An Epidemiological Assessment of Cryptosporidium and Giardia spp. Infection in Pet Animals from Taiwan
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
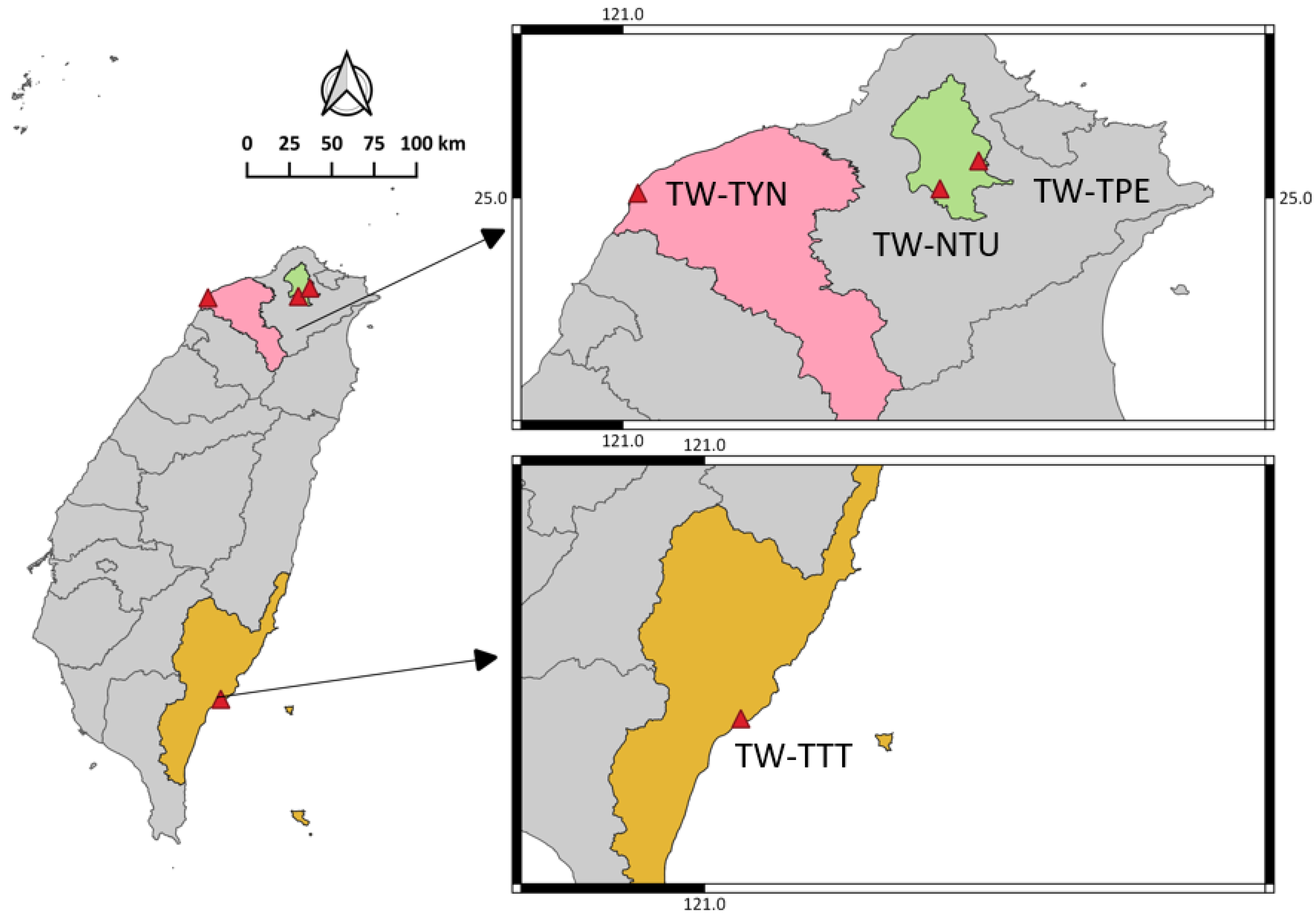
2.2. Sample Collection
2.3. DNA Extraction
2.4. Nested Polymerase Chain Reaction (PCR) Amplification
2.5. Phylogenetic Analysis
2.6. Software and Statistical Analysis
3. Results
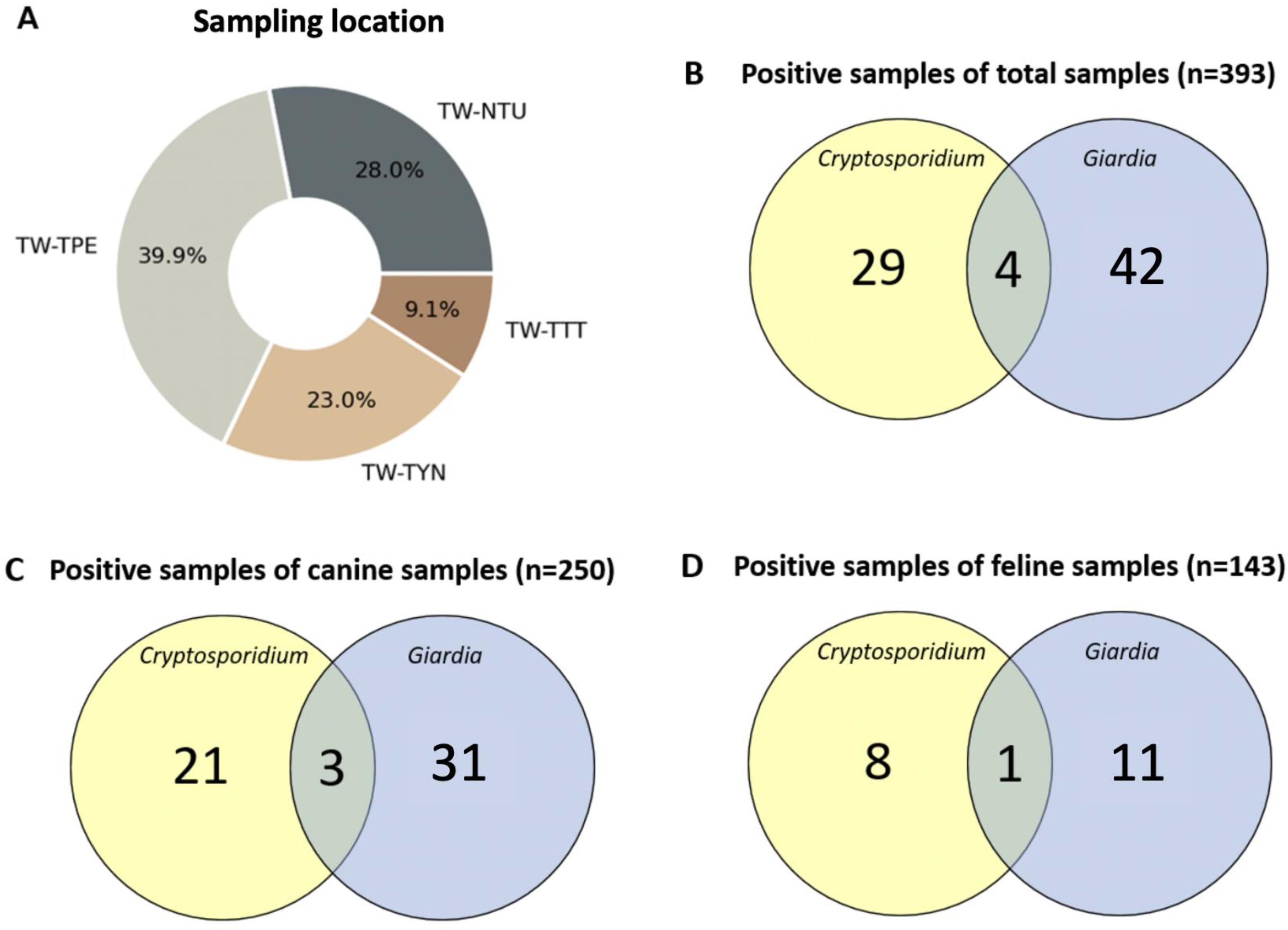
3.1. Prevalence of Cryptosporidium and Giardia Infection and Co-Infection Pattern in Companion Animals
3.2. Risk Factor Analysis
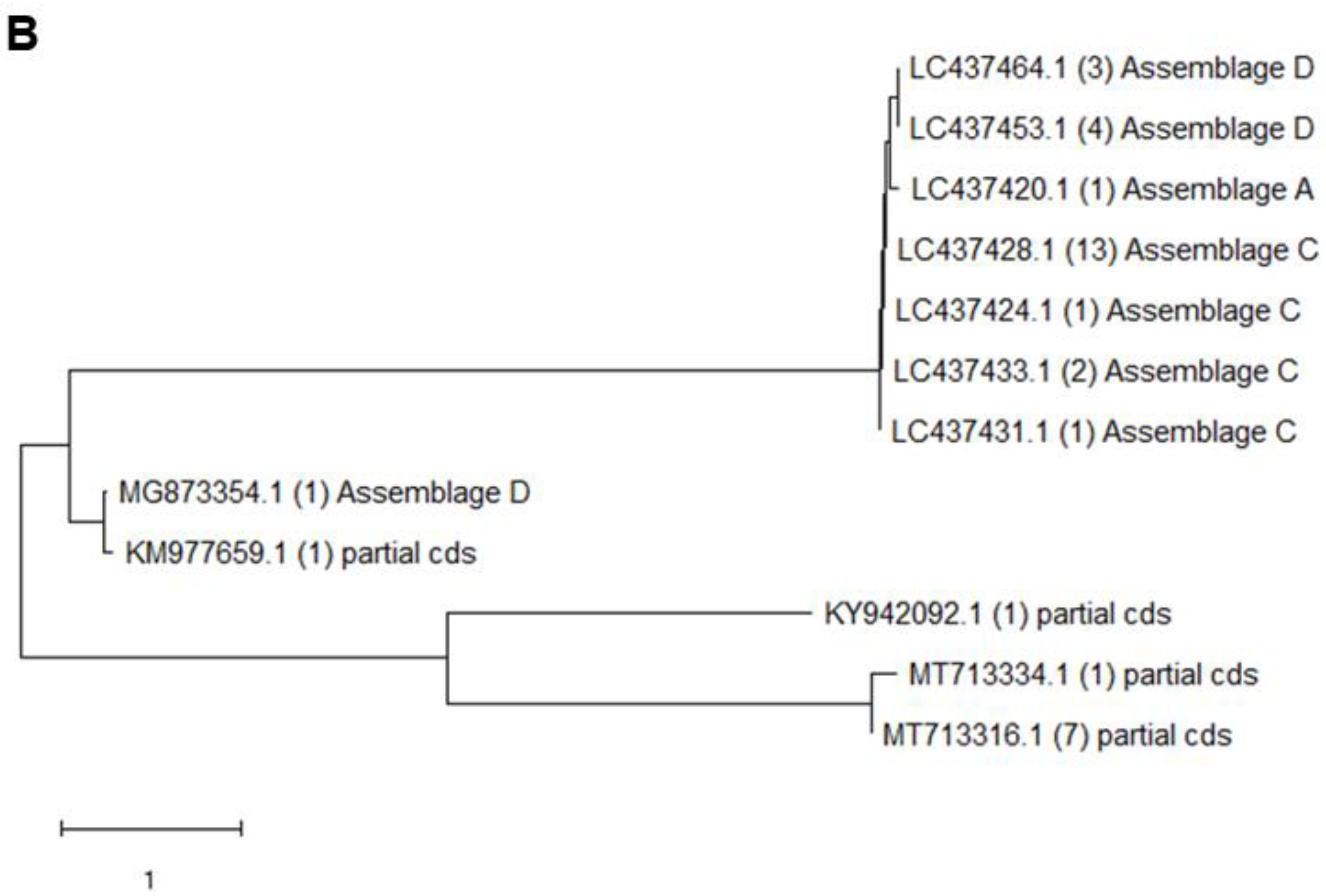
3.3. Phylogenetic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rossle, N.F.; Latif, B. Cryptosporidiosis as threatening health problem: A review. Asian Pac. J. Trop. Biomed. 2013, 3, 916–924. [Google Scholar] [CrossRef]
- Fayer, R.; Speer, C.; Dubey, J. The General Biology of Cryptosporidium; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Wang, J.; Liew, C. Prevalence of Cryptosporidium spp. in birds in Taiwan. Taiwan J. Vet. Med. Anim. Husb. 1990, 56, 45–57. [Google Scholar]
- Bodley-Tickell, A.; Kitchen, S.; Sturdee, A. Occurrence of Cryptosporidium in agricultural surface waters during an annual farming cycle in lowland UK. Water Res. 2002, 36, 1880–1886. [Google Scholar] [CrossRef] [PubMed]
- Gerace, E.; Presti, V.D.M.L.; Biondo, C. Cryptosporidium infection: Epidemiology, pathogenesis, and differential diagnosis. Eur. J. Microbiol. Immunol. 2019, 9, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Ryan, U.M.; Xiao, L. Genetic Diversity and Population Structure of Cryptosporidium. Trends Parasitol. 2018, 34, 997–1011. [Google Scholar] [CrossRef]
- Feng, Y.; Xiao, L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin. Microbiol. Rev. 2011, 24, 110–140. [Google Scholar] [CrossRef]
- Bouzid, M.; Halai, K.; Jeffreys, D.; Hunter, P.R. The prevalence of Giardia infection in dogs and cats, a systematic review and meta-analysis of prevalence studies from stool samples. Vet. Parasitol. 2015, 207, 181–202. [Google Scholar] [CrossRef]
- Santin, M. Clinical and subclinical infections with Cryptosporidium in animals. N. Z. Vet. J. 2013, 61, 1–10. [Google Scholar] [CrossRef]
- Alves, M.E.M.; Martins, F.D.C.; Braunig, P.; Pivoto, F.L.; Sangioni, L.A.; Vogel, F.S.F. Molecular detection of Cryptosporidium spp. and the occurrence of intestinal parasites in fecal samples of naturally infected dogs and cats. Parasitol. Res. 2018, 117, 3033–3038. [Google Scholar] [CrossRef]
- Yang, R.; Ying, J.L.; Monis, P.; Ryan, U. Molecular characterisation of Cryptosporidium and Giardia in cats (Felis catus) in Western Australia. Exp. Parasitol. 2015, 155, 13–18. [Google Scholar] [CrossRef]
- Gil, H.; Cano, L.; de Lucio, A.; Bailo, B.; de Mingo, M.H.; Cardona, G.A.; Fernandez-Basterra, J.A.; Aramburu-Aguirre, J.; Lopez-Molina, N.; Carmena, D. Detection and molecular diversity of Giardia duodenalis and Cryptosporidium spp. in sheltered dogs and cats in Northern Spain. Infect. Genet. Evol. 2017, 50, 62–69. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Song, M.; Lu, Y.; Yang, J.; Tao, W.; Jiang, Y.; Wan, Q.; Zhang, S.; Xiao, L. Prevalence and genetic characteristics of Cryptosporidium, Enterocytozoon bieneusi and Giardia duodenalis in cats and dogs in Heilongjiang province, China. Vet. Parasitol. 2015, 208, 125–134. [Google Scholar] [CrossRef]
- Lucio-Forster, A.; Griffiths, J.K.; Cama, V.A.; Xiao, L.; Bowman, D.D. Minimal zoonotic risk of cryptosporidiosis from pet dogs and cats. Trends Parasitol. 2010, 26, 174–179. [Google Scholar] [CrossRef]
- Xu, H.; Jin, Y.; Wu, W.; Li, P.; Wang, L.; Li, N.; Feng, Y.; Xiao, L. Genotypes of Cryptosporidium spp., Enterocytozoon bieneusi and Giardia duodenalis in dogs and cats in Shanghai, China. Parasites Vectors 2016, 9, 121. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.A.; Palmer, C.S.; O’Handley, R. The public health and clinical significance of Giardia and Cryptosporidium in domestic animals. Vet. J. 2008, 177, 18–25. [Google Scholar] [CrossRef]
- Steneroden, K.; Hill, A.E.; Salman, M. Zoonotic disease awareness in animal shelter workers and volunteers and the effect of training. Zoonoses Public Health 2011, 58, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.S.; Bruckner, D.A.; Brewer, T.C.; Shimizu, R.Y. Techniques for the Recovery and Identification of Cryptosporidium Oocysts from Stool Specimens. J. Clin. Microbiol. 1983, 18, 185–190. [Google Scholar] [CrossRef]
- Hunter, P.R.; Thompson, R.A. The zoonotic transmission of Giardia and Cryptosporidium. Int. J. Parasitol. 2005, 35, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Coupe, S.; Sarfati, C.; Hamane, S.; Derouin, F. Detection of Cryptosporidium and identification to the species level by nested PCR and restriction fragment length polymorphism. J. Clin. Microbiol. 2005, 43, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Hawash, Y. DNA extraction from protozoan oocysts/cysts in feces for diagnostic PCR. Korean J. Parasitol. 2014, 52, 263. [Google Scholar] [CrossRef]
- Xiao, L.; Escalante, L.; Yang, C.; Sulaiman, I.; Escalante, A.A.; Montali, R.J.; Fayer, R.; Lal, A.A. Phylogenetic analysis of Cryptosporidium parasites based on the small-subunit rRNA gene locus. Appl. Environ. Microbiol. 1999, 65, 1578–1583. [Google Scholar] [CrossRef] [PubMed]
- Lalle, M.; Pozio, E.; Capelli, G.; Bruschi, F.; Crotti, D.; Cacciò, S.M. Genetic heterogeneity at the β-giardin locus among human and animal isolates of and identification of potentially zoonotic subgenotypes. Int. J. Parasitol. 2005, 35, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Cacciò, S.M.; Beck, R.; Lalle, M.; Marinculic, A.; Pozio, E. Multilocus genotyping of reveals striking differences between assemblages A and B. Int. J. Parasitol. 2008, 38, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Itoh, N.; Kimura, Y.; Kanai, K. Prevalence of intestinal parasites in breeding cattery cats in Japan. J. Feline Med. Surg. 2016, 18, 834–837. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lee, S.; Song, K. Prevalence of canine giardiosis in South Korea. Res. Vet. Sci. 2008, 84, 416–418. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, P.; Wang, P.; Alsarakibi, M.; Zhu, H.; Liu, Y.; Meng, X.; Li, J.; Guo, J.; Li, G. Genotype identification and prevalence of Giardia duodenalis in pet dogs of Guangzhou, Southern China. Vet. Parasitol. 2012, 188, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L. Molecular epidemiology of cryptosporidiosis: An update. Exp. Parasitol. 2010, 124, 80–89. [Google Scholar] [CrossRef]
- Beser, J.; Toresson, L.; Eitrem, R.; Troell, K.; Winiecka-Krusnell, J.; Lebbad, M. Possible zoonotic transmission of Cryptosporidium felis in a household. Infect. Ecol. Epidemiol. 2015, 5, 28463. [Google Scholar] [CrossRef]
- Cama, V.A.; Ross, J.M.; Crawford, S.; Kawai, V.; Chavez-Valdez, R.; Vargas, D.; Vivar, A.; Ticona, E.; Navincopa, M.; Williamson, J. Differences in clinical manifestations among Cryptosporidium species and subtypes in HIV-infected persons. J. Infect. Dis. 2007, 196, 684–691. [Google Scholar] [CrossRef]
- Gatei, W.; Suputtamongkol, Y.; Waywa, D.; Ashford, R.; Bailey, J.; Greensill, J.; Beeching, N.; Hart, C.A. Zoonotic species of Cryptosporidium are as prevalent as the anthroponotic in HIV-infected patients in Thailand. Ann. Trop. Med. Parasitol. 2002, 96, 797–802. [Google Scholar] [CrossRef]
- Xiao, L.; Cama, V.A.; Cabrera, L.; Ortega, Y.; Pearson, J.; Gilman, R.H. Possible transmission of Cryptosporidium canis among children and a dog in a household. J. Clin. Microbiol. 2007, 45, 2014–2016. [Google Scholar] [CrossRef]
- Koompapong, K.; Mori, H.; Thammasonthijarern, N.; Prasertbun, R.; Pintong, A.-r.; Popruk, S.; Rojekittikhun, W.; Chaisiri, K.; Sukthana, Y.; Mahittikorn, A. Molecular identification of Cryptosporidium spp. in seagulls, pigeons, dogs, and cats in Thailand. Parasite 2014, 21, 52. [Google Scholar] [CrossRef]
- Erickson, M.C.; Ortega, Y.R. Inactivation of protozoan parasites in food, water, and environmental systems. J. Food Prot. 2006, 69, 2786–2808. [Google Scholar] [CrossRef] [PubMed]
- Taiwan Open Weather Data Communications. Central Weather Administration. 2020. Available online: https://opendata.cwb.gov.tw/index (accessed on 15 October 2020).
- Watanabe, Y.; Kimura, K.; Yang, C.-H.; Ooi, H.-K. Detection of Cryptosporidium sp. oocyst and Giardia sp. cyst in faucet water samples from cattle and goat farms in Taiwan. J. Vet. Med. Sci. 2005, 67, 1285–1287. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hsu, B.-M.; Huang, C.; Hsu, C.-L.L.; Hsu, Y.-F.; Yeh, J. Occurrence of Giardia and Cryptosporidium in the Kau-Ping River and its watershed in southern Taiwan. Water Res. 1999, 33, 2701–2707. [Google Scholar] [CrossRef]
- Okhuysen, P.C.; Chappell, C.L.; Crabb, J.H.; Sterling, C.R.; DuPont, H.L. Virulence of three distinct Cryptospovidium parvum isolates for healthy adults. J. Infect. Dis. 1999, 180, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Hamnes, I.S.; Gjerde, B.K.; Robertson, L.J. A longitudinal study on the occurrence of Cryptosporidium and Giardia in dogs during their first year of life. Acta Vet. Scand. 2007, 49, 22. [Google Scholar] [CrossRef]
- Uehlinger, F.D.; Greenwood, S.J.; McClure, J.T.; Conboy, G.; O’Handley, R.; Barkema, H.W. Zoonotic potential of Giardia duodenalis and Cryptosporidium spp. and prevalence of intestinal parasites in young dogs from different populations on Prince Edward Island, Canada. Vet. Parasitol. 2013, 196, 509–514. [Google Scholar] [CrossRef]
- Paris, J.K.; Wills, S.; Balzer, H.-J.; Shaw, D.J.; Gunn-Moore, D.A. Enteropathogen co-infection in UK cats with diarrhoea. BMC Vet. Res. 2014, 10, 13. [Google Scholar] [CrossRef]
- da Rocha Gizzi, A.B.; Oliveira, S.T.; Leutenegger, C.M.; Estrada, M.; Kozemjakin, D.A.; Stedile, R.; Marcondes, M.; Biondo, A.W. Presence of infectious agents and co-infections in diarrheic dogs determined with a real-time polymerase chain reaction-based panel. BMC Vet. Res. 2014, 10, 23. [Google Scholar]

| Primer Name | Primer Sequence (5’→3’) | Annealing (°C/s) | Product Size (bp) | Reference |
|---|---|---|---|---|
| Nested-PCR primers for Cryptosporidium SSU genes | ||||
| SSU-F1 | TTCTAGAGCTAATACATGCG | 55/45 | 1325 | (Xiao et al., 1999) [22] |
| SSU-R1 | CCCATTTCCTTCGAAACAGGA | |||
| SSU-F2 | GGAAGGGTTGTATTTATTAGATAAAG | 55/45 | 840 | |
| SSU-R2 | CTCATAAGGTGCTGAAGGAGTA | |||
| Nested-PCR primers for Giardia BG genes | ||||
| BG-F1 | AAGCCCGACGACCTCACCCGCAGTGC | 65/30 | 735 | (MarcoLalle et al., 2005) [23] |
| BG-R1 | GAGGCCGCCCTGGATCTTCGAGACGAC | |||
| BG-F2 | GAACGAACGAGATCGAGGTCCG | 65/30 | 511 | |
| BG-R2 | CTCGACGAGCTTCGTGTT | |||
| Nested-PCR primers for Giardia GDH genes | ||||
| GDH-F1 | TTCCGTRTYCAGTACAACTC | 57.8/30 | 754 | (S.M.Cacciò et al., 2008) [24] |
| GDH-R1 | ACCTCGTTCTGRGTGGCGCA | |||
| GDH-F2 | ATGACYGAGCTYCAGAGGCACGT | 57.8/30 | 530 | |
| GDH-R2 | GTGGCGCARGGCATGATGCA | |||
| Category | No. Examined | Canine | No. Examined | Feline | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pathogen | Cryptosporidium (%) | Giardia (%) | Cryptosporidium (%) | Giardia (%) | ||||||
| Source-1 TW-TPE | 82 | 9 | 11.0% | 19 | 23.2% | 76 | 4 | 5.3% | 6 | 7.9% |
| Source-2 TW-TYN | 55 | 2 | 3.6% | 7 | 12.7% | 36 | 1 | 2.8% | 3 | 8.3% |
| Source-3 TW-TTT | 26 | 3 | 11.5% | 2 | 7.7% | 10 | 0 | 0.0% | 1 | 10.0% |
| Subtotal | 163 | 14 | 8.6% | 28 | 17.2% | 122 | 5 | 4.1% | 10 | 8.2% |
| Source-4 TW-NTU | 87 | 7 | 8.0% | 3 | 3.4% | 21 | 3 | 14.3% | 1 | 4.8% |
| Total | 250 | 21 | 8.4% | 31 | 12.4% | 143 | 8 | 5.6% | 11 | 7.7% |
| Category | No. Examined | Assemblages (n) of Canine | No. Examined | Assemblages (n) of Feline | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pathogen | Overall | BG | GDH | Overall | BG | GDH | ||||||
| Source-1 TW-TPE | 82 | 19 | 16 | C (8), D (6) | 7 | C (3), D (3) | 76 | 6 | 6 | A (1) | 0 | |
| Source-2 TW-TYN | 55 | 7 | 6 | C (5), D (1) | 3 | C (3) | 36 | 3 | 3 | C (2) | 1 | F (1) |
| Source-3 TW-TTT | 26 | 2 | 1 | D (1) | 2 | D (2) | 10 | 1 | 0 | 1 | D (1) | |
| Source-4 TW-NTU | 87 | 3 | 3 | C (2) | 0 | 21 | 1 | 1 | 0 | |||
| Total | 250 | 31 | 26 | 12 | 143 | 11 | 10 | 2 | ||||
| Factor | n | Cryptosporidium spp. | G. duodenalis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. Positive | OR | 95%CI | p-Value | No. Positive | OR | 95%CI | p-Value | ||
| Gender | |||||||||
| Female | 113 | 11 | 1.4 | (0.6–3.4) | 0.49 | 12 | 0.7 | (0.3–1.6) | 0.44 |
| Male | 106 | 7 | 0.7 | (0.3–1.7) | 0.38 | 12 | 0.8 | (0.4–1.8) | 0.66 |
| Unknown | 31 | 3 | 1.2 | (0.3–4.3) | 0.78 | 7 | 2.4 | (0.9–6.1) | 0.07 |
| Total | 250 | 21 | 31 | ||||||
| Source | |||||||||
| Veterinary Hospital | 87 | 7 | 1 | (0.4–2.4) | 0.88 | 3 | 0.2 | (0.1–0.6) | 0.0048 ** |
| Animal Shelter | 163 | 14 | 1.1 | (0.4–2.8) | 28 | 5.8 | (1.7–19.7) | ||
| Total | 250 | 21 | 31 | ||||||
| Breed | |||||||||
| Purebred | 64 | 4 | 0.7 | (0.2–2.1) | 0.47 | 3 | 0.3 | (0.1–0.9) | 0.0406 * |
| Mixed | 186 | 17 | 1.5 | (0.5–4.7) | 28 | 3.6 | (1.1–12.3) | ||
| Total | 250 | 21 | 31 | ||||||
| Clinical Sign | |||||||||
| Diarrhea | 34 | 6 | 2.9 | (1.0–8.0) | 0.0439 * | 7 | 2.1 | (0.8–5.3) | 0.13 |
| No notable sign | 216 | 15 | 0.3 | (0.1–1.0) | 24 | 0.5 | (0.2–1.2) | ||
| Total | 250 | 21 | 31 | ||||||
| Factor | n | Cryptosporidium spp. | G. duodenalis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. Positive | OR | 95% CI | p-Value | No. Positive | OR | 95% CI | p-Value | ||
| Gender | |||||||||
| Female | 50 | 3 | 1.1 | (0.3–4.9) | 0.88 | 1 | 0.17 | (0.0–1.4) | 0.10 |
| Male | 46 | 4 | 2.2 | (0.5–9.3) | 0.28 | 4 | 1.22 | (0.3–4.4) | 0.76 |
| Unknown | 47 | 1 | 0.3 | (0.0–2.3) | 0.24 | 6 | 2.66 | (0.8–9.2) | 0.12 |
| Total | 143 | 8 | 11 | ||||||
| Source | |||||||||
| Veterinary Hospital | 21 | 3 | 3.9 | (0.9–17.7) | 0.08 | 1 | 0.61 | (0.1–5.0) | 0.65 |
| Animal Shelter | 122 | 5 | 0.3 | (0.1–1.2) | 10 | 1.6393 | (0.2–13.5) | ||
| Total | 143 | 8 | 11 | ||||||
| Breed | |||||||||
| Purebred | 11 | 3 | 9.5 | (1.9–47.1) | 0.0058 ** | 0 | 0.4594 | (0.0–8.3) | 0.60 |
| Mixed | 132 | 5 | 0.1 | (0.0–0.5) | 11 | 2.177 | (0.1–39.3) | ||
| Total | 143 | 8 | 11 | ||||||
| Clinical Sign | |||||||||
| Diarrhea | 20 | 0 | 0.3 | (0.0–6.0) | 0.45 | 1 | 0.5947 | (0.1–4.9) | 0.63 |
| No notable sign | 123 | 8 | 3 | (0.2–54.3) | 10 | 1.6814 | (0.2–13.9) | ||
| Total | 143 | 8 | 11 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-H.; Liang, C.; Chi, S.-C.; Lee, K.-J.; Chou, C.-H.; Lin, C.-S.; Yang, W.-Y. An Epidemiological Assessment of Cryptosporidium and Giardia spp. Infection in Pet Animals from Taiwan. Animals 2023, 13, 3373. https://doi.org/10.3390/ani13213373
Hsu C-H, Liang C, Chi S-C, Lee K-J, Chou C-H, Lin C-S, Yang W-Y. An Epidemiological Assessment of Cryptosporidium and Giardia spp. Infection in Pet Animals from Taiwan. Animals. 2023; 13(21):3373. https://doi.org/10.3390/ani13213373
Chicago/Turabian StyleHsu, Chia-Hui, Chi Liang, Shi-Chien Chi, Kuan-Ju Lee, Chung-Hsi Chou, Chen-Si Lin, and Wen-Yuan Yang. 2023. "An Epidemiological Assessment of Cryptosporidium and Giardia spp. Infection in Pet Animals from Taiwan" Animals 13, no. 21: 3373. https://doi.org/10.3390/ani13213373
APA StyleHsu, C.-H., Liang, C., Chi, S.-C., Lee, K.-J., Chou, C.-H., Lin, C.-S., & Yang, W.-Y. (2023). An Epidemiological Assessment of Cryptosporidium and Giardia spp. Infection in Pet Animals from Taiwan. Animals, 13(21), 3373. https://doi.org/10.3390/ani13213373










