Effects of Different Types of Dietary Fibers on Lipid Metabolism and Bile Acids in Weaned Piglets
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Diets and Experimental Design
2.3. Sample Collection
2.4. Biochemical Analysis
2.5. Analysis of Bile Acids in Bile by Ultra-Performance Liquid Chromatography Tandem Mass Spectrometry (UPLC-MS/MS)
2.6. Real-Time Quantitative PCR
2.7. 16S rRNA Gene Sequencing
2.8. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Serum Biochemistry
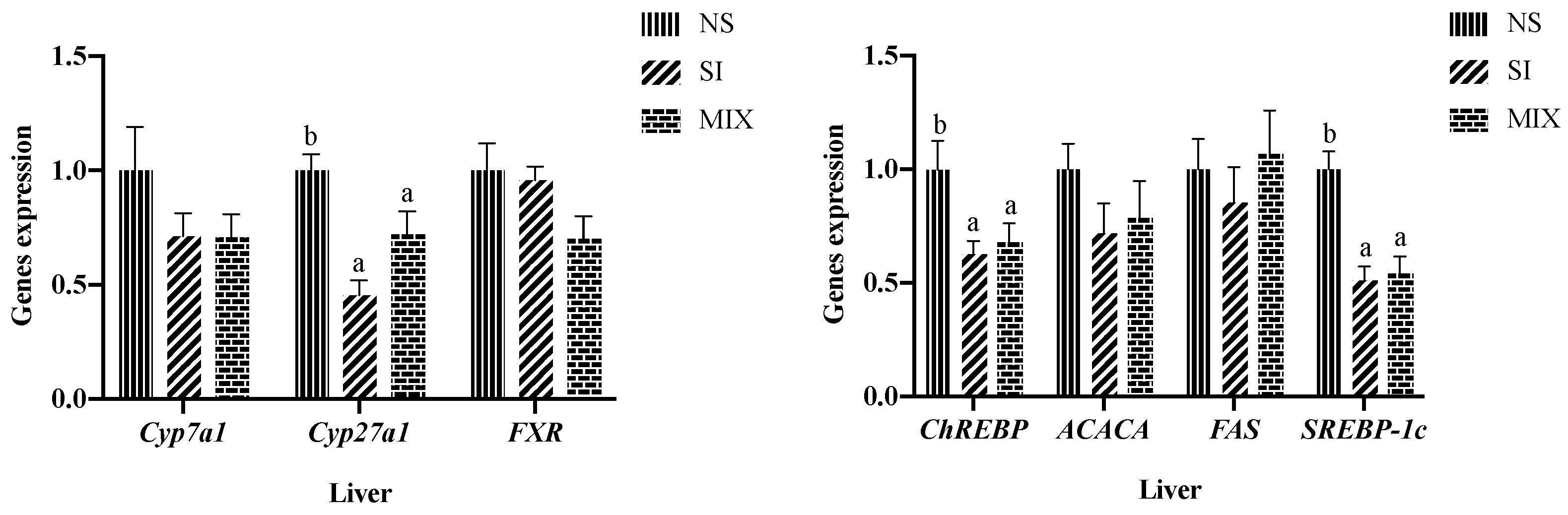
3.3. Hepatic Lipid Metabolism-Related Genes Expression
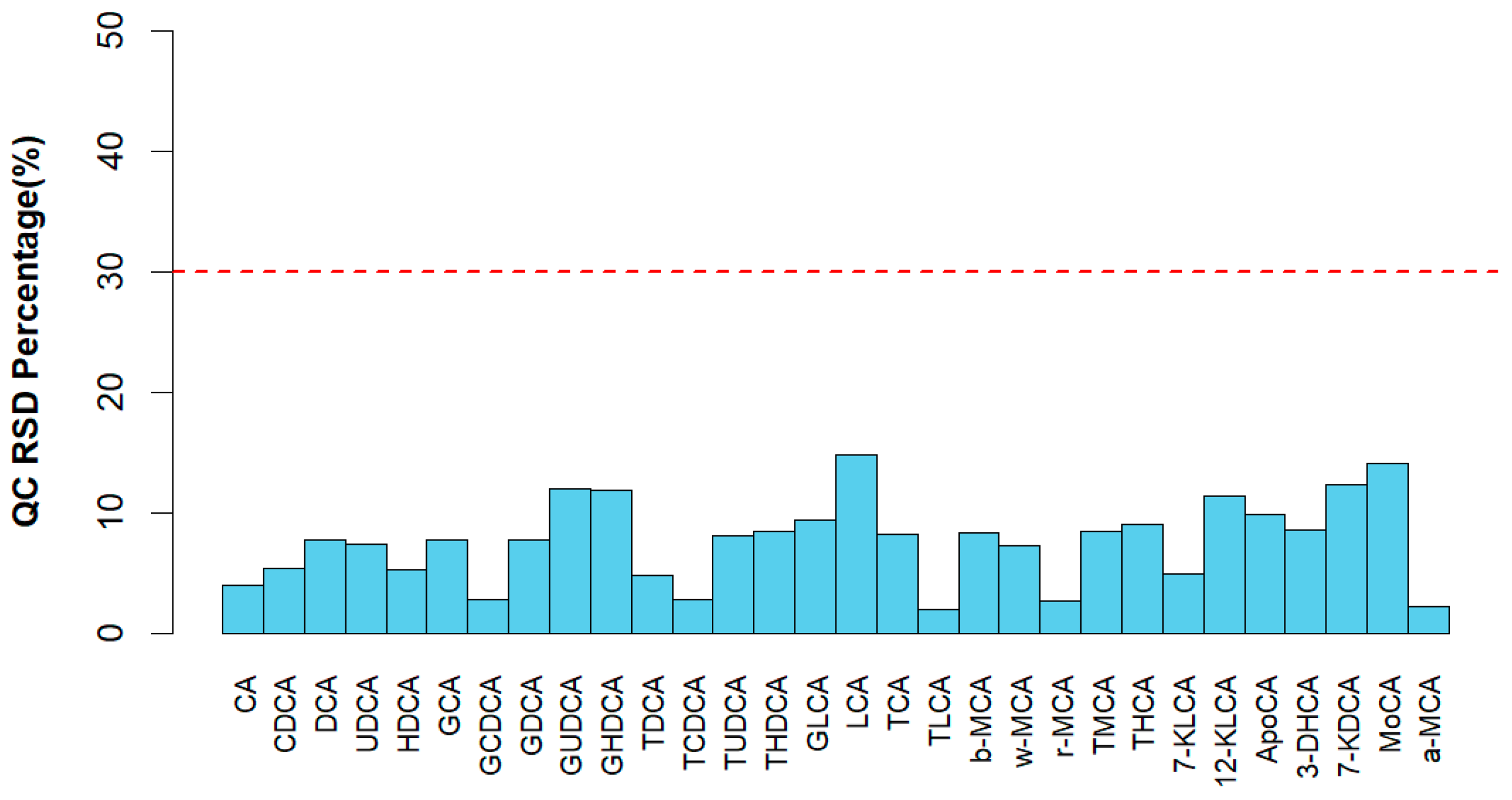
3.4. Bile Acid Profiles of Bile
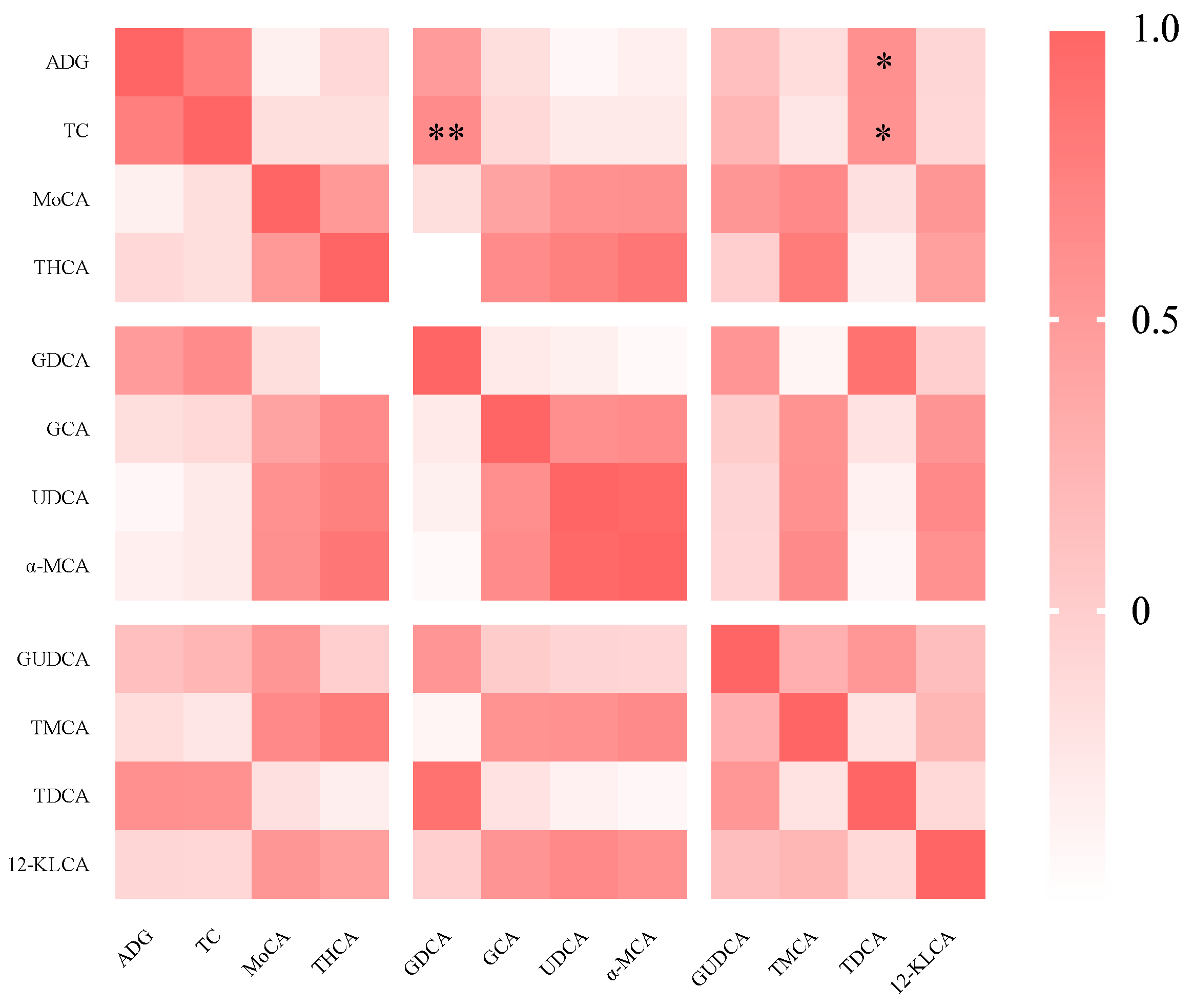
3.5. Spearman’s Correlation Analysis
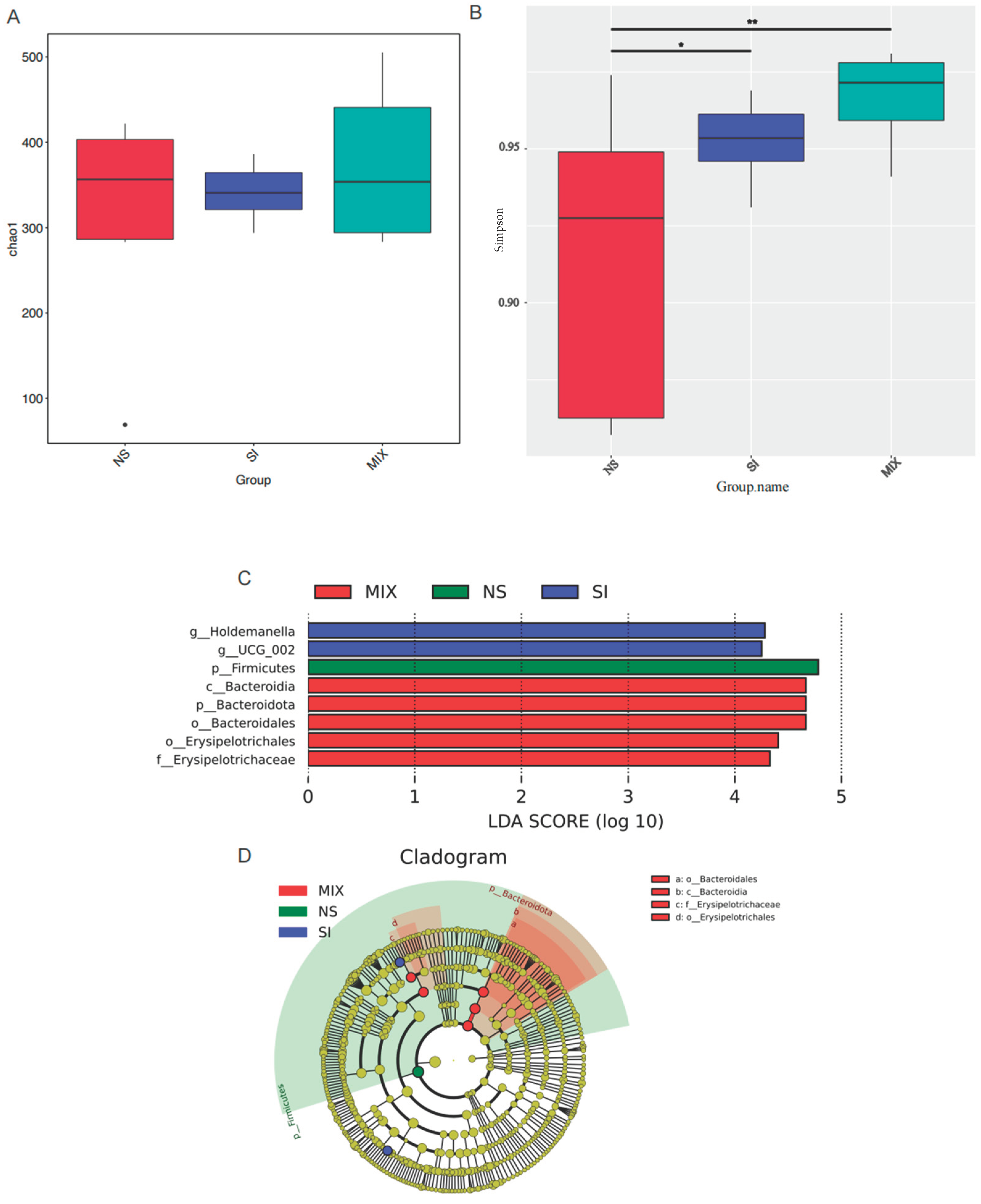
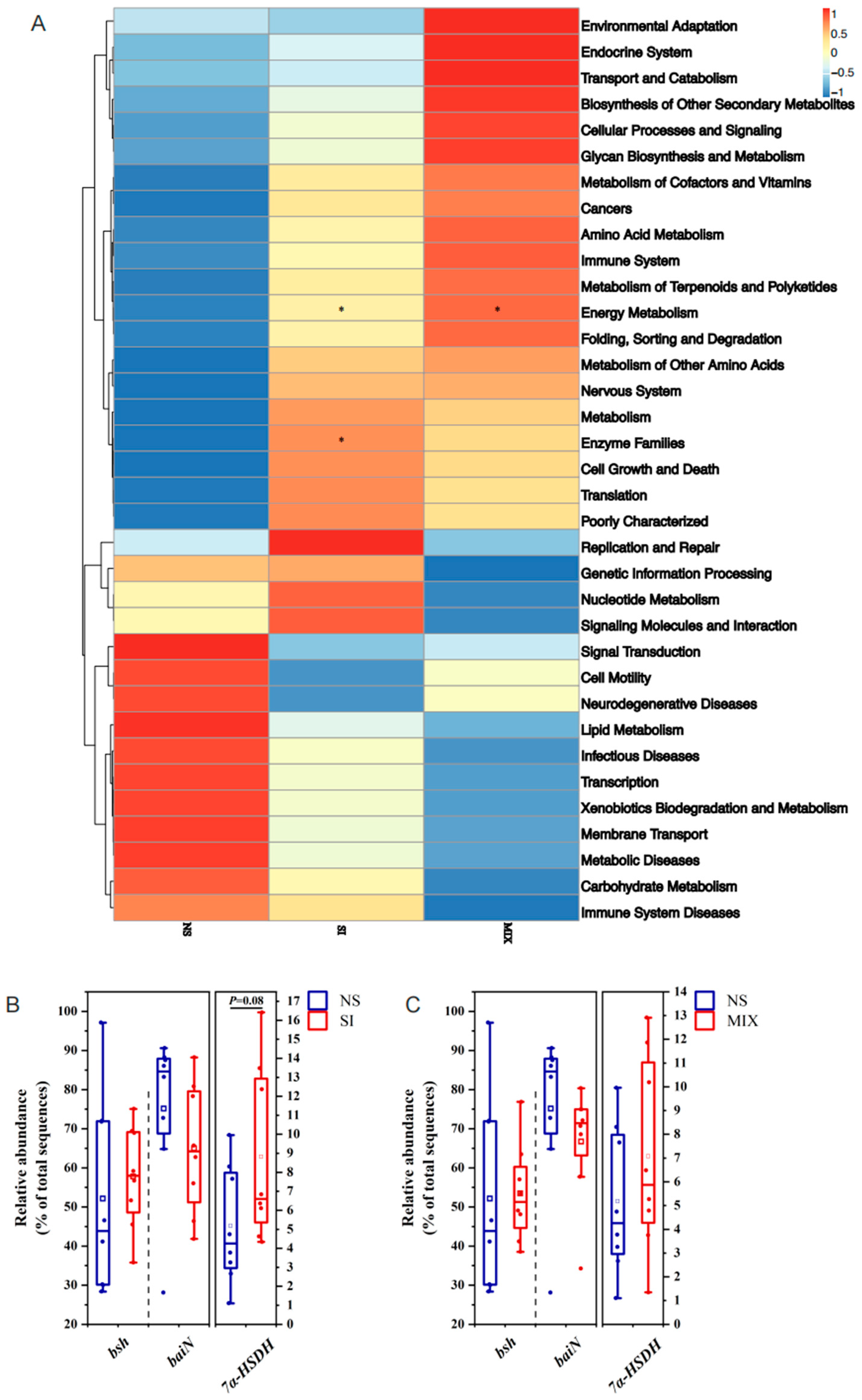
3.6. The Composition and Function of Gut Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.; Fouhse, J.M.; Tiwari, U.P.; Li, L.; Willing, B.P. Dietary Fiber and Intestinal Health of Monogastric Animals. Front. Vet. Sci. 2019, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xia, D.; Chen, J.; Zhang, X.; Wang, H.; Huang, L.; Shen, J.; Wang, S.; Feng, Y.; He, D.; et al. Dietary fibers with different viscosity regulate lipid metabolism via ampk pathway: Roles of gut microbiota and short-chain fatty acid. Poult. Sci. 2022, 101, 101742. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Xie, J.; Zhang, H. Dietary fibers influence the intestinal SCFAs and plasma metabolites profiling in growing pigs. Food Funct. 2016, 7, 4644–4654. [Google Scholar] [CrossRef] [PubMed]
- Scholz-Ahrens, K.E.; Schrezenmeir, J. Inulin and Oligofructose and Mineral Metabolism: The Evidence from Animal Trials. J. Nutr. 2007, 137, 2513S–2523S. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Richardson, R.I.; Sheard, P.R. Manipulating Meat Quality and Composition. Proc. Nutr. Soc. 1999, 58, 363–370. [Google Scholar] [CrossRef]
- Zhou, P.; Zhao, Y.; Zhang, P.; Gui, T.; Wang, J.; Jin, C.; Che, L.; Li, J.; Lin, Y.; Xu, S.; et al. Microbial Mechanistic Insight into the Role of Inulin in Improving Maternal Health in a Pregnant Sow Model. Front. Microbiol. 2017, 8, 2242. [Google Scholar] [CrossRef]
- Grela, E.R.; Świątkiewicz, M.; Florek, M.; Bąkowski, M.; Skiba, G. Effect of Inulin Source and a Probiotic Supplement in Pig Diets on Carcass Traits, Meat Quality and Fatty Acid Composition in Finishing Pigs. Animals 2021, 11, 2438. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, H.W.; Na, L.; Zhou, X.H.; Li, X.; Zhao, Y.R.; Wen, Z.; He, Q.H. Methionine restriction at the post-weanling period promotes muscle fiber transition in piglets and improves intramuscular fat content in growing-finishing pigs. Amino Acids 2019, 51, 1657–1666. [Google Scholar] [CrossRef]
- Dritz, S.S.; Owen, K.Q.; Nelssen, J.L.; Goodband, R.D.; Tokach, M.D. Influence of weaning age and nursery diet complexity on growth performance and carcass characteristics and composition of high-health status pigs from weaning to 109 kilograms. J. Anim. Sci. 1996, 74, 2975–2984. [Google Scholar] [CrossRef]
- Yang, J.; Kurnia, P.; Henning, S.M.; Lee, R.; Huang, J.; Garcia, M.C.; Surampudi, V.; Heber, D.; Li, Z. Effect of Standardized Grape Powder Consumption on the Gut Microbiome of Healthy Subjects: A Pilot Study. Nutrients 2021, 13, 3965. [Google Scholar] [CrossRef] [PubMed]
- Smits, C.H.; Veldman, A.; Verkade, H.J.; Beynen, A.C. The inhibitory effect of carboxymethylcellulose with high viscosity on lipid absorption in broiler chickens coincides with reduced bile salt concentration and raised microbial numbers in the small intestine. Poult. Sci. 1998, 77, 1534–1539. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.W. The Enzymes, Regulation, and Genetics of Bile Acid Synthesis. Annu. Rev. Biochem. 2003, 72, 137–174. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Li, L.; Yang, M.; Liu, T.; Song, X.; Qin, X.; Xu, X.; Liu, J.; Wang, B.; Cao, H. Bile acid–gut microbiota crosstalk in irritable bowel syndrome. Crit. Rev. Microbiol. 2022, 49, 350–369. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.L.; Stine, J.G.; Bisanz, J.E.; Okafor, C.D.; Patterson, A.D. Bile acids and the gut microbiota: Metabolic interactions and impacts on disease. Nat. Rev. Microbiol. 2022, 21, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Guzior, D.; Quinn, R. Review: Microbial transformations of human bile acids. Microbiome 2021, 9, 140. [Google Scholar] [CrossRef]
- Lefebvre, P.; Cariou, B.; Lien, F.; Kuipers, F.; Staels, B. Role of Bile Acids and Bile Acid Receptors in Metabolic Regulation. Physiol. Rev. 2009, 89, 147–191. [Google Scholar] [CrossRef]
- Pi, Y.; Wu, Y.; Zhang, X.; Lu, D.; Han, D.; Zhao, J.; Zheng, X.; Zhang, S.; Ye, H.; Lian, S.; et al. Gut microbiota-derived ursodeoxycholic acid alleviates low birth weight-induced colonic inflammation by enhancing M2 macrophage polarization. Microbiome 2023, 11, 19. [Google Scholar] [CrossRef]
- Liu, Y.; Azad, M.A.K.; Ding, S.; Zhu, Q.; Blachier, F.; Yu, Z.; Gao, H.; Kong, X. Dietary bile acid supplementation in weaned piglets with intrauterine growth retardation improves colonic microbiota, metabolic activity, and epithelial function. J. Anim. Sci. Biotechnol. 2023, 14, 1–18. [Google Scholar] [CrossRef]
- Kuang, J.; Wang, J.; Li, Y.; Li, M.; Zhao, M.; Ge, K.; Zheng, D.; Cheung, K.C.P.; Liao, B.; Wang, S.; et al. Hyodeoxycholic acid alleviates non-alcoholic fatty liver disease through modulating the gut-liver axis. Cell Metab. 2023, 35, 1752–1766.e8. [Google Scholar] [CrossRef]
- Makki, K.; Brolin, H.; Petersen, N.; Henricsson, M.; Christensen, D.P.; Khan, M.T.; Wahlström, A.; Bergh, P.-O.; Tremaroli, V.; Schoonjans, K.; et al. 6α-hydroxylated bile acids mediate TGR5 signalling to improve glucose metabolism upon dietary fiber supplementation in mice. Gut 2022, 72, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Weng, K.; Guo, Y.; Peng, J.; Zhu, Z.-J. An integrated targeted metabolomic platform for high-throughput metabolite profiling and automated data processing. Metabolomics 2015, 11, 1575–1586. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Slavin, J. Fiber and Prebiotics: Mechanisms and Health Benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef]
- McRorie, J.W., Jr.; McKeown, N.M. Understanding the Physics of Functional Fibers in the Gastrointestinal Tract: An Evidence-Based Approach to Resolving Enduring Misconceptions about Insoluble and Soluble Fiber. J. Acad. Nutr. Diet. 2017, 117, 251–264. [Google Scholar] [CrossRef]
- Sobolewska, S.; Grela, E.R. Effect of inulin extraction method and level in growing-finishing pig diets on performance, carcass traits and nutrients digestibility. J. Anim. Sci. Biol. Bioecon. 2013, 31, 56–64. [Google Scholar]
- Samolińska, W.; Kowalczuk-Vasilev, E.; Grela, E.R. Comparative effect of different dietary inulin sources and probiotics on growth performance and blood characteristics in growing–finishing pigs. Arch. Anim. Nutr. 2018, 72, 379–395. [Google Scholar] [CrossRef]
- Cieœlik, E.; Kopeæ, A.; Pisulewski, P.M. Effects of fructooligosaccharides and longchain inulin on serum lipids in rats. Pol. J. Food Nutr. Sci. 2005, 14, 437–441. [Google Scholar] [CrossRef]
- Mudgil, D. Chapter 3—The Interaction between Insoluble and Soluble Fiber. In Dietary Fiber for the Prevention of Cardiovascular Disease; Academic Press: Cambridge, MA, USA, 2017; pp. 35–59. [Google Scholar] [CrossRef]
- Huang, F.; Zheng, X.; Ma, X.; Jiang, R.; Zhou, W.; Zhou, S.; Zhang, Y.; Lei, S.; Wang, S.; Kuang, J.; et al. Theabrownin from Pu-erh tea attenuates hypercholesterolemia via modulation of gut microbiota and bile acid metabolism. Nat. Commun. 2019, 10, 4971. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, K.; Takao, K.; Yabe, D. ChREBP-Mediated Regulation of Lipid Metabolism: Involvement of the Gut Microbiota, Liver, and Adipose Tissue. Front. Endocrinol. 2020, 11, 587189. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, K.; Zhao, Q. Fructooligosaccharide enhanced absorption and anti-dyslipidemia capacity of tea flavonoids in high sucrose-fed mice. Int. J. Food Sci. Nutr. 2019, 70, 311–322. [Google Scholar] [CrossRef]
- Huang, S.; Dong, S.; Lin, L.; Ma, Q.; Xu, M.; Ni, L.; Fan, Q. Inulin ameliorates metabolic syndrome in high-fat diet-fed mice by regulating gut microbiota and bile acid excretion. Front. Pharmacol. 2023, 14, 1226448. [Google Scholar] [CrossRef] [PubMed]
- Trauner, M.; Claudel, T.; Fickert, P.; Moustafa, T.; Wagner, M. Bile Acids as Regulators of Hepatic Lipid and Glucose Metabolism. Dig. Dis. 2010, 28, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; He, X.; Gao, X.; Liu, Q.; Zhao, Y.; Hong, Y.; Zhu, W.; Yan, J.; Li, Y.; Li, Y.; et al. Hyodeoxycholic acid ameliorates nonalcoholic fatty liver disease by inhibiting RAN-mediated PPARα nucleus-cytoplasm shuttling. Nat. Commun. 2023, 14, 5451. [Google Scholar] [CrossRef]
- MahmoudianDehkordi, S.; Arnold, M.; Nho, K.; Ahmad, S.; Jia, W.; Xie, G.X.; Louie, G.; Kueider-Paisley, A.; Moseley, M.A.; Thompson, J.W.; et al. Altered bile acid profile associates with cognitive impairment in Alzheimer’s disease—An emerging role for gut microbiome. Alzheimer’s Dement. 2019, 15, 76–92. [Google Scholar] [CrossRef]
- Byrd, D.A.; Sinha, R.; Weinstein, S.J.; Albanes, D.; Freedman, N.D.; Sampson, J.; Loftfield, E. An investigation of cross-sectional associations of a priori–selected dietary components with circulating bile acids. Am. J. Clin. Nutr. 2021, 114, 1802–1813. [Google Scholar] [CrossRef]
- Jiao, N.; Baker, S.S.; Chapa-Rodriguez, A.; Liu, W.; Nugent, C.A.; Tsompana, M.; Mastrandrea, L.; Buck, M.J.; Baker, R.D.; Genco, R.J.; et al. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut 2018, 67, 1881–1891. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, T.; Zhao, A.; Ning, Z.; Kuang, J.; Wang, S.; You, Y.; Bao, Y.; Ma, X.; Yu, H.; et al. Hyocholic acid species as novel biomarkers for metabolic disorders. Nat. Commun. 2021, 12, 1487. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, T.; Jiang, R.; Zhao, A.; Wu, Q.; Kuang, J.; Sun, D.; Ren, Z.; Li, M.; Zhao, M.; et al. Hyocholic acid species improve glucose homeostasis through a distinct TGR5 and FXR signaling mechanism. Cell Metab. 2020, 33, 791–803.e7. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhou, Y.; Zhou, K.; Cai, W. Targeted Metabolomics Reveals Birth Screening Biomarkers for Biliary Atresia in Dried Blood Spots. J. Proteome Res. 2021, 21, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Xie, G.; Jia, W. Bile acid–microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2017, 15, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Wardman, J.F.; Bains, R.K.; Rahfeld, P.; Withers, S.G. Carbohydrate-active enzymes (CAZymes) in the gut microbiome. Nat. Rev. Microbiol. 2022, 20, 542–556. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Wang, C.; Li, D.; Peng, Y.; Deng, L.; Zhao, Y.; Zhang, Z.; Wei, M.; Wu, K.; Zhao, J.; et al. The unique gut microbiome of giant pandas involved in protein metabolism contributes to the host’s dietary adaption to bamboo. Microbiome 2023, 11, 180. [Google Scholar] [CrossRef]
- Huang, X.Q.; Chen, Q.Y.; Fan, Y.Y.; Yang, R.Z.; Gong, G.Y.; Yan, C.S.; Song, Y.; Zhang, B.Z.; Xi, S.Y.; Huang, Y.P.; et al. Fructooligosaccharides attenuate non-alcoholic fatty liver disease by remodeling gut microbiota and association with lipid metabolism. Biomed. Pharmacother. 2023, 159, 114300. [Google Scholar] [CrossRef]
- Zhang, C.H.; Liang, D.; Li, X.X.; Liu, J.; Fan, M.Y.; Jing, M.; Wang, Y.F.; Zhang, Y.; Fang, Y.Q.; Li, D. Characteristics of Gut Microbial Profiles of Offshore Workers and Its Associations with Diet. Front. Nutr. 2022, 9, 904927. [Google Scholar] [CrossRef]
- Burakova, I.; Smirnova, Y.; Gryaznova, M.; Syromyatnikov, M.; Chizhkov, P.; Popov, E.; Popov, V. The Effect of Short-Term Consumption of Lactic Acid Bacteria on the Gut Microbiota in Obese People. Nutrients 2022, 14, 3384. [Google Scholar] [CrossRef]
- Jia, E.-T.; Liu, Z.-Y.; Pan, M.; Lu, J.-F.; Ge, Q.-Y. Regulation of bile acid metabolism-related signaling pathways by gut microbiota in diseases. J. Zhejiang Univ. B 2019, 20, 781–792. [Google Scholar] [CrossRef]
- Huang, Z.; Boekhorst, J.; Fogliano, V.; Capuano, E.; Wells, J.M. Distinct effects of fiber and colon segment on microbiota-derived indoles and short-chain fatty acids. Food Chem. 2023, 398, 133801. [Google Scholar] [CrossRef]
- Kiriyama, Y.; Nochi, H. The Biosynthesis, Signaling, and Neurological Functions of Bile Acids. Biomolecules 2019, 9, 232. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.T.; Lei, Y.; Qu, Y.; Fan, Z.; Zhang, T.; Xu, Y.B.; Du, Q.; Brugger, D.; Chen, Y.L.; Zhang, K.; et al. Bacteroides uniformis-induced perturbations in colonic microbiota and bile acid levels inhibit TH17 differentiation and ameliorate colitis developments. NPJ Biofilms Microbiomes 2023, 9, 56. [Google Scholar] [CrossRef] [PubMed]
| Items | Content | Nutrient Level 3 | |
|---|---|---|---|
| Corn starch | 61.90 | DE (MJ/kg) | 16.07 |
| Fish meal | 5.00 | Crude Protein | 19.05 |
| Soybean protein isolate (90%) | 17.80 | Calcium | 0.75 |
| Whey powder | 8.00 | Total phosphorus | 0.52 |
| Lactose | 2.00 | Lysine | 1.29 |
| Sucrose | 3.00 | Methionine | 0.38 |
| dl-Methionin (99%) | 0.08 | Tryptophan | 0.26 |
| l-Lysine-HCl (78%) | 0.05 | Threonine | 0.76 |
| l-Threonine | 0.01 | ||
| l-Tryptophan | 0.01 | ||
| Limestone | 0.80 | ||
| Choline chloride (50%) | 0.15 | ||
| NaCl | 0.25 | ||
| Ca(H2PO4)2 | 0.70 | ||
| Vitamin premix 1 | 0.05 | ||
| Mineral premix 2 | 0.20 | ||
| Total | 100.00 | ||
| Genes | Primers | ATc (°C) | Size, bp |
|---|---|---|---|
| SREBP-1c | F: AAGCGGACGGCTCACAATG R: GCAAGACGGCGGATTTATTCA | 56.03 | 121 |
| FAS | F: GCCGAGTACAGCGTCAACAACC R: TGGTCCTTCTTCATCAGCGGGAT | 59.18 | 173 |
| ACACA | F: CAACAATGGCATCGCAGCAGTG R: GGCTTTCAGGTCTTCGGGTGTG | 58.44 | 121 |
| ChREBP | F: ACAGACGCCTACACCTTCAAACTTC R: CCAGGACCCCACTGCTAAGGAC | 58.19 | 83 |
| Cyp7a1 | F: GCATTTGGGCACAGAAGCATTGAC R: GGCAAGCAAATTCAAGGCGTCAC | 58.67 | 105 |
| Cyp27a1 | F: AGAGTCATGGTACCGGCTGC R: AGAGCATTGGTGTAGAGCGCA | 56.96 | 81 |
| FXR | F: GACCACGAAGATCAGATTGCTTTGC R: ATGTCCAGCCGGAAGTTTCCTATTG | 57.86 | 99 |
| β-actin | F: TCTGGCACCACACCTTCT R: TGATCTGGGTCATCTTCTCAC | 53.01 | 114 |
| Items | NS | SI | MIX | SEM | p-Value |
|---|---|---|---|---|---|
| 1–21 d | |||||
| Initial weight, kg | 10.53 | 10.52 | 10.53 | 0.23 | 1.00 |
| Final weight, kg | 21.00 | 17.92 | 19.32 | 0.54 | 0.06 |
| ADFI, g/d | 826 | 691 | 774 | 31.18 | 0.21 |
| ADG, g/d | 486 b | 357 a | 421 ab | 18.42 | 0.01 |
| F/G | 1.70 b | 1.94 a | 1.85 ab | 0.04 | 0.02 |
| Items | NS | SI | MIX | SEM | p-Value |
|---|---|---|---|---|---|
| TG (mmol/L) | 0.38 | 0.32 | 0.36 | 0.02 | 0.47 |
| TC (mmol/L) | 1.32 | 1.05 | 1.14 | 0.05 | 0.08 |
| TBA (μmol/L) | 17.99 b | 13.17 ab | 11.47 a | 1.18 | 0.04 |
| LDL-C (mmol/L) | 4.67 | 6.09 | 4.54 | 0.59 | 0.50 |
| HDL-C (mmol/L) | 3.29 | 3.18 | 3.52 | 0.13 | 0.57 |
| Items | NS | SI | MIX | SEM | p-Value |
|---|---|---|---|---|---|
| THCA | 223.11 a | 354.10 b | 411.84 b | 28.57 | 0.01 |
| GDCA | 703.93 b | 457.13 a | 442.85 a | 43.55 | 0.02 |
| GCA | 439.85 a | 582.92 ab | 712.13 b | 43.65 | 0.03 |
| UDCA | 1.18 ab | 2.79 b | 0.45 a | 0.39 | 0.04 |
| α-MCA | 12.35 | 42.56 | 13.02 | 6.16 | 0.07 |
| GUDCA | 996.62 | 1147.11 | 707.29 | 86.01 | 0.10 |
| TMCA | 13.08 | 20.92 | 25.38 | 2.48 | 0.12 |
| GHDCA | 31,557.63 | 33,730.90 | 26,025.04 | 1674.02 | 0.15 |
| 12-KLCA | 1.74 | 1.25 | 0.44 | 0.28 | 0.16 |
| 7-KDCA | 27.85 | 28.93 | 20.61 | 1.96 | 0.17 |
| DCA | 1.96 | 0.46 | 0.20 | 0.42 | 0.19 |
| HDCA | 109.77 | 120.17 | 25.92 | 24.63 | 0.24 |
| TCA | 171.22 | 163.29 | 231.20 | 17.86 | 0.25 |
| TDCA | 24.97 | 16.28 | 20.55 | 5.01 | 0.28 |
| GCDCA | 6661.24 | 7018.93 | 6216.24 | 212.85 | 0.32 |
| LCA | 0.33 | 0.16 | 0.13 | 0.06 | 0.32 |
| ω-MCA | 12.35 | 18.57 | 11.77 | 2.03 | 0.33 |
| CDCA | 12.79 | 11.19 | 4.36 | 2.52 | 0.37 |
| GLCA | 212.31 | 132.88 | 158.87 | 24.02 | 0.41 |
| TLCA | 7.88 | 5.31 | 5.76 | 0.86 | 0.44 |
| TUDCA | 45.83 | 70.77 | 65.24 | 8.20 | 0.45 |
| CA | 41.53 | 47.62 | 23.69 | 9.32 | 0.57 |
| THDCA | 1656.30 | 1495.12 | 1447.70 | 107.11 | 0.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; He, D.; Yu, B.; Chen, D. Effects of Different Types of Dietary Fibers on Lipid Metabolism and Bile Acids in Weaned Piglets. Animals 2023, 13, 3266. https://doi.org/10.3390/ani13203266
Hu Y, He D, Yu B, Chen D. Effects of Different Types of Dietary Fibers on Lipid Metabolism and Bile Acids in Weaned Piglets. Animals. 2023; 13(20):3266. https://doi.org/10.3390/ani13203266
Chicago/Turabian StyleHu, Yaolian, Dongting He, Bing Yu, and Daiwen Chen. 2023. "Effects of Different Types of Dietary Fibers on Lipid Metabolism and Bile Acids in Weaned Piglets" Animals 13, no. 20: 3266. https://doi.org/10.3390/ani13203266
APA StyleHu, Y., He, D., Yu, B., & Chen, D. (2023). Effects of Different Types of Dietary Fibers on Lipid Metabolism and Bile Acids in Weaned Piglets. Animals, 13(20), 3266. https://doi.org/10.3390/ani13203266





