Origin and Distribution of the Brachial Plexus in Two Procyonids (Procyon cancrivorus and Nasua nasua, Carnivora)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimens
2.2. Fixation and Vascular Repletion of Specimens
2.3. Dissection and Documentation
3. Results
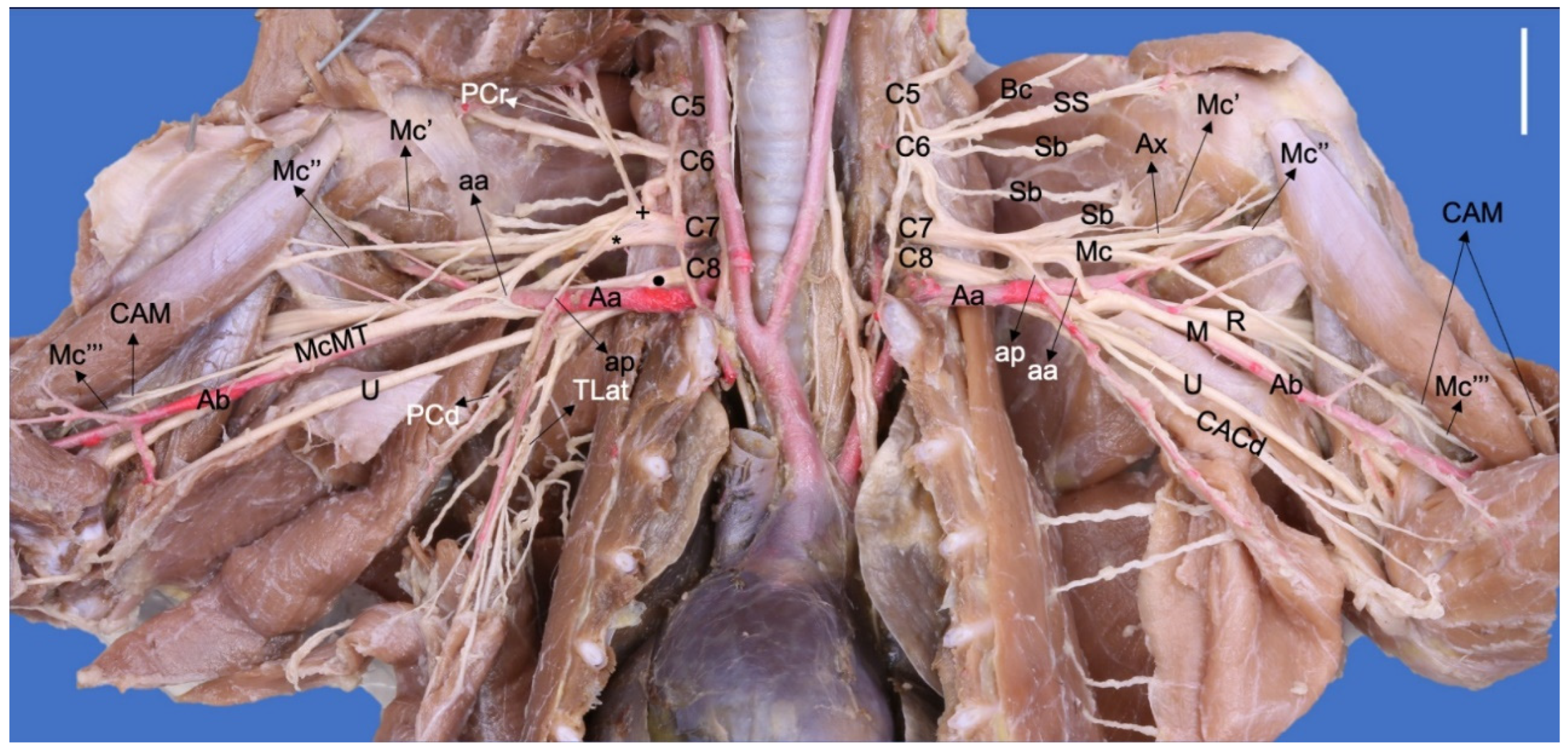
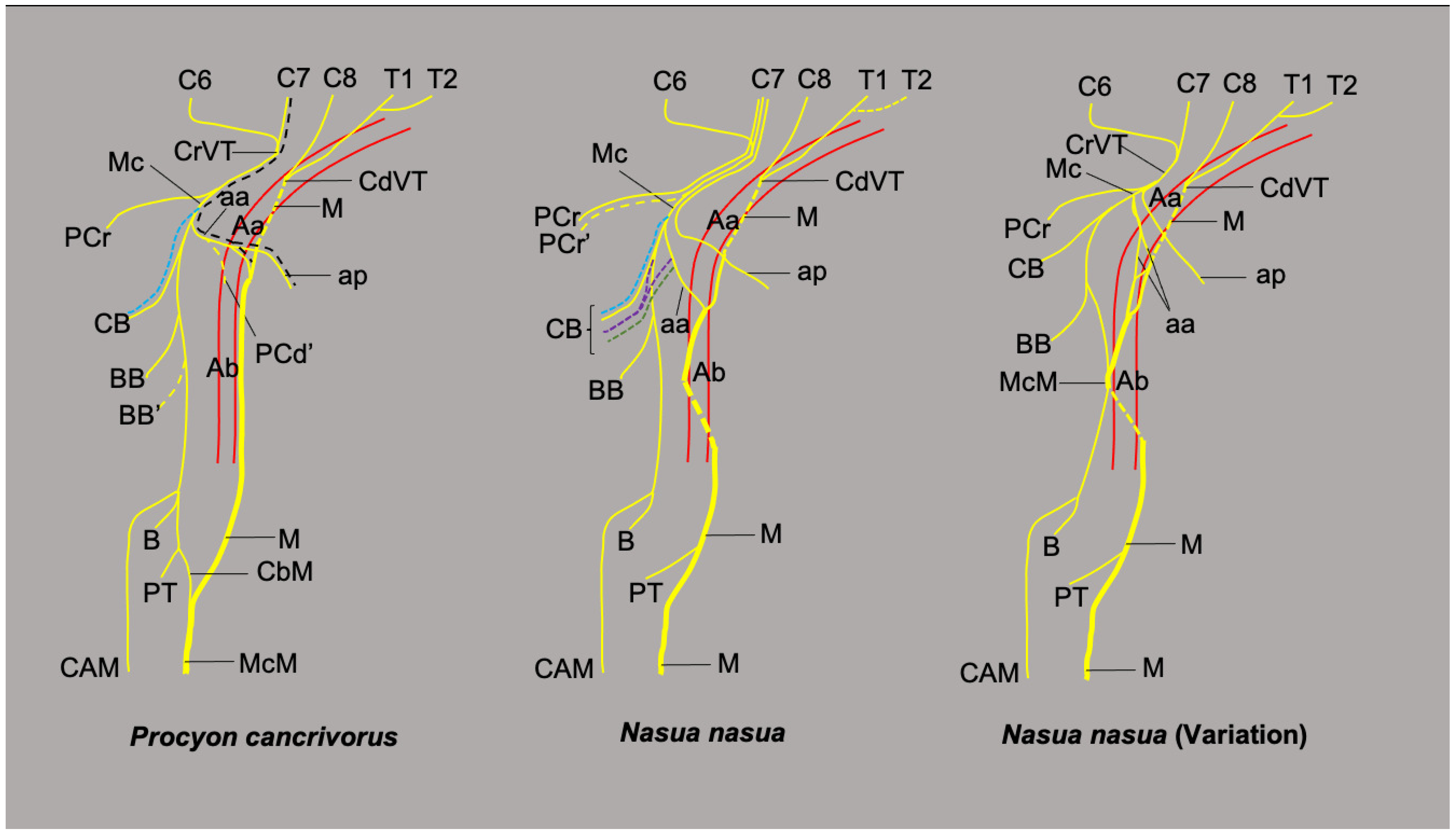
3.1. Origin of the Brachial Plexus in Procyon cancrivorus and Nasua nasua
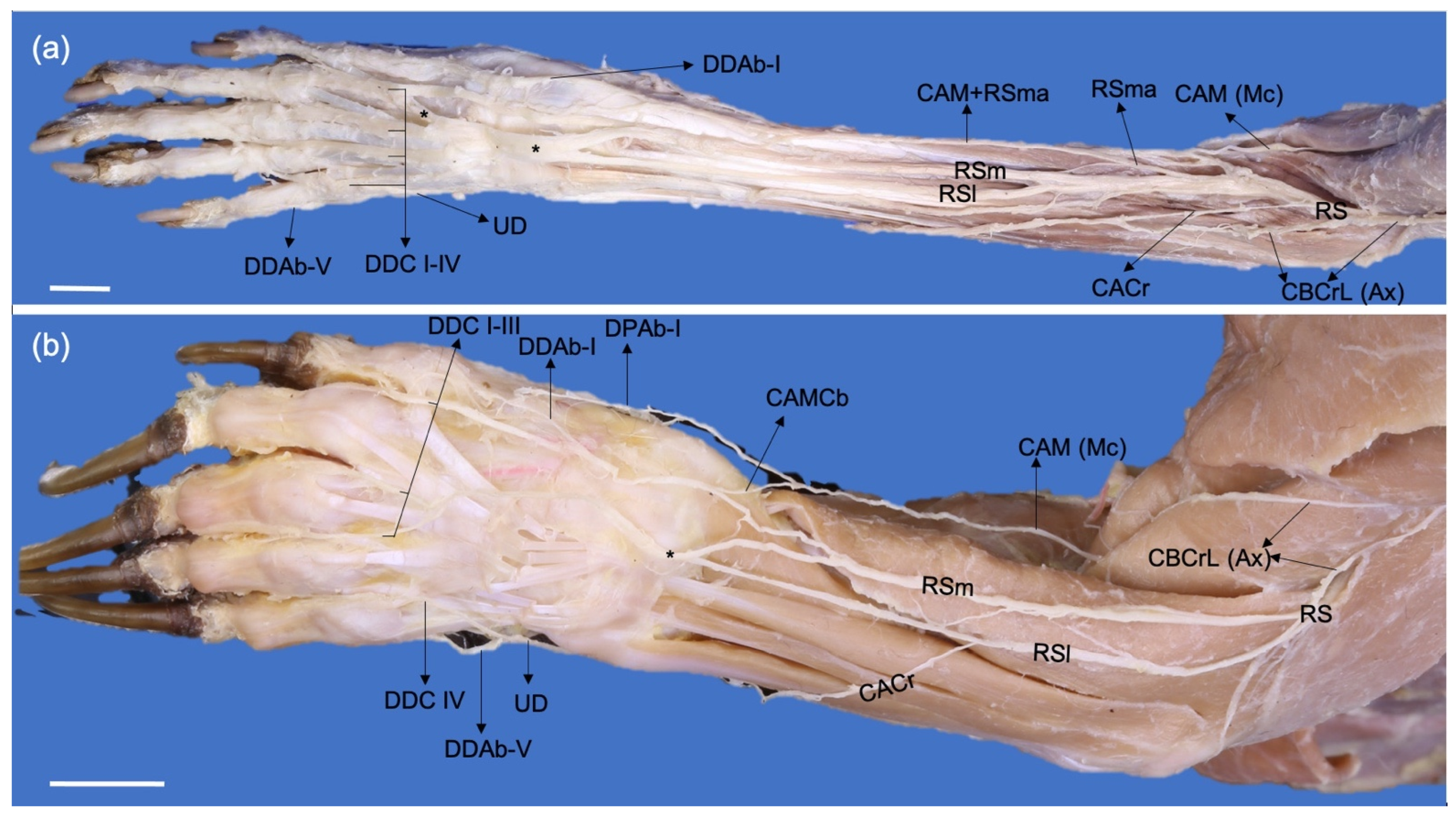
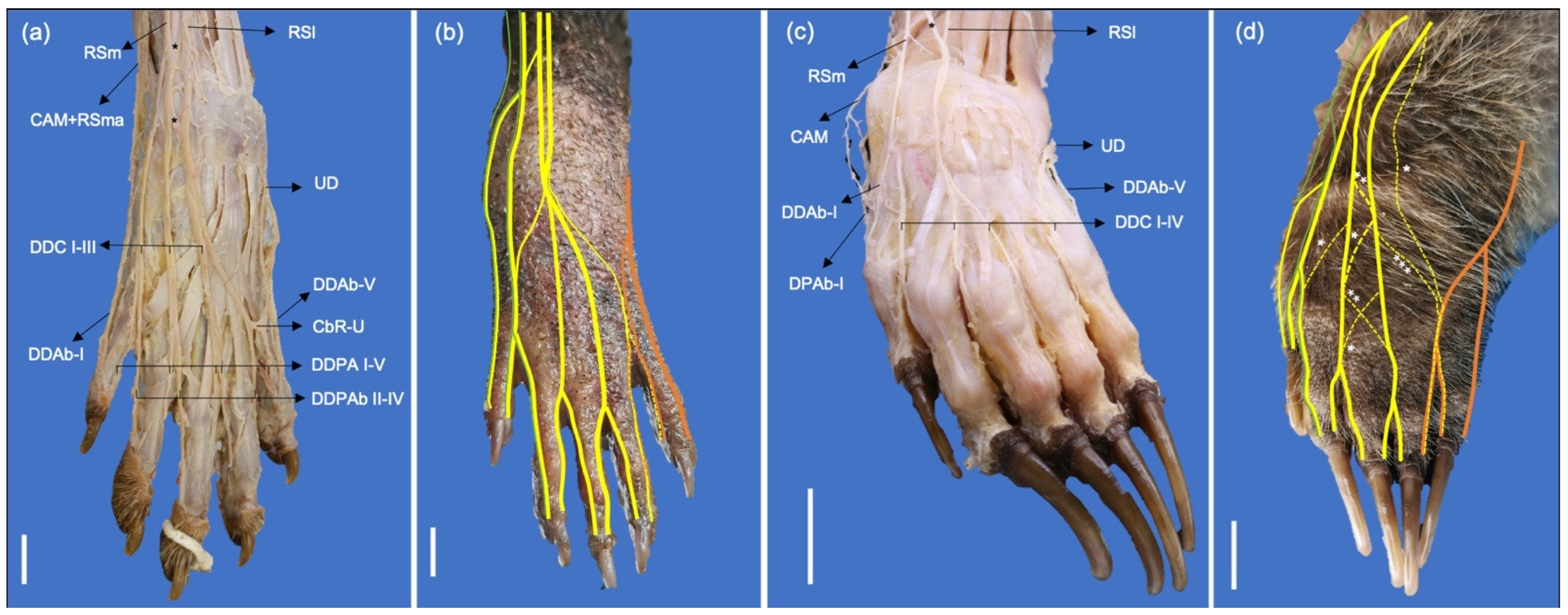
3.2. Origin and Distribution of the Brachial Plexus Nerves in Procyon cancrivorus and Nasua nasua
4. Discussion
4.1. Comparative Origin of the Brachial Plexus in Carnivorans
4.2. Comparative Distribution of the Brachial Plexus Nerves in Carnivorans and Evolutionary Comments
4.3. Comparative Nerve Branching on the Manus of Carnivorans
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Nerve | Pc1 (JF) | Pc2 (JF) | Pc3 (AM) | Pc4 (AM) | Pc5 (AM) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Right | Left | Right | Left | Right | Left | Right | Left | Right | Left | |
| Brachial plexus | C6-T2 | C6-T2 | C6-T2 | C6-T2 | C6-T2 | C6-T2 | C6-T2 | C6-T2 | C5-T1 | C5- T1 |
| Suprascapularis | C6 | C6 | C6-C7 | C6 | C6 | C6 | C6 | C6 | C5-C7 | C5-C6 |
| Subscapularis cranialis | C6 | C6 | C6-C7 | C6 | C6 | C6 | C6 | C6 | C6-C7 | C6-C7 |
| Subscapularis medium | --- | --- | --- | --- | --- | --- | --- | C6 | --- | --- |
| Subscapularis caudalis | C6-C7 | C6-C7 | C6-C7 | C6-C7 | C6-C7 | C6-C7 | C6-C7 | C6-C7 | C6-C7 | C6-C7 |
| Musculocutaneus | C6-C7 | C6-C7 | C7 | C6-C7 | C6-C7 | C6-C7 | C6-C7 | C6-C7 | C6-C7 | C6-C7 |
| Axilaris | C6-C7 | C6-C7 | C6-C7 | C6-C7 | C6-C7 | C6-C7 | C6-C7 | C6-C7 | C6-C7 | C6-C7 |
| Radialis | C6-T2 | C6-T2 | C6-T2 | C6-T2 | C6-C8 | C6-C8 | C6-T2 | C6-T2 | C6-T1 | C6-T1 |
| Medianus | C6-T2 | C6-T2 | C7-T2 | C6-T2 | C6-T2 | C6-T2 | C7-T2 | C7-T2 | C6-T1 | C6-T1 |
| Ulnaris | C8-T2 | C8-T2 | C8-T2 | C8-T2 | C8-T2 | C8-T2 | C8-T2 | C8-T2 | C8-T1 | C8-T1 |
| Cutaneus antebrachii caudalis | C8-T2 | C8-T2 | C8-T2 | C8-T2 | C8-T2 | C8-T2 | T1-T2 | T1-T2 | T1 | T1 |
| Brachiocephalicus | C6 | C6 | C6-C7 | C6 | C5-C6 | C5-C6 | C5-C6 | C5-C6 | C5-C6 | C5-C6 |
| Thoracicus longus | C7 | C7 | C7 | C7 | C7 | C7 | C7 | C7 | C7 | C7 |
| Thoracicus lateralis | C6- T2 | C6- T2 | C6-T2 | C6- T2 | C8-T2 | C8-T2 | C8-T2 | C8-T2 | C8-T1 | C8-T1 |
| Thoracodorsalis | C7-T2 | C7-T2 | C7-C8 | C7-C8 | C6-C8 | C6-C8 | C6-T2 | C6-T2 | C6-T1 | C6-T1 |
| Pectorales craniales | C6-C7 | C6-C7 | C7-T2 | C6-C7 | C6-C7 | C6-C7 | C6-C7 | C6-C7 | C6-C7 | C6-C7 |
| Pectorales caudales | C6-T2 | C6-T2 | C7-T2 | C6-T2 | C6-T2 | C6-T2 | C7-T2 | C7-T2 | C6-T1 | C6-T1 |
| Nerve | Nn1 (IM) | Nn2 (JM) | Nn3 (AF) | Nn4 (AM) | Nn5 (AM) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Right | Left | Right | Left | Right | Left | Right | Left | Right | Left | |
| Brachial plexus | C5-T2 | C5-T2 | C5-T2 | C5-T1 | C5-T1 | C5-T2 | C6-T1 | C6-T1 | C5-T2 | C5-T2 |
| Suprascapularis | C5-C6 | C5-C6 | C5-C6 | C5-C6 | C5-C6 | C5-C6 | C6-C7 | C6 | C5-C6 | C5-C6 |
| Subscapularis cranialis | C5-C6 | C5-C6 | C6 | C6 | C5-C6 | C5-C6 | C6-C7 | C6 | C5-C6 | C5-C6 |
| Subscapularis medium | C6 | C6 | C6-C7 | C6-C7 | C6-C7 | C6-C7 | --- | --- | C6 | C6-C7 |
| Subscapularis caudalis | C6-C7 | C6-C7 | C6-C7 | C6-C7 | C6-C7 | C6-C7 | C6-C7 | C6-C7 | C6-C7 | C6-C7 |
| Musculocutaneus | C6-C7 | C6-C7 | C6-C7 | C6-C7 | C6-C7 | C6-C7 | C6-C7 | C6-C7 | C6-C7 | C6-C7 |
| Axilaris | C6-C7 | C6-C7 | C6-C7 | C6-C7 | C6-C7 | C6-C7 | C6-C7 | C6-C7 | C6-C7 | C6-C7 |
| Radialis | C6-T1 | C6-T1 | C7-T1 | C7-T1 | C7-T1 | C7-T1 | C6-T1 | C6-T1 | C6-T1 | C6-T1 |
| Medianus | C6-T2 | C6-T2 | C6-T2 | C6-T1 | C6-T1 | C6-T2 | C6-T1 | C6-T1 | C6-T1 | C6-T1 |
| Ulnaris | C8-T2 | C8-T2 | C8-T1 | C8-T2 | C8-T1 | C8-T2 | C8-T1 | C8-T1 | C8-T1 | C8-T1 |
| Cutaneus antebrachii caudalis | C8-T2 | C8-T2 | T1 | C8-T1 | T1 | T1 | T1 | T1 | T1 | T1 |
| Brachiocephalicus | C5-C6 | C5-C6 | C5-C6 | C5-C6 | C5-C6 | C5-C6 | C6 | C6 | C5-C6 | C5-C6 |
| Thoracicus longus | C7 | C7-C8 | C7 | C7 | C7 | C7 | C7 | C7 | C7 | C7 |
| Thoracicus lateralis | C8-T1 | C8-T1 | C8-T1 | C8-T1 | C7-T1 | C8-T1 | C8-T1 | C8-T1 | C8-T1 | C8-T1 |
| Thoracodorsalis | C6-T1 | C6-C8 | C7-C8 | C7-C8 | C7-C8 | C7-C8 | C7-C8 | C7-C8 | C7-C8 | C7-C8 |
| Pectorales craniales | C6-C7 | C6-C7 | C7 | C7 | C7 | C7 | C7 | C7 | C6-C7 | C6-C7 |
| Pectorales caudales | C6-T1 | C6-T1 | C7-T1 | C7-T1 | C7-T1 | C7-T1 | C7-T1 | C7-T1 | C7-T1 | C7-T1 |
References
- Nyakatura, K.; Bininda-Emonds, O.R. Updating the Evolutionary History of Carnivora (Mammalia): A New Species-Level Supertree Complete with Divergence Time Estimates. BMC Biol. 2012, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Reid, F.; Helgen, K.; González-Maya, J.F. Procyon Cancrivorus. Available online: https://www.iucnredlist.org/species/41685/45216426 (accessed on 19 December 2022).
- Emmons, L.; Helgen, K. Nasua nasuai. Available online: https://www.iucnredlist.org/species/41684/45216227 (accessed on 19 December 2022).
- Hassanin, A.; Veron, G.; Ropiquet, A.; Jansen van Vuuren, B.; Lécu, A.; Goodman, S.M.; Haider, J.; Nguyen, T.T. Evolutionary History of Carnivora (Mammalia, Laurasiatheria) Inferred from Mitochondrial Genomes. PLoS ONE 2021, 16, e0240770. [Google Scholar] [CrossRef]
- Whiteside, D.P. Nutrition and Behavior of Coatis and Raccoons. Vet. Clin. N. Am. Exot. Anim. Pract. 2009, 12, 187–195. [Google Scholar] [CrossRef]
- Arispe, R.; Venegas, C.; Rumiz, D. Abundancia y Patrones de Actividad Del Mapache (Procyon cancrivorus) En Un Bosque Chiquitano de Bolivia. Mastozool. Neotrop. 2008, 15, 323–333. [Google Scholar]
- Hirsch, B. Nasua nasua (Ring-Tailed Coati). CABI Compend. 2022. [Google Scholar] [CrossRef]
- Gatti, A.; Bianchi, R.; Rosa, C.R.X.; Mendes, S.L. Diet of Two Sympatric Carnivores, Cerdocyon thous and Procyon cancrivorus, in a Restinga Area of Espirito Santo State, Brazil. J. Trop. Ecol. 2006, 22, 227–230. [Google Scholar] [CrossRef] [Green Version]
- Quintela, F.M.; Iob, G.; Artioli, L.G.S. Diet of Procyon Cancrivorus (Carnivora, Procyonidae) in Restinga and Estuarine Environments of Southern Brazil. Iheringia. Série Zool. 2014, 104, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Alves-Costa, C.P.; Fonseca, G.; Christófaro, C. Variation in the Diet of the Brown-Nosed Coati (Nasua nasua) in Southeastern Brazil. J. Mammal. 2004, 85, 478–482. [Google Scholar] [CrossRef]
- Beisiegel, B.M. Notes on the Coati, Nasua nasua (Carnivora: Procyonidae) in an Atlantic Forest Area. Braz. J. Biol. 2001, 61, 689–692. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, B.T. Seasonal Variation in the Diet of Ring-Tailed Coatis (Nasua nasua) in Iguazu, Argentina. J. Mammal. 2009, 90, 136–143. [Google Scholar] [CrossRef] [Green Version]
- Campos, Z.; Mourão, G. Camera Traps Capture Images of Predators of Caiman Crocodilus Yacare Eggs (Reptilia: Crocodylia) in Brazil’s Pantanal Wetlands. J. Nat. Hist. 2015, 49, 977–982. [Google Scholar] [CrossRef]
- Desbiez, A.L.; Borges, P.A. Density, Habitat Selection and Observations of South American Coati Nasua Nasua in the Central Region of the Brazilian Pantanal Wetland. Small Carniv. Conserv. 2010, 42, 14–18. [Google Scholar]
- Olifiers, N.; de Bianchi, R.C.; de Mourão, G.M.; Gompper, M.E. Construction of Arboreal Nests by Brown-Nosed Coatis, Nasua nasua (Carnivora: Procyonidae) in the Brazilian Pantanal. Zoologia 2009, 26, 571–574. [Google Scholar] [CrossRef]
- Nowak, R.M. Walker’s Carnivores of the World; The John Hopkins University Presss: Baltimore, MD, USA, 2005. [Google Scholar]
- McClearn, D. Locomotion, Posture, and Feeding Behavior of Kinkajous, Coatis, and Raccoons. J. Mammal. 1992, 73, 245–261. [Google Scholar] [CrossRef]
- Santos, C.M.D.; Santos, S.M.D.; Pizzutto, C.S.; Custódio, A.E.I. Enriquecimento Ambiental Para Guaxinim, Procyon cancrivorus (Cuvier, 1798). Biosci. J. 2015, 31, 275–282. [Google Scholar] [CrossRef] [Green Version]
- Demczuk Thomas, L.; Piccoli, R.J.; Quintana Bernardi, P.E.; Sinotti, J.F.; Andrade Silva, V.; Fucks de Souza, C.; Bono Fukushima, F. Braquial Plexus Block and Lumbosacral Epidural in a South American Coati (Nasua nasua). Acta Sci. Vet. 2021, 49 (Suppl. S1), 651. [Google Scholar] [CrossRef]
- Pinheiro, L.L.; Branco, É.; Souza, D.C.; Pereira, L.H.C.; Lima, A.R. Descrição Do Plexo Braquial Do Cachorro-Do-Mato (Cerdocyon thous Linnaeus, 1766). Ciência Anim. Bras. 2014, 15, 213–219. [Google Scholar] [CrossRef] [Green Version]
- Chagas, K.L.S.; Moura Fé, L.C.; Pereira, L.C.; Lima, A.R.; Branco, É. Descrição Morfológica Do Plexo Braquial de Jaguatirica (Leopardus pardalis). Biotemas 2014, 27, 171. [Google Scholar] [CrossRef]
- Haligur, A.; Ozkadif, S. Macroanatomical Investigation of the Plexus Brachialis in the Red Fox (Vulpes vulpes). Pak. J. Zool. 2021, 53, 1617–1622. [Google Scholar] [CrossRef]
- Souza Junior, P.; da Carvalho, N.C.; Medeiros-do-Nascimento, R.; de Dantas, P.O.; Bernardes, F.C.S.; Abidu-Figueiredo, M. Brachial Plexus Formation in Jaguarundi (Puma yagouaroundi). Anat. Histol. Embryol. 2022, 51, 746–755. [Google Scholar] [CrossRef]
- Sánchez, H.L.; Silva, L.B.; Rafasquino, M.E.; Mateo, A.G.; Zuccolilli, G.O.; Portiansky, E.L.; Alonso, C.R. Anatomical Study of the Forearm and Hand Nerves of the Domestic Cat (Felis catus), Puma (Puma concolor) and Jaguar (Panthera onca). Anat. Histol. Embryol. 2013, 42, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, L.L.; Branco, É.R.; De Souza, D.C.; De Souza, A.C.B.; Pereira, L.C.; Lima, A.R. De Descrição Do Plexo Braquial Do Cachorro-Do-Mato-de-Orelhas-Curtas (Atelocynus microtis—Sclater, 1882): Relato de Caso. Biotemas 2013, 26, 203–209. [Google Scholar] [CrossRef]
- De Souza, D.A.S.; de Castro, T.F.; Franceschi, R.D.C.; Silva Filho, R.P.; Pereira, M.A.M. Formação Do Plexo Braquial e Sistematização Dos Territórios Nervosos Em Membros Torácicos de Lobos-Marinhos Arctocephalus australis. Braz. J. Vet. Res. Anim. Sci. 2010, 47, 168–174. [Google Scholar] [CrossRef] [Green Version]
- Barreto-Mejía, R.; Ceballos, C.P.; Tamayo-Arango, L.J. Anatomical Description of the Origin and Distribution of the Brachial Plexus to the Antebrachium in One Puma (Puma concolor) (Linnaeus, 1771). Anat. Histol. Embryol. 2022, 51, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Hakkı Nur, İ.; Keleş, H.; Pérez, W. Origin and Distribution of the Brachial Plexus of the Van Cats. Anat. Histol. Embryol. 2020, 49, 251–259. [Google Scholar] [CrossRef]
- Roos, H.; Vollmerhaus, B. Konstruktionsprinzipien an Der Vorder- Und Hinterpfote Der Hauskatze (Felis catus). 4. Mitteilung: Muskelinnervation Und Bewegungsanalyse+. Anat. Histol. Embryol. 2005, 34, 2–14. [Google Scholar] [CrossRef]
- Enciso-García, L.M.; Vélez-García, J.F. Origin and Distribution of the Brachial Plexus in Kinkajou (Potos flavus—Schreber, 1774). Anat. Histol. Embryol. 2022, 51, 221–235. [Google Scholar] [CrossRef]
- Grzeczka, A.; Zdun, M. The Structure of the Brachial Plexus in Selected Representatives of the Caniformia Suborder. Animals 2022, 12, 566. [Google Scholar] [CrossRef]
- Souza-Junior, P.; Wronski, J.G.; Carvalho, N.C.; Abidu-Figueiredo, M. Brachial Plexus in the Leopardus geoffroyi. Ciência Anim. Bras. 2018, 19, 1–14. [Google Scholar] [CrossRef] [Green Version]
- International Committee on Veterinary Gross Anatomical Nomenclature. Nomina Anatómica Veterinaria, 6th ed.; World Association of Veterinary Anatomists, Ed.; World Association of Veterinary Anatomists: Hannover, Germany, 2017; ISBN 0-9600444-7-7. [Google Scholar]
- Hermanson, J.; Evans, H.; De Lahunta, A. Miller and Evan’s Anatomy of the Dog, 5th ed.; Hermanson, J.W., Evans, H., De Lahunta, A., Eds.; Elsevier Inc.: St. Louis, MO, USA, 2020; ISBN 978-0-323-54601-0. [Google Scholar]
- Davis, D.D. The Giant Panda: A Morphological Study of Evolutionary Mechanisms. Fieldiana 1964, 3, 1–339. [Google Scholar]
- Langworthy, O.R. The Panniculus Carnosus in Cat and Dog and Its Genetical Relation to the Pectoral Musculature. J. Mammal. 1924, 5, 49. [Google Scholar] [CrossRef]
- Vélez García, J.F.; Miglino, M.A. Evolutionary Comparative Analysis of the Extrinsic Thoracic Limb Muscles in Three Procyonids (Procyon cancrivorus Cuvier, 1798, Nasua nasua Linnaeus, 1766, and Potos flavus Schreber, 1774) Based on Their Attachments and Innervation. Anat. Sci. Int. 2022. [Google Scholar] [CrossRef] [PubMed]
- Barone, R. Anatomie Comparée Des Mammifères Domestiques. Tome 2: Arthrologie et Myoulogie, 4th ed.; Association Centrale D’Entraide Vétérinaire: Paris, France, 2020; ISBN 978-2-9571960-1-2. [Google Scholar]
- Perdomo-Cárdenas, V.; Patiño-Holguín, C.; Vélez-García, J.F. Evolutionary and Terminological Analysis of the Flexor Digitorum Superficialis, Interflexorii and Palmaris Longus Muscles in Kinkajou (Potos flavus) and Crab-Eating Racoon (Procyon Cancrivorus). Anat. Histol. Embryol. 2021, 50, 520–533. [Google Scholar] [CrossRef] [PubMed]
- Allam, M.W.; Lee, D.G.; Nulsen, F.E.; Fortune, E.A. The Anatomy of the Brachial Plexus of the Dog. Anat. Rec. 1952, 114, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Aubert, L.; Carozzo, C.; Devillaire, A.-C.; Crevier-Denoix, N.; Moissonnier, P. Macro- and Microanatomical Characterization of the Cat Brachial Plexus. Cells Tissues Organs 2004, 176, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Singh, B. Dyce, Sack and Wensing’ Textbook of Veterinary Anatomy, 5th ed.; Elsevier: Saint Louis, MO, USA, 2018. [Google Scholar]
- Demiraslan, Y.; Aykut, M.; Özgel, Ö. Macroanatomical Characteristics of Plexus Brachialis and Its Branches in Martens (Martes foina). Turk. J. Vet. Anim. Sci. 2015, 39, 693–698. [Google Scholar] [CrossRef]
- Backus, T.C.; Solounias, N.; Mihlbachler, M.C. The Brachial Plexus of the Sumatran Rhino (Dicerorhinus Sumatrensis) and Application of Brachial Plexus Anatomy Toward Mammal Phylogeny. J. Mamm. Evol. 2016, 23, 71–79. [Google Scholar] [CrossRef]
- Souza-Junior, P.; Carvalho, N.C.; Mattos, K.; Santos, A.L.Q. Origens e Ramificações Do Plexo Braquial No Cachorro-Do-Mato Cerdocyon thous (Linnaeus, 1766). Pesqui. Vet. Bras. 2014, 34, 1011–1023. [Google Scholar] [CrossRef] [Green Version]
- Souza-Junior, P.; da Cruz de Carvalho, N.; de Mattos, K.; Abidu Figueiredo, M.; Luiz Quagliatto Santos, A. Brachial Plexus in the Pampas Fox (Lycalopex gymnocercus): A Descriptive and Comparative Analysis. Anat. Rec. 2017, 300, 537–548. [Google Scholar] [CrossRef]
- Silva, L.B.; Sánchez, H.L. La Inervación Del Miembro Torácico En Felinos. AnAlectA Vet. 2013, 33, 10–17. [Google Scholar]
- Felipe, R.; Silva, F.; França, G.L.; Silva, E.M.; Leonel, L.C.; Carvalho-Barros, R.A.; Silva, D.C.; Silva, Z. Anatomia Descritiva Do Nervo Musculocutâneo Em Quatis (Nasua nasua, Linnaeus, 1766). Enciclopédia Biosf. 2014, 10, 92–98. [Google Scholar]
- Vélez-García, J.F.; Patiño-Holguín, C.; Duque-Parra, J.E. Anatomical Variations of the Caudomedial Antebrachial Muscles in the Crab-Eating Fox (Cerdocyon thous). Int. J. Morphol. 2018, 36, 1193–1196. [Google Scholar] [CrossRef] [Green Version]
- Reighard, J.; Jennings, H.S. Anatomy of the Cat; Henry Holt and Company: New York, NY, USA, 1901. [Google Scholar]
- König, H.E. Anatomie Der Katze: Mit Hinweisen Für Die Tierärztliche Praxis; Gustav Fischer: Portland, OR, USA, 1992. [Google Scholar]
- Hudson, L.C.; Hamilton, W.C. Atlas of Feline Anatomy for Veterinarians, 2nd ed.; Teton NewMedia: Jackson, MI, USA, 2010. [Google Scholar]
- König, H.; Mülling, C.; Seeger, J.; Liebich, H. Nervous System (Systema Nervosum). In Veterinary Anatomy of Domestic Animals: Textbook and Colour Atlas; König, H.E., Liebich, H.G., Eds.; Thieme: Stuttgart, Germany, 2020; pp. 515–586. [Google Scholar]
- Arłamowska-Palider, A. Morphological Studies on the Main Branches of the Radial Nerve in Mammals. Acta Theriol. 1970, 15, 185–197. [Google Scholar] [CrossRef] [Green Version]
- Hermanson, J. The Muscular System. In Miller’s Anatomy of the Dog; Hermanson, J.W., Evans, H., De Lahunta, A., Eds.; Elsevier Inc.: St. Louis, MO, USA, 2020; pp. 444–658. ISBN 978-0-323-54601-0. [Google Scholar]
- Kamali, Y. Aberrant Arrangement of the Musculocutaneous and Median Nerves in the Thoracic Limbs of a Mixed-breed Dog Cadaver. Anat. Histol. Embryol. 2022, 51, 419–423. [Google Scholar] [CrossRef]
- Vélez García, J.F.; Ospina Orozco, A.; Duque Parra, J.E. Origen de Los Nervios Del Plexo Braquial Del Venado Coliblanco (Odocoileus virginianus) En Comparación Con Otros Rumiantes. Rev. Investig. Vet. Del Perú 2018, 29, 713–722. [Google Scholar] [CrossRef]
- Arłamowska-Palider, A. Comparative Anatomical Studies of Nervus Musculocutaneus in Mammals. Acta Theriol. 1970, 15, 343–356. [Google Scholar] [CrossRef] [Green Version]
- Kawashima, T.; Thorington, R.W.; Bohaska, P.W.; Chen, Y.-J.; Sato, F. Anatomy of Shoulder Girdle Muscle Modifications and Walking Adaptation in the Scaly Chinese Pangolin (Manis pentadactylapPentadactyla: Pholidota) Compared with the Partially Osteoderm-Clad Armadillos (Dasypodidae). Anat. Rec. 2015, 298, 1217–1236. [Google Scholar] [CrossRef]
- Upham, N.S.; Esselstyn, J.A.; Jetz, W. Inferring the Mammal Tree: Species-Level Sets of Phylogenies for Questions in Ecology, Evolution, and Conservation. PLoS Biol. 2019, 17, e3000494. [Google Scholar] [CrossRef]
- Liebich, H.G.; Maierl, J.; König, H.E. Forelimbs or Thoracic Limbs (Membra Thoracica). In Veterinary Anatomy of Domestic Animals: Textbook and Colour Atlas; König, H.E., Liebich, H.G., Eds.; Georg Thieme Verlag: Stuttgart, Germany, 2020; pp. 171–242. ISBN 978-3-13-242933-8. [Google Scholar]
- Windle, B.; Parsons, F. Myology of the Terrestrial Carnivora. Part I. Muscles of the Head, Neck, and Fore-Limb. Proc. Zool. Soc. Lond. 1897, 65, 370–409. [Google Scholar] [CrossRef]
- Abdala, V.; Diogo, R. Comparative Anatomy, Homologies and Evolution of the Pectoral and Forelimb Musculature of Tetrapods with Special Attention to Extant Limbed Amphibians and Reptiles. J. Anat. 2010, 217, 536–573. [Google Scholar] [CrossRef]
- Vélez-García, J.F.; Arbeláez-Quiñones, A.C.; Montealegre-Hurtado, K.D. Evolutionary Adaptations in the Flexor Digitorum Profundus Muscle in Tamandua mexicana (Xenarthra, Myrmecophagidae). Anat. Rec. 2021, 304, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Niederschuh, S.J.; van Beesel, J.; Schmidt, M. The Role of Sensory Feedback from Carpal Sinus Hairs in Locomotor Kinematics of Rats (Rattus norvegicus, Rodentia) during Walking on Narrow Substrates. Zoology 2022, 155, 126055. [Google Scholar] [CrossRef] [PubMed]
- Fundin, B.T.; Arvidsson, J.; Rice, F.L. Innervation of Nonmystacial Vibrissae in the Adult Rat. J. Comp. Neurol. 1995, 357, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.S.; Kitchell, R.L.; Johnson, R.D. Spinal Nerve Root Origins of the Cutaneous Nerves Arising from the Canine Brachial Plexus. Am. J. Vet. Res. 1982, 43, 820–825. [Google Scholar] [PubMed]
- Numata, N.; Kida, M.Y.; Kudoh, H. Ramification Patterns of the Nerves Innervating the Forearm Extensors in Mammals and Reptiles. Okajimas Folia Anat. Jpn. 1996, 73, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, M. Comparative Anatomy of the Subscapularis, Teres Major and Latissimus Dorsi Muscles from Salamanders to Mammals with Special Reference to Their Innervations from the Brachial Plexus. Anat. Sci. Int. 2022, 97, 124–137. [Google Scholar] [CrossRef]
- Vélez-García, J.F.; Chunganá-Caicedo, D.; Saavedra-Montealegre, S. Gross Anatomy of the Craniolateral Antebrachial Muscles in Kinkajou (Potos flavus, Carnivora): Intra- and Interspecific Variants within the Family Procyonidae. Anat. Histol. Embryol. 2022, 51, 308–313. [Google Scholar] [CrossRef]
- Vélez-García, J.F.; Marín-González, L.; Monroy-Cendales, M.J.; Miglino, M.A. Craniolateral Forearm Muscles of the Crab-Eating Raccoon (Procyon cancrivorus) and a Comparative Review with Other Carnivorans. Iheringia. Série Zool. 2022, 112, e2022012. [Google Scholar] [CrossRef]
- Echeverry, J.; Vélez, J.; Sánchez, C. Descripción Anatómica de Los Músculos Cráneo-Laterales Superficiales Del Antebrazo Del Zorro Perruno (Cerdocyon thous). Rev. Colomb. Cienc. Anim. 2015, 8, 44–51. [Google Scholar]
- Diogo, R.; Abdala, V. Muscles of Vertebrates: Comparative Anatomy, Evolution, Homologies and Development, 1st ed.; CRC Press: Enfield, NH, USA, 2010; ISBN 9781578086825. [Google Scholar]
- Koizumi, M.; Sakai, T. On the Morphology of the Brachial Plexus of the Platypus (Ornithorhynchus anatinus) and the Echidna (Tachyglossus aculeatus). J. Anat. 1997, 190, 447–455. [Google Scholar] [CrossRef]
- Turnbull, B.G.; Rasmusson, D.D. Sensory Innervation of the Raccoon Forepaw: 1. Receptor Types in Glabrous and Hairy Skin and Deep Tissue. Somatosens. Res. 1986, 4, 43–62. [Google Scholar] [CrossRef] [PubMed]
- Munger, B.L.; Pubols, L.M. The Sensorineural Organization of the Digital Skin of the Raccoon (Part 1 of 2). Brain. Behav. Evol. 1972, 5, 367–379. [Google Scholar] [CrossRef] [PubMed]




| Nerve | Origin | Procyon cancrivorus (%) | Nasua nasua (%) | Muscle Innervation | Skin Innervation |
|---|---|---|---|---|---|
| Suprascapularis | C5-C6 | 10 | 80 | Supraspinatus and infraspinatus | |
| C5-C7 | 10 | 0 | |||
| C6 | 70 | 10 | |||
| C6-C7 | 10 | 10 | |||
| Subscapularis cranialis | C5-C6 | 0 | 60 | Subscapularis | |
| C6 | 70 | 30 | |||
| C6-C7 | 30 | 10 | |||
| Subscapularis medium | absent | 90 | 20 | ||
| C6 | 10 | 30 | |||
| C6-C7 | 0 | 50 | |||
| Subscapularis caudalis | C6-C7 | 100 | 100 | ||
| Teres major | C6-C7 | 20 | 100 | Teres major and subscapularis | |
| Axillaris | C6-C7 | 100 | 100 | Subscapularis, teres, minor, deltoideus (Pars scapularis, pars acromialis, pars clavicularis -m. Cleidobrachialis-), teres major (only in P. cancrivorus). | Cranial, lateral, and medial surfaces of the distal half of the brachium and proximal extreme of the antebrachium. |
| Musculocutaneus | C6-C7 | 90 | 100 | Coracobrachialis, biceps brachii, brachialis, pronator teres (only in P. cancrivorus), and flexor carpi radialis (only in P. cancrivorus). | Medial surfaces of the antebrachium and manus. Abaxial and axial dorsal surfaces of the digit I, and abaxial dorsal surface of the digit II (via communicating branches with the radial nerve). Abaxial and axial palmar surfaces of the digits I and II, and abaxial palmar surface of the digit III (via common trunk with the median nerve in P. cancrivorus) Abaxial palmar surface of the digit I (N. nasua) |
| C7 | 10 | 0 | |||
| Radialis | C6-C8 | 20 | 0 | Tensor fasciae antebrachii, triceps brachii (Caput longum, caput mediale, caput accessorium, and caput laterale), anconeus lateralis (anconeus), brachioradialis, extensor carpi radialis, extensor digitorum communis, extensor digitorum lateralis, extensor carpi ulnaris, supinator, abductor digiti I longus, and extensor digiti I et II. | Cranial and lateral surfaces of the antebrachium, and dorsal and medial surfaces of the manus Axial and abaxial dorsal surfaces of the digits I to III. Abaxial dorsal surface of the digit IV and axial dorsal surface of the digit V (100% in P. cancrivorus and 40% in N. nasua) |
| C6-T1 | 20 | 60 | |||
| C6-T2 | 60 | 0 | |||
| C7-T1 | 0 | 40 | |||
| Medianus | C6-T1 | 20 | 60 | Pronator teres (only in N. nasua), flexor carpi radialis, flexor digitorum superficialis, palmaris longus, flexor digitorum profundus (caput humerale and caput radiale), interflexorii, pronator quadratus, abductor digiti I brevis, flexor digiti I brevis, and the most medial lumbricalis. | Axial and abaxial palmar surfaces of the digits II to III. Abaxial palmar surface of the digit I (P. cancrivorus) Abaxial palmar surface of the digit IV and axial palmar surface of the digit V (10% of N. nasua) |
| C6-T2 | 50 | 40 | |||
| C7-T2 | 30 | 0 | |||
| Ulnaris | C8-T1 | 20 | 60 | Anconeus medialis (Anconeus epitrochlearis), flexor carpi ulnaris (Caput humerale and caput ulnare), flexor digitorum profundus (Caput humerale and caput ulnare), flexor digitorum brevis, adductor digiti I, adductor digiti II, abductor digiti V, flexor digiti V, adductor digiti V, interossei, and the three lateral lumbricales. | Caudal surface of the antebrachium. Carpal tactile hairs and distal extreme of the antebrachium. Lateral dorsal and palmar surfaces of the manus. Axial and abaxial palmar surfaces of the digit V. Abaxial palmar and dorsal surfaces of the digit IV. Axial and abaxial dorsal surfaces of the digit V. |
| C8-T2 | 80 | 40 | |||
| Cutaneus antebrachii caudalis | C8-T1 | 0 | 10 | Caudal surface of the antebrachium | |
| C8-T2 | 60 | 20 | |||
| T1 | 20 | 70 | |||
| T1-T2 | 20 | 0 | |||
| Brachiocephalicus | C5-C6 | 60 | 80 | Supraspinatus (only 10% in P. cancrivorus) | Cranial surface of the shoulder and proximal half of the cranial surface of the brachium |
| C6 | 30 | 20 | |||
| C6-C7 | 10 | 0 | |||
| Thoracicus longus | C7 | 100 | 90 | Serratus ventralis thoracis | |
| C7-C8 | 0 | 10 | |||
| Thoracodorsalis | C6-C8 | 20 | 10 | Latissimus dorsi | |
| C6-T1 | 20 | 10 | |||
| C6-T2 | 20 | 0 | |||
| C7-C8 | 20 | 80 | |||
| C7-T2 | 20 | 0 | |||
| Pectorales craniales | C6-C7 | 90 | 40 | Pectoralis descendens and pectoralis transversus | |
| C7 | 0 | 60 | |||
| C7-T2 | 10 | 0 | |||
| Pectorales caudales | C6-T1 | 20 | 20 | Pectoralis profundus and pectoralis transversus | |
| C6-T2 | 50 | 0 | |||
| C7-T1 | 0 | 80 | |||
| C7-T2 | 30 | 0 | |||
| Thoracicus lateralis | C6-T2 | 40 | 0 | Cutaneus trunci and pectoralis abdominalis | |
| C7-T1 | 0 | 10 | |||
| C8-T1 | 20 | 90 | |||
| C8-T2 | 40 | 0 |
| Suborder | Infraorder | Superfamily | Family | Species | Number of Limbs (Number of Specimens) | Brachial Plexus Origin (%) |
|---|---|---|---|---|---|---|
| Caniformia | Arctoidea | Musteloidea | Procyonidae | Procyon cancrivorus | 10 (2 females and 3 males) | C5-T1 (20%) C6-T2 (80%) |
| Nasua nasua | 10 (1 female and 4 males) | C5-T1 (20%) C5-T2 (60%) C6-T1 (20%) | ||||
| Bassariscus astutus [35] | 2 (1 unreported sex) | C6-T2 (100%) | ||||
| Potos flavus [30] | 10 (3 females and 2 males) | C5-T2 (30%) C6-T2 (70%) | ||||
| Mustelidae | ||||||
| Martes foina [43] | 12 (3 females and 3 males) | C6-T1 (100%) | ||||
| Martes martes [31] | 16 (6 female and 2 male) | C6-T1 (100%) | ||||
| Neovison vison [31] | 60 (18 female and 12 male) | C6-T1 (26.67%) C6-T2 (73.33%) | ||||
| Neovison vison [44] | 52 (26 unreported sexes) | C6-T1 (100%) | ||||
| Meles meles [31] | 50 (15 females and 10 males) | C5-T1 (10%) C6-T1 (40%) C6-T2 (50%) | ||||
| Ursoidea | Ursidae | Ailuropoda melanoleuca [35] | 2 (1 male) | C5-T2 (100%) | ||
| Ursus americanus [35] | 2 (1 unreported sex) | C6-T1 (100%) | ||||
| Canoidea | Canidae | Nyctereutes procyonoides [31] | 40 (10 female and 10 males) | C5-T1 (12.5%) C6-T1 (25%) C6-T2 (62.5%) | ||
| Vulpes vulpes [31] | 52 (10 females and 16 male) | C5-T1 (7.69%) C6-T1 (61.54%) C6-T2 (30.77%) | ||||
| Vulpes vulpes [22] | 12 (6 males) | C5-T2 (33.33%) C6-T1 (66.67%) | ||||
| Canis lupus familiaris [40] | 58 (unreported specimens and sexes) | C6-T1 (58.62%) C6-T1 (20.69%) C5-T1 (17.24%) C5-T2 (0.034%) | ||||
| Atelocynus microtis [25] | 1 female | C6-T1 (100%) | ||||
| Cerdocyon thous [45] | 20 (8 females and 2 males) | C6-T1 (100%) | ||||
| Cerdocyon thous [20] | 6 (3 males) | C6-T1 (100%) | ||||
| Lycalopex gymnocercus [46] | 20 (3 females and 7 males) | C6-T1 (100%) | ||||
| Pinnipedia | Otaridae | Arctocephalus australis [26] | 4 (1 female and 1 male) | C6-T1 (100%) | ||
| Feliformia | Aeluroidea | Feloidea | Felidae | Felis catus [47] | 12 (6 unreported sexes) | C6-T1 (100%) |
| Felis catus [28] | 10 (5 females) | C6-T1 (100%) | ||||
| Panthera onca [47] | 4 (2 unreported sexes) | C6-T1 (100%) | ||||
| Puma concolor [47] | 6 (3 unreported sexes) | C6-T1 (100%) | ||||
| Puma concolor [27] | 2 (1 female) | C6-T1 (100%) | ||||
| Puma yagouaroundi [23] | 14 (5 males and 2 females) | C5-T1 (57%) | ||||
| C6-T1 (43%) | ||||||
| Leopardus geoffroyi [32] | 6 (4 females) | C5-T1 (66.66%) C6-T1 (33.33%) | ||||
| Leopardus pardalis [21] | 4 (1 male and 1 female) | C6-T1 (100%) |
| Nerves | Origin | Species |
|---|---|---|
| Suprascapularis | C5-C6 | P. cancrivorus (10%), N. nasua (80%), P. flavus (20%), L. geofffroyi (50%), and C. l. familiaris. |
| C5-C7 | P. cancrivorus (10%), P. flavus (10%), V. vulpes* (33.33%), M. meles, N. procyonoides, V. vulpes, and C. l. familiaris. | |
| C6 | P. cancrivorus (70%), N. nasua (10%), C. thous (25%), L. gymnocercus (20%), C. l. familiaris, F. catus* (80%), P. concolor*, P. yagouaroundi (85.7%), and L. geofffroyi (50%). | |
| C6-C7 | P. cancrivorus (10%), N. nasua (10%), P. flavus (70%), M. meles, M. foina, M. martes, N. vison, N. procyonoides, C. l. familiaris, A. microtis, C. thous (75%), C. thous*, L. gymnocercus (75%), A. australis, V. vulpes* (33.33%), V. vulpes, F. catus* (20%), P. yagouaroundi (14.3%), and L. pardalis (100%). | |
| Subscapulares | C5-C7 | V. vulpes (16.67%) and N. nasua (60%). |
| C6-C7 | P. cancrivorus (100%), N. nasua (40%), P. flavus (100%), N. vison, M. meles, C. l. familiaris, C. thous (45%), C. thous*, L. gymnocercus (90%), V. vulpes* (83.33%), V. vulpes, P. yagouaroundi (78.7%), L. geofffroyi (100%), P. concolor*, and L. pardalis (100%). | |
| Musculocutaneus | C6-C7 | P. cancrivorus (90%), N. nasua (100%), P. flavus (100%), N. vison, M. martes, M. meles, V. vulpes, N. procyonoides, C. l. familiaris, A. microtis, C. thous (30%), C. thous*, L. gymnocercus (20%), F. catus* (100%), F. catus, P. onca, P. concolor, P. concolor*, P. yagouaroundi (57.3%), L. geofffroyi (50%), and L. pardalis (100%). |
| C7 | P. cancrivorus (10%), N. nasua* (100%). | |
| Axillaris | C6-C7 | P. cancrivorus (100%), N. nasua (100%), P. flavus (80%), A. microtis, C. thous (20%), V. vulpes, F. catus* (100%), F. catus, P. onca, P concolor, P concolor*, P. yagouaroundi (57.3%), and L. geofffroyi (50%) |
| Radialis | C6-C8 | P. cancrivorus (20%) and C. thous (5%). |
| C6-T1 | P. cancrivorus (20%), N. nasua (60%), and P. yagouaroundi (14.3%). | |
| C6-T2 | P. cancrivorus (60%), and P. flavus (90%). | |
| C7-T1 | N. nasua (40%), N. vison, M. martes, M. meles, C. l. familiaris, C. thous (70%), C. thous*, L. gymnocercus (70%), V. vulpes, N. procyonoides, F. catus, P. onca, P. concolor, P. concolor*, L. geoffroyi (66.67%), and P. yagouaroundi (64.3%), and L. pardalis (100%). | |
| Medianus | C6-T1 | N. nasua (60%) and P. cancrivorus (20%). |
| C6-T2 | P. cancrivorus (50%), N. nasua (40%), and P. flavus (100%). | |
| C7-T2 | P. cancrivorus (30%), N. vison, and C. l. familiaris. | |
| Ulnaris | C8-T1 | P. cancrivorus (20%), N. nasua (60%), N. vison, M. meles, M. martes, N. procyonoides, C. l. familiaris, A. microtis, C. thous (80%), C. thous*, L. gymnocercus (70%), V. vulpes, A. australis, F. catus* (100%), F. catus, P. onca, P concolor, P. yagouaroundi (85.7%), L. geoffroyi (100%), and L. pardalis (100%). |
| C8-T2 | P. cancrivorus (80%), N. nasua (40%), P. flavus (100%), N. vison, M. meles, N. procyonoides, and C. l. familiaris. | |
| Brachiocephalicus | C5-C6 | P. cancrivorus (60%), N. nasua (80%), P. flavus (30%), M. meles, N. procyonoides, and C. l. familiaris. |
| C5-C7 | P. cancrivorus (10%). | |
| C6 | P. cancrivorus (30%), N. nasua (20%), P. flavus (60%), N. vison, M. martes, M. foina, C. l. familiaris, C. thous (100%), L. gymnocercus (100%), V. vulpes, P. yagouaroundi (29.6%), and L. geoffroyi (33.34%). | |
| C6-C7 | P. cancrivorus (10%) and L. pardalis (100%). | |
| Thoracicus longus | C7 | P. cancrivorus (100%), N. nasua (90%), P. flavus (100%), M. meles, C. l. familiaris, C. thous (100%), L. gymnocercus (100%), V. vulpes, F. catus* (100%), P. concolor*, P. yagouaroundi (92.9%), and L. geoffroyi (100%). |
| C7-C8 | N. nasua (10%), N. vison, M. martes, A. australis, and V. vulpes* (83.33%). | |
| Thoracodorsalis | C6-C8 | P. cancrivorus (20%), N. nasua (10%), and P. flavus (10%). |
| C6-T1 | P. cancrivorus (20%) and N. nasua (10%). | |
| C6-T2 | P. cancrivorus (20%) and P. flavus (60%). | |
| C7-C8 | P. cancrivorus (20%), N. nasua (80%), M. foina, M. martes, M. meles, C. l. familiaris, C. thous (5%), L. gymnocercus (50%), V. vulpes, F. catus *(80%), P. concolor*, P. yagouaroundi (7.1%), and L. geoffroyi (83.33%). | |
| Thoracicus lateralis | C6-T2 | P. cancrivorus (40%) and P. flavus (10%). |
| C7-T1 | N. nasua (10%), C. thous (25%), L. gymnocercus (35%), and P. yagouaroundi (14.2%). | |
| C8-T1 | P. cancrivorus (20%), N. nasua (90%), M. martes, N. procyonoides, C. l. familiaris, C. thous (40%), C. thous*, L. gymnocercus (45%), F. catus* (100%), P. yagouaroundi (50%), and L. geoffroyi (16.67%). | |
| C8-T2 | P. cancrivorus (40%), P. flavus (90%), N. vison, N. procyonoides. | |
| Pectorales craniales | C6-C7 | P. cancrivorus (90%), N. nasua (40%), P. flavus (60%), C. thous (25%), L. gymnocercus (5%), V. vulpes* (33.33%), V. vulpes, P. yagouaroundi (21.4%), and L. geoffroyi (16.67%). |
| C7 | N. nasua (60%), P. flavus (10%), N. vison, M. meles, M. martes, M. foina, N. procyonoides, C. thous (25%), L. gymnocercus (40%), F. catus* (100%), P. concolor*, P. yagouaroundi (35.7%), and L. geoffroyi (50%). | |
| C7-T2 | P. cancrivorus (10%). | |
| Pectorales caudales | C6-T1 | P. cancrivorus (10%) and N. nasua (10%). |
| C6-T2 | P. cancrivorus (50%) and P. flavus (20%). | |
| C7-T1 | N. nasua (80%), M. martes, N. vison, C. thous (20%), and L. gymnocercus (35%). | |
| C7-T2 | P. cancrivorus (30%). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vélez García, J.F.; de Carvalho Barros, R.A.; Miglino, M.A. Origin and Distribution of the Brachial Plexus in Two Procyonids (Procyon cancrivorus and Nasua nasua, Carnivora). Animals 2023, 13, 210. https://doi.org/10.3390/ani13020210
Vélez García JF, de Carvalho Barros RA, Miglino MA. Origin and Distribution of the Brachial Plexus in Two Procyonids (Procyon cancrivorus and Nasua nasua, Carnivora). Animals. 2023; 13(2):210. https://doi.org/10.3390/ani13020210
Chicago/Turabian StyleVélez García, Juan Fernando, Roseãmely Angélica de Carvalho Barros, and Maria Angélica Miglino. 2023. "Origin and Distribution of the Brachial Plexus in Two Procyonids (Procyon cancrivorus and Nasua nasua, Carnivora)" Animals 13, no. 2: 210. https://doi.org/10.3390/ani13020210
APA StyleVélez García, J. F., de Carvalho Barros, R. A., & Miglino, M. A. (2023). Origin and Distribution of the Brachial Plexus in Two Procyonids (Procyon cancrivorus and Nasua nasua, Carnivora). Animals, 13(2), 210. https://doi.org/10.3390/ani13020210






