | AAGCTTAGGTATGTTATCTCGCTGAACCACC | |
| Flag-pMID2-∆RING-Rev | GTCGACCTAGAGAGCCGGTGAATTATATGCT | |
| Flag-pMID2-∆BBC-For | AAGCTTATGGGTGAAAGCCCAGCC | |
| Flag-pMID2-∆BBC-Rev | GTCGACCTAGAGAGCCGGTGAATTATATGCT | |
| Flag-pMID2-∆COS-For | AAGCTTATGGGTGAAAGCCCAGCC | |
| Flag-pMID2-∆COS-Rev | GTCGACCTAGAGAGCCGGTGAATTATATGCT | |
| Flag-pMID2-∆FN3-For | AAGCTTATGGGTGAAAGCCCAGCC | |
| Flag-pMID2-∆FN3-Rev | GTCGACCTAGAGAGCCGGTGAATTATATGCT | |
| Flag-pMID2-∆SPRY-For | AAGCTTATGGGTGAAAGCCCAGCC | |
| Flag-pMID2-∆SPRY-Rev | GTCGACTTAGTCAATGAATATATTTCCTGCTGC | |
| Luc1(−1852/+18)-For | GGTACCGTTATTGGATTGCAGTTCTTTACG | promoter |
| Luc1(−1852/+18)-Rev | AAGCTTTTTACCCGGAGCATTCCCG | |
| Luc2(−1366/+18)-For | GGTACCCCATGTTCTGAAAAGAATCTCCTCT | |
| Luc2(−1366/+18)-Rev | AAGCTTTTTACCCGGAGCATTCCCG | |
| Luc3(−890/+18)-For | GGTACCGTTAACTTTGCATAGAGCAGCTTCG | |
| Luc3(−890/+18)-Rev | AAGCTTTTTACCCGGAGCATTCCCG | |
| Luc4(−444/+18)-For | GGTACCAGAAAACATGTAAACGCGCCT | |
| Luc4(−444/+18)-Rev | AAGCTTTTTACCCGGAGCATTCCCG | |
| Luc5(−343/+18)-For | GGTACCCCGGGCCCTTCCCAGCGCT | |
| Luc5(−343/+18)-Rev | AAGCTTTTTACCCGGAGCATTCCCG | |
| Luc6(−235/+18)-For | GGTACCGCGGAAATGACAGTGTGGTG | |
| Luc6(−235/+18)-Rev | AAGCTTTTTACCCGGAGCATTCCCG | |
| Luc7(−152/+18)-For | GGTACCAGATCCAGCCGCGGTAGC | |
| Luc7(−152/+18)-Rev | AAGCTTTTTACCCGGAGCATTCCCG | |
| Luc5-Mut-NF-κB-For | CTCCCGCTCTGCGGCGGGGGAAATGACAGTGTGGT | site-directed |
| Luc5-Mut-NF-κB-Rev | ACCACACTGTCATTTCCCCCGCCGCAGAGCGGGAG | |
| pMID2-For | GGGAATGCTCCGGGTGAA | RT-qPCR |
| pMID2-Rev | CAGGAGGGGGTCTTCAAACA | |
| pGAPDH-For | ACATGGCCTCCAAGGAGTAAGA | |
| pGAPDH-Rev | GATCGAGTTGGGGCTGTGACT |
| siRNA | Sequence (5′-3′) |
|---|---|
| NC-For | UUCUCCGAACGUGUCACGUTT |
| NC-Rev | ACGUGACACGUUCGGAGAATT |
| siMID2-For | GGCAACUGCAUCUUCUCAATT |
| siMID2-Rev | UUGAGAAGAUGCAGUUGCCTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Zhou, L.; Yang, Z.; Zhao, S.; Li, W.; Zhang, Y.; Xia, P. The Molecular and Function Characterization of Porcine MID2. Animals 2023, 13, 2853. https://doi.org/10.3390/ani13182853
Chen J, Zhou L, Yang Z, Zhao S, Li W, Zhang Y, Xia P. The Molecular and Function Characterization of Porcine MID2. Animals. 2023; 13(18):2853. https://doi.org/10.3390/ani13182853
Chicago/Turabian StyleChen, Jing, Likun Zhou, Zhuosong Yang, Shijie Zhao, Wen Li, Yina Zhang, and Pingan Xia. 2023. "The Molecular and Function Characterization of Porcine MID2" Animals 13, no. 18: 2853. https://doi.org/10.3390/ani13182853
APA StyleChen, J., Zhou, L., Yang, Z., Zhao, S., Li, W., Zhang, Y., & Xia, P. (2023). The Molecular and Function Characterization of Porcine MID2. Animals, 13(18), 2853. https://doi.org/10.3390/ani13182853





