Variations in Composition, Antioxidant Profile, and Physical Traits of Goat Milk within the Semi-Intensive Production System in Mountainous Areas during the Post-Weaning to End-of-Lactation Period
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Milk Chemical Composition and Microbiological Evaluation
2.3. Determination of Fatty Acid Composition
Nutritional Indices
2.4. Total Phenolic Content and Antioxidant Profile
2.5. Milk Physicochemical Properties
2.6. Statistical Analysis
3. Results and Discussion
3.1. Farm and Flock Characteristics—Diets
3.2. Milk Yield and Composition—Milk microbiology
3.3. Milk Fatty Acid Composition and Nutritional Value
3.4. Milk Total Phenolic Content and Antioxidant Profile
3.5. Milk Physicochemical Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cifuni, G.F.; Claps, S.; Signorelli, F.; Di Francia, A.; Di Napoli, M.A. Fatty Acid and Terpenoid Profile: A Signature of Mountain Milk. Int. Dairy J. 2022, 127, 105301. [Google Scholar] [CrossRef]
- Bergamaschi, M.; Cipolat-Gotet, C.; Stocco, G.; Valorz, C.; Bazzoli, I.; Sturaro, E.; Ramanzin, M.; Bittante, G. Cheesemaking in Highland Pastures: Milk Technological Properties, Cream, Cheese and Ricotta Yields, Milk Nutrients Recovery, and Products Composition. J. Dairy Sci. 2016, 99, 9631–9646. [Google Scholar] [CrossRef]
- Liechti, K.; Biber, J.-P. Pastoralism in Europe: Characteristics and Challenges of Highland–Lowland Transhumance: -EN- -FR- Le Pastoralisme En Europe: Caractéristiques et Défis de La Transhumance de La Montagne Vers La Plaine -ES- El Pastoreo En Europa: Características y Problemas de La Trashumancia de Tierras Altas-Tierras Bajas. Rev. Sci. Tech. OIE 2016, 35, 561–575. [Google Scholar] [CrossRef]
- Zendri, F.; Ramanzin, M.; Bittante, G.; Sturaro, E. Transhumance of Dairy Cows to Highland Summer Pastures Interacts with Breed to Influence Body Condition, Milk Yield and Quality. Ital. J. Anim. Sci. 2016, 15, 481–491. [Google Scholar] [CrossRef]
- Kühl, S.; Flach, L.; Gauly, M. Economic Assessment of Small-Scale Mountain Dairy Farms in South Tyrol Depending on Feed Intake and Breed. Ital. J. Anim. Sci. 2020, 19, 41–50. [Google Scholar] [CrossRef]
- Coppa, M.; Chassaing, C.; Sibra, C.; Cornu, A.; Verbič, J.; Golecký, J.; Engel, E.; Ratel, J.; Boudon, A.; Ferlay, A.; et al. Forage System Is the Key Driver of Mountain Milk Specificity. J. Dairy Sci. 2019, 102, 10483–10499. [Google Scholar] [CrossRef] [PubMed]
- Revello Chion, A.; Tabacco, E.; Giaccone, D.; Peiretti, P.G.; Battelli, G.; Borreani, G. Variation of Fatty Acid and Terpene Profiles in Mountain Milk and “Toma Piemontese” Cheese as Affected by Diet Composition in Different Seasons. Food Chem. 2010, 121, 393–399. [Google Scholar] [CrossRef]
- Leiber, F.; Jouven, M.; Martin, B.; Priolo, A.; Coppa, M.; Prache, S.; Heckendorn, F.; Baumont, R. Potentials and Challenges for Future Sustainable Grassland Utilisation in Animal Production. Options Méditerranéennes Série A: Séminaires Méditerranéens 2014, 109, 33–48. [Google Scholar]
- Bernués, A.; Ruiz, R.; Olaizola, A.; Villalba, D.; Casasús, I. Sustainability of Pasture-Based Livestock Farming Systems in the European Mediterranean Context: Synergies and Trade-Offs. Livest. Sci. 2011, 139, 44–57. [Google Scholar] [CrossRef]
- EFSA Panel on Animal Health and Welfare (AHAW) Scientific Opinion on the Welfare Risks Related to the Farming of Sheep for Wool, Meat and Milk Production. EFSA J. 2014, 12, 3933. [CrossRef]
- Morand-Fehr, P.; Fedele, V.; Decandia, M.; Le Frileux, Y. Influence of Farming and Feeding Systems on Composition and Quality of Goat and Sheep Milk. Small Rumin. Res. 2007, 68, 20–34. [Google Scholar] [CrossRef]
- Santini, F.; Guri, F.; Gomez y Paloma, S. Labelling of Agricultural and Food Products of Mountain Farming; Publications Office of the European Union: Luxembourg, 2013. [Google Scholar]
- Strzałkowska, N.; Jóźwik, A.; Bagnicka, E.; Krzyżewski, J.; Horbańczuk, K.; Pyzel, B.; Horbańczuk, J.O. Chemical Composition, Physical Traits and Fatty Acid Profile of Goat Milk as Related to the Stage of Lactation. Anim. Sci. Pap. Rep. 2009, 27, 311–320. [Google Scholar]
- Fekadu, B.; Soryal, K.; Zeng, S.; Hekken, D.V.; Bah, B.; Villaquiran, M. Changes in Goat Milk Composition during Lactation and Their Effect on Yield and Quality of Hard and Semi-Hard Cheeses. Small Rumin. Res. 2005, 59, 55–63. [Google Scholar] [CrossRef]
- Kasapidou, E.; Karatzia, M.-A.; Mitlianga, P.; Basdagianni, Z. Effects of Production Systems and Seasons on Retail-Goat-Milk Fatty-Acid Composition and Nutritional Indices in Greece. Animals 2022, 12, 2204. [Google Scholar] [CrossRef] [PubMed]
- Peña-Avelino, L.Y.; Ceballos-Olvera, I.; Rosales-Martinez, G.N.; Hernández-Melendez, J.; Alva-Pérez, J. Milk Composition of Creole Goats Raised at Different Altitudes in an Extensive Production System in Northeast Mexico. Animals 2023, 13, 1738. [Google Scholar] [CrossRef]
- Pamukova, D.; Naydenova, N.; Mihaylova, G. Fatty Acid Profile and Healthy Lipid Indices of Bulgarian Goat Milk from Breeds, Pasture-Raised in a Mountain Region. Trakia J. Sci. 2018, 16, 313–319. [Google Scholar] [CrossRef]
- Delgado-Pertíñez, M.; Gutiérrez-Peña, R.; Mena, Y.; Fernández-Cabanás, V.M.; Laberye, D. Milk Production, Fatty Acid Composition and Vitamin E Content of Payoya Goats According to Grazing Level in Summer on Mediterranean Shrublands. Small Rumin. Res. 2013, 114, 167–175. [Google Scholar] [CrossRef]
- Margatho, G.; Rodríguez-Estévez, V.; Medeiros, L.; Simões, J. Seasonal Variation of Serrana Goat Milk Contents in Mountain Grazing System for Cheese Manufacture. Rev. Méd. Vét. 2018, 169, 157–165. [Google Scholar]
- Nordic Centre for Spatial Development. Mountain Areas in Europe: Analysis of Mountain Areas in EU Member States, Acceding and Other European Countries; Nordregio report/Nordic Centre for Spatial Development; Nordregio: Stockholm, Sweden, 2004; ISBN 978-91-89332-35-5. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- ISO 15884:2002; IDF 182:2002—Milk Fat—Preparation of Fatty Acid Methyl Esters. International Organization for Standardization: Geneva, Switzerland, 2002.
- Hanuš, O.; Samková, E.; Křížová, L.; Hasoňová, L.; Kala, R. Role of Fatty Acids in Milk Fat and the Influence of Selected Factors on Their Variability—A Review. Molecules 2018, 23, 1636. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef] [PubMed]
- Slinkard, K.; Singleton, V.L. Total Phenol Analysis: Automation and Comparison with Manual Methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A Procedure to Measure the Antiradical Efficiency of Polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant Activity of Dietary Polyphenols As Determined by a Modified Ferric Reducing/Antioxidant Power Assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Tsiouni, M.; Aggelopoulos, S.; Pavloudi, A.; Siggia, D. Economic and Financial Sustainability Dependency on Subsidies: The Case of Goat Farms in Greece. Sustainability 2021, 13, 7441. [Google Scholar] [CrossRef]
- Pappa, E.C.; Kondyli, E.; Sotirakoglou, K.; Bosnea, L.; Mataragas, M.; Allouche, L.; Tsiplakou, E.; Pappas, A.C. Farmers Profile and Characterization of Sheep and Goat Dairy Chain in Northwestern Greece. Sustainability 2021, 13, 833. [Google Scholar] [CrossRef]
- Gelasakis, A.I.; Rose, G.; Giannakou, R.; Valergakis, G.E.; Theodoridis, A.; Fortomaris, P.; Arsenos, G. Typology and Characteristics of Dairy Goat Production Systems in Greece. Livest. Sci. 2017, 197, 22–29. [Google Scholar] [CrossRef]
- Chebli, Y.; El Otmani, S.; Hornick, J.-L.; Keli, A.; Bindelle, J.; Chentouf, M.; Cabaraux, J.-F. Using GPS Collars and Sensors to Investigate the Grazing Behavior and Energy Balance of Goats Browsing in a Mediterranean Forest Rangeland. Sensors 2022, 22, 781. [Google Scholar] [CrossRef]
- Soryal, K.A.; Zeng, S.S.; Min, B.R.; Hart, S.P.; Beyene, F.A. Effect of Feeding Systems on Composition of Goat Milk and Yield of Domiati Cheese. Small Rumin. Res. 2004, 54, 121–129. [Google Scholar] [CrossRef]
- Hellenic Agricultural Organisation. Milk Quality-Location-Month. Available online: https://www.elgo.gr/images/ELOGAK_files/POIOTHTA/QUALITY_LOCATION_%CE%9C%CE%9F%CE%9D%CE%A4%CE%97_2022.xls (accessed on 22 July 2023).
- Ataşoğlu, C.; Uysal-Pala, Ç.; Karagül-Yüceer, Y. Changes in Milk Fatty Acid Composition of Goats during Lactation in a Semi-Intensive Production System. Arch. Anim. Breed. 2009, 52, 627–636. [Google Scholar] [CrossRef]
- Pulina, G.; Milán, M.J.; Lavín, M.P.; Theodoridis, A.; Morin, E.; Capote, J.; Thomas, D.L.; Francesconi, A.H.D.; Caja, G. Invited Review: Current Production Trends, Farm Structures, and Economics of the Dairy Sheep and Goat Sectors. J. Dairy Sci. 2018, 101, 6715–6729. [Google Scholar] [CrossRef]
- Lock, A.L.; Garnsworthy, P.C. Seasonal Variation in Milk Conjugated Linoleic Acid and Δ9-Desaturase Activity in Dairy Cows. Livest. Prod. Sci. 2003, 79, 47–59. [Google Scholar] [CrossRef]
- Sandrucci, A.; Bava, L.; Tamburini, A.; Gislon, G.; Zucali, M. Management Practices and Milk Quality in Dairy Goat Farms in Northern Italy. Ital. J. Anim. Sci. 2019, 18, 1–12. [Google Scholar] [CrossRef]
- Lianou, D.T.; Michael, C.K.; Vasileiou, N.G.C.; Liagka, D.V.; Mavrogianni, V.S.; Caroprese, M.; Fthenakis, G.C. Association of Breed of Sheep or Goats with Somatic Cell Counts and Total Bacterial Counts of Bulk-Tank Milk. Appl. Sci. 2021, 11, 7356. [Google Scholar] [CrossRef]
- Kondyli, E.; Svarnas, C.; Samelis, J.; Katsiari, M.C. Chemical Composition and Microbiological Quality of Ewe and Goat Milk of Native Greek Breeds. Small Rumin. Res. 2012, 103, 194–199. [Google Scholar] [CrossRef]
- Žan, M.; Stibilj, V.; Rogelj, I. Milk Fatty Acid Composition of Goats Grazing on Alpine Pasture. Small Rumin. Res. 2006, 64, 45–52. [Google Scholar] [CrossRef]
- Decandia, M.; Cabiddu, A.; Molle, G.; Branca, A.; Epifani, G.; Pintus, S.; Tavera, F.; Piredda, G.; Pinna, G.; Addis, M. Effect of Different Feeding Systems on Fatty Acid Composition and Volatile Compound Content in Goat Milk. Options Méditérr. 2007, 74, 129–134. [Google Scholar]
- Chilliard, Y.; Toral, P.G.; Shingfield, K.J.; Rouel, J.; Leroux, C.; Bernard, L. Effects of Diet and Physiological Factors on Milk Fat Synthesis, Milk Fat Composition and Lipolysis in the Goat: A Short Review. Small Rumin. Res. 2014, 122, 31–37. [Google Scholar] [CrossRef]
- Alipanahi, Z.; Fatahnia, F.; Jafari, H.; Taasoli, G.; Mirzaei-Alamouti, H.; Barrett, D.; Pormalekshahi, A. Effect of Oak Acorn with or without Polyethylene Glycol in Diets Containing Extruded Soybean on Milk Fatty Acid Profile, Ruminal Fermentation and Plasma Metabolites of Lactating Goats. Livest. Sci. 2019, 221, 57–62. [Google Scholar] [CrossRef]
- Ayeb, N.; Addis, M.; Fiori, M.; Khorchani, S.; Atigui, M.; Khorchani, T. Quality and Fatty Acid Profile of the Milk of Indigenous Goats Subjected to Different Local Diets in Tunisian Arid Lands. J. Anim. Physiol. Anim. Nutr. 2016, 100, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Paszczyk, B.; Łuczyńska, J. The Comparison of Fatty Acid Composition and Lipid Quality Indices in Hard Cow, Sheep, and Goat Cheeses. Foods 2020, 9, 1667. [Google Scholar] [CrossRef] [PubMed]
- Sinanoglou, V.J.; Koutsouli, P.; Fotakis, C.; Sotiropoulou, G.; Cavouras, D.; Bizelis, I. Assessment of Lactation Stage and Breed Effect on Sheep Milk Fatty Acid Profile and Lipid Quality Indices. Dairy Sci. Technol. 2015, 95, 509–531. [Google Scholar] [CrossRef]
- Basdagianni, Z.; Papaloukas, L.; Kyriakou, G.; Karaiskou, C.; Parissi, Z.; Sinapis, E.; Kasapidou, E. A Comparative Study of the Fatty Acid and Terpene Profiles of Ovine and Caprine Milk from Greek Mountain Sheep Breeds and a Local Goat Breed Raised under a Semi-Extensive Production System. Food Chem. 2019, 278, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Barlowska, J.; Pastuszka, R.; Domaradzki, P.; Król, J.; Brodziak, A.; Teter, A.; Rysiak, A. Fat Dispersion and Fatty Acid Profile, Including Health Indicators in Goat Milk from Different Flora Composition of Grazing Sites. Anim. Sci. Pap. Rep. 2019, 37, 4. [Google Scholar]
- Bodnár, Á.; Egerszegi, I.; Kuchtik, J.; Penksza, K.; Póti, P.; Pajor, F. Effect of Grazing on Composition, Fatty Acid Profile and Nutritional Indices of the Goat Milk and Cheese. J. Anim. Feed Sci. 2021, 30, 320–328. [Google Scholar] [CrossRef]
- Cardiovascular Review Group—Great Britain. Department of Health. Nutritional Aspects of Cardiovascular Disease; HMSO: London, UK, 1994. [Google Scholar]
- Gibson, R.A.; Makrides, M.; Smithers, L.G.; Voevodin, M.; Sinclair, A.J. The Effect of Dairy Foods on CHD: A Systematic Review of Prospective Cohort Studies. Br. J. Nutr. 2009, 102, 1267–1275. [Google Scholar] [CrossRef]
- Chávez-Servín, J.L.; Andrade-Montemayor, H.M.; Velázquez Vázquez, C.; Aguilera Barreyro, A.; García-Gasca, T.; Ferríz Martínez, R.A.; Olvera Ramírez, A.M.; de la Torre-Carbot, K. Effects of Feeding System, Heat Treatment and Season on Phenolic Compounds and Antioxidant Capacity in Goat Milk, Whey and Cheese. Small Rumin. Res. 2018, 160, 54–58. [Google Scholar] [CrossRef]
- Cabiddu, A.; Delgadillo-Puga, C.; Decandia, M.; Molle, G. Extensive Ruminant Production Systems and Milk Quality with Emphasis on Unsaturated Fatty Acids, Volatile Compounds, Antioxidant Protection Degree and Phenol Content. Animals 2019, 9, 771. [Google Scholar] [CrossRef] [PubMed]
- Delgadillo-Puga, C.; Cuchillo-Hilario, M.; León-Ortiz, L.; Ramírez-Rodríguez, A.; Cabiddu, A.; Navarro-Ocaña, A.; Morales-Romero, A.M.; Medina-Campos, O.N.; Pedraza-Chaverri, J. Goats’ Feeding Supplementation with Acacia Farnesiana Pods and Their Relationship with Milk Composition: Fatty Acids, Polyphenols, and Antioxidant Activity. Animals 2019, 9, 515. [Google Scholar] [CrossRef]
- Amrit, B.; Ponnampalam, E.N.; Macwan, S.; Wu, H.; Aziz, A.; Muir, S.; Dunshea, F.R.; Suleria, H.A.R. Comprehensive Screening and Characterization of Polyphenol Compounds from Pasture Grasses Used for Livestock Production under Temperate Region. Anim. Feed Sci. Technol. 2023, 300, 115657. [Google Scholar] [CrossRef]
- Vázquez, C.V.; Rojas, M.G.V.; Ramírez, C.A.; Chávez-Servín, J.L.; García-Gasca, T.; Ferriz Martínez, R.A.; García, O.P.; Rosado, J.L.; López-Sabater, C.M.; Castellote, A.I.; et al. Total Phenolic Compounds in Milk from Different Species. Design of an Extraction Technique for Quantification Using the Folin–Ciocalteu Method. Food Chem. 2015, 176, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, A.; Frazier, R.A. Characterization of Protein–Polyphenol Interactions. Trends Food Sci. Technol. 2004, 15, 186–190. [Google Scholar] [CrossRef]
- Mal, G.; Singh, B.; Mane, B.G.; Sharma, V.; Sharma, R.; Bhar, R.; Dhar, J.B. Milk Composition, Antioxidant Activities and Protein Profile of Gaddi Goat Milk. J. Food Biochem. 2018, 42, e12660. [Google Scholar] [CrossRef]
- Di Trana, A.; Bonanno, A.; Cecchini, S.; Giorgio, D.; Di Grigoli, A.; Claps, S. Effects of Sulla Forage (Sulla Coronarium L.) on the Oxidative Status and Milk Polyphenol Content in Goats. J. Dairy Sci. 2015, 98, 37–46. [Google Scholar] [CrossRef]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z.; Scheerens, J.C.; Miller, A.R. Modified 2,2-Azino-Bis-3-Ethylbenzothiazoline-6-Sulfonic Acid (ABTS) Method to Measure Antioxidant Capacity of Selected Small Fruits and Comparison to Ferric Reducing Antioxidant Power (FRAP) and 2,2‘-Diphenyl-1-Picrylhydrazyl (DPPH) Methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for Antioxidant Assays for Food Components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
- Park, Y.W.; Juárez, M.; Ramos, M.; Haenlein, G.F.W. Physico-Chemical Characteristics of Goat and Sheep Milk. Small Rumin. Res. 2007, 68, 88–113. [Google Scholar] [CrossRef]
- Foschino, R.; Invernizzi, A.; Barucco, R.; Stradiotto, K. Microbial Composition, Including the Incidence of Pathogens, of Goat Milk from the Bergamo Region of Italy during a Lactation Year. J. Dairy Res. 2002, 69, 213–225. [Google Scholar] [CrossRef]
- Barlowska, J.; Pastuszka, R.; Król, J.; Brodziak, A.; Teter, A.; Litwinczuk, Z. Differences in Physico-Chemical Parameters of Goat Milk Depending on Breed Type,Physiological and Environmental Factors. Turk. J. Vet. Anim. Sci. 2020, 44, 720–728. [Google Scholar] [CrossRef]
- Kandeel, S.A.; Megahed, A.A.; Ebeid, M.H.; Constable, P.D. Ability of Milk pH to Predict Subclinical Mastitis and Intramammary Infection in Quarters from Lactating Dairy Cattle. J. Dairy Sci. 2019, 102, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Mabrook, M.F.; Petty, M.C. Effect of Composition on the Electrical Conductance of Milk. J. Food Eng. 2003, 60, 321–325. [Google Scholar] [CrossRef]
- Fox, P.F.; Uniacke-Lowe, T.; McSweeney, P.L.H.; O’Mahony, J.A. Physical Properties of Milk. In Dairy Chemistry and Biochemistry; Fox, P.F., Uniacke-Lowe, T., McSweeney, P.L.H., O’Mahony, J.A., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 321–343. ISBN 978-3-319-14892-2. [Google Scholar]
- Voutsinas, L.; Pappas, C.; Katsiari, M. The Composition of Alpine Goats’ Milk during Lactation in Greece. J. Dairy Res. 1990, 57, 41–51. [Google Scholar] [CrossRef]
- Stergiadis, S.; Nørskov, N.P.; Purup, S.; Givens, I.; Lee, M.R.F. Comparative Nutrient Profiling of Retail Goat and Cow Milk. Nutrients 2019, 11, 2282. [Google Scholar] [CrossRef]
- Norberg, E.; Hogeveen, H.; Korsgaard, I.R.; Friggens, N.C.; Sloth, K.H.M.N.; Løvendahl, P. Electrical Conductivity of Milk: Ability to Predict Mastitis Status. J. Dairy Sci. 2004, 87, 1099–1107. [Google Scholar] [CrossRef]
- Haenlein, G.F.W. Relationship of Somatic Cell Counts in Goat Milk to Mastitis and Productivity. Small Rumin. Res. 2002, 45, 163–178. [Google Scholar] [CrossRef]
- Tangorra, F.M.; Zaninelli, M.; Costa, A.; Agazzi, A.; Savoini, G. Milk Electrical Conductivity and Mastitis Status in Dairy Goats: Results from a Pilot Study. Small Rumin. Res. 2010, 90, 109–113. [Google Scholar] [CrossRef]
- Moore, D.A.; Taylor, J.; Hartman, M.L.; Sischo, W.M. Quality Assessments of Waste Milk at a Calf Ranch. J. Dairy Sci. 2009, 92, 3503–3509. [Google Scholar] [CrossRef]
- Kljajevic, N.V.; Tomasevic, I.B.; Miloradovic, Z.N.; Nedeljkovic, A.; Miocinovic, J.B.; Jovanovic, S.T. Seasonal Variations of Saanen Goat Milk Composition and the Impact of Climatic Conditions. J. Food Sci. Technol. 2018, 55, 299–303. [Google Scholar] [CrossRef]
- Greek Government. Code of Foodstuffs, Beverages and Objects of Common Use—Part A, Foodstuffs and Beverages, Article 80 (Types of Milk); Greek Government Official Publication: Athens, Greece, 2016.
- Janštová, B.; Dračková, M.; Navrátilová, P.; Hadra, L.; Vorlová, L. Freezing Point of Raw and Heat-Treated Goat Milk. Czech J. Anim. Sci. 2007, 52, 394–398. [Google Scholar] [CrossRef]
- Raynal-Ljutovac, K.; Gaborit, P.; Lauret, A. The Relationship between Quality Criteria of Goat Milk, Its Technological Properties and the Quality of the Final Products. Small Rumin. Res. 2005, 60, 167–177. [Google Scholar] [CrossRef]
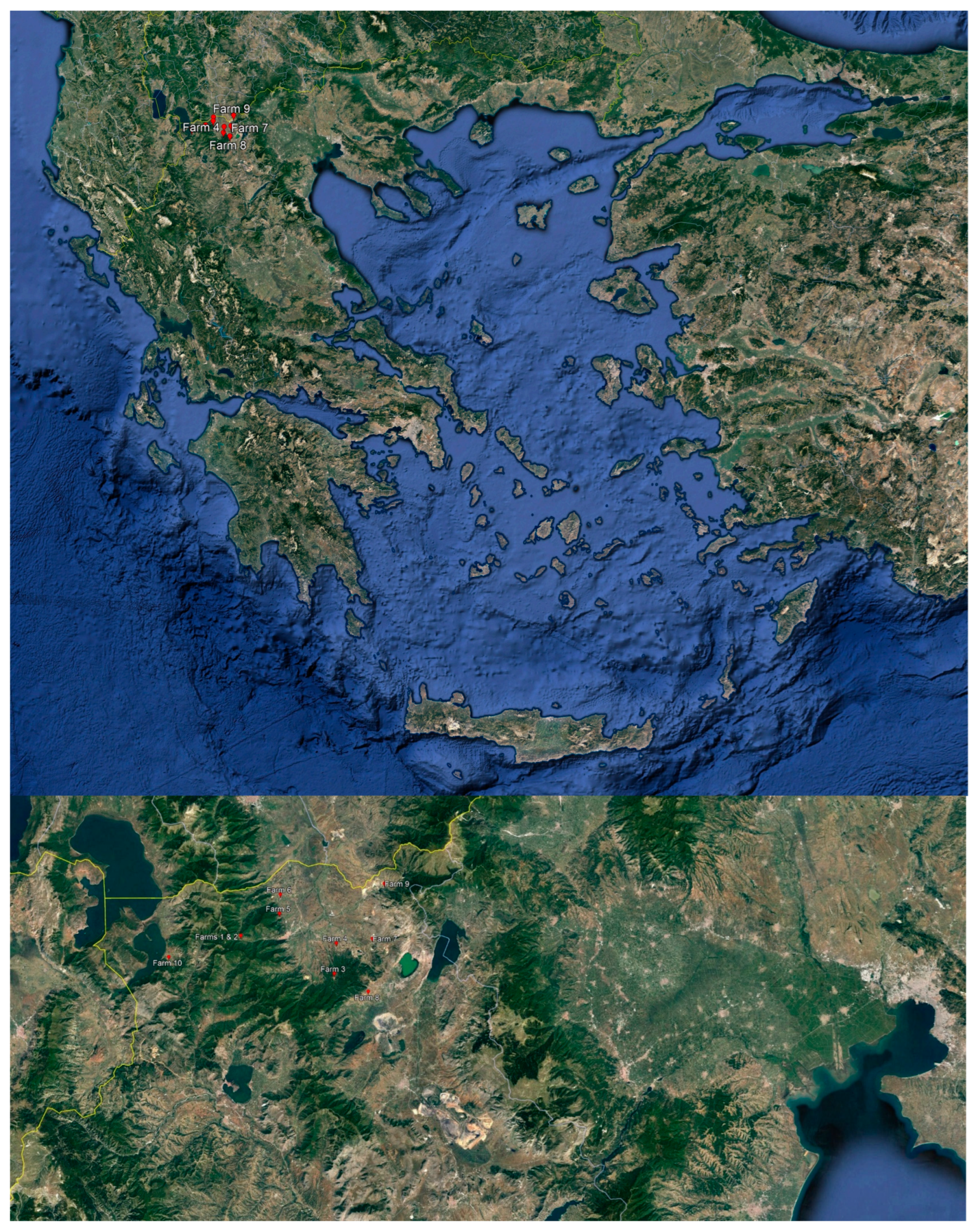

| Farm No | Altitude (m) | Number of Goats in the Flock | Average Daily Milk Yield (kg) | Breed of Goats |
|---|---|---|---|---|
| 1 | 989 | 120 | 110.00 | Damascus |
| 2 | 989 | 130 | 207.23 | Damascus |
| 3 | 777 | 200 | 149.58 | Indigenous Greek (Capra prisca) |
| 4 | 673 | 250 | 412.93 | Indigenous Greek (Capra prisca), Alpine, Damascus |
| 5 | 679 | 150 | 145.13 | Cross breeds (Indigenous Greek (Capra prisca), Alpine, Damascus) |
| 6 | 618 | 130 | 174.57 | Indigenous Greek (Capra prisca), Damascus |
| 7 | 732 | 150 | 122.23 | Indigenous Greek (Capra prisca) |
| 8 | 643 | 90 | 98.36 | Indigenous Greek (Capra prisca), Alpine |
| 9 | 753 | 150 | 108.80 | Indigenous Greek (Capra prisca), Alpine, Damascus |
| 10 | 869 | 140 | 126.38 | Indigenous Greek (Capra prisca) |
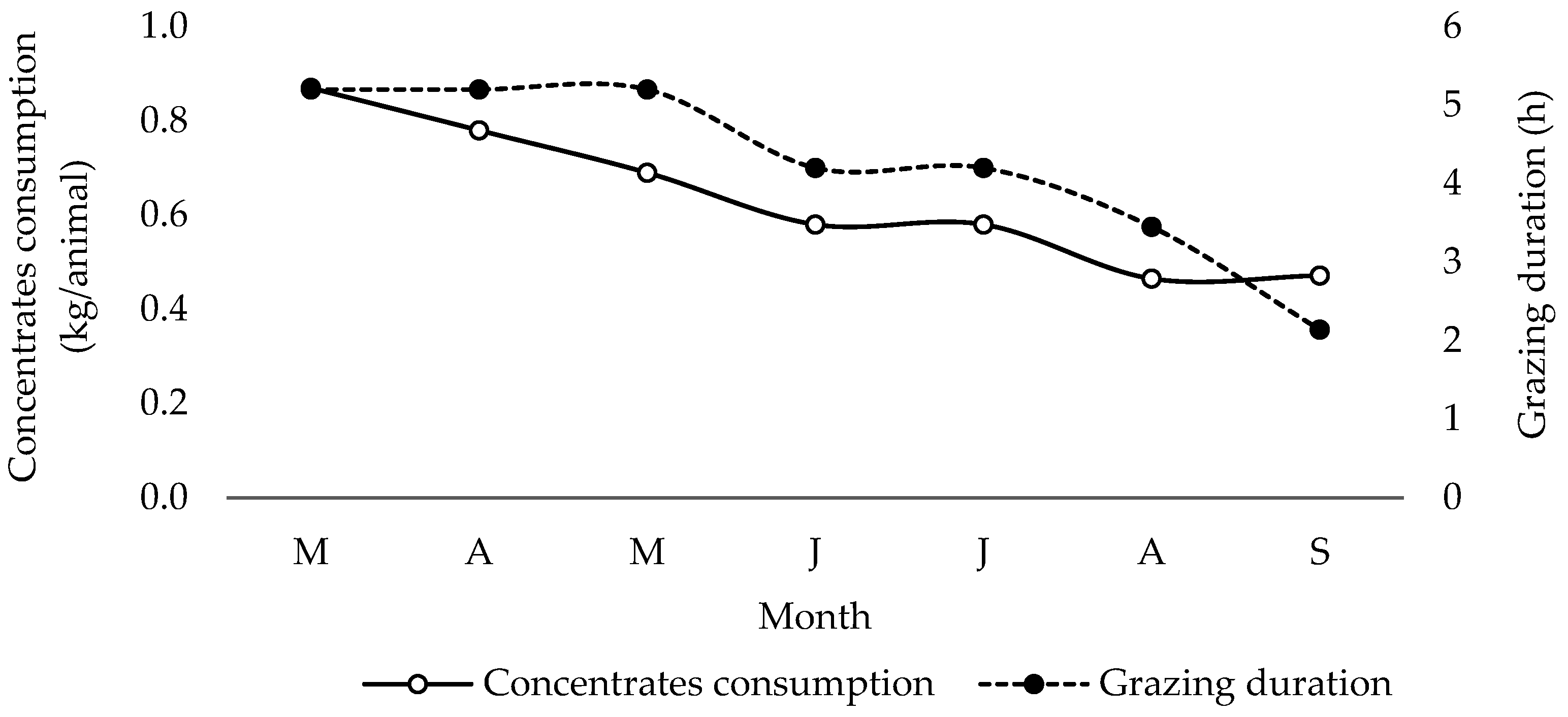
| Farm No | Concentrates (Description) | Average Daily Intake (Concentrates) (kg) | Forage (Description) | Average Grazing Duration (h) |
|---|---|---|---|---|
| 1 | Commercial pellet for dairy goats | 0.59 | Oak, rowan, low bushes (kermes oak) wild vetch, tree spurge | 4.23 |
| 2 | Commercial pellet for dairy goats | 0.65 | Oak, rowan, low bushes (kermes oak) wild vetch, tree spurge | 3.31 |
| 3 | Corn, straw, grassland grasses | 0.58 | Oak, rowan, low bushes (kermes oak) wild vetch, tree spurge | 3.42 |
| 4 | Commercial concentrate | 0.89 | Corn stalks, oats | 3.13 |
| 5 | Commercial concentrate | 0.57 | Oak, rowan, low bushes (kermes oak) wild vetch, tree spurge | 3.87 |
| 6 | Commercial concentrate | 0.64 | Oak, rowan, low bushes (kermes oak) wild vetch, tree spurge | 3.21 |
| 7 | Corn, straw, grassland grasses | 0.57 | Oak, rowan, low bushes (kermes oak) wild vetch, tree spurge | 5.08 |
| 8 | Corn, straw, grassland grasses | 0.66 | Oak, rowan, low bushes (kermes oak) wild vetch, tree spurge | 5.79 |
| 9 | Commercial concentrate | 0.55 | Oak, rowan, low bushes (kermes oak) wild vetch, tree spurge | 4.67 |
| 10 | Corn, beans, straw, grassland grasses, alfalfa | 0.67 | Oak, rowan, low bushes (kermes oak) wild vetch, tree spurge | 6.08 |
| Variable | Sampling Month | SEM | Significance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| March (n = 20) | April (n = 20) | May (n = 20) | June (n = 20) | July (n = 20) | August (n = 20) | September (n = 14) | |||
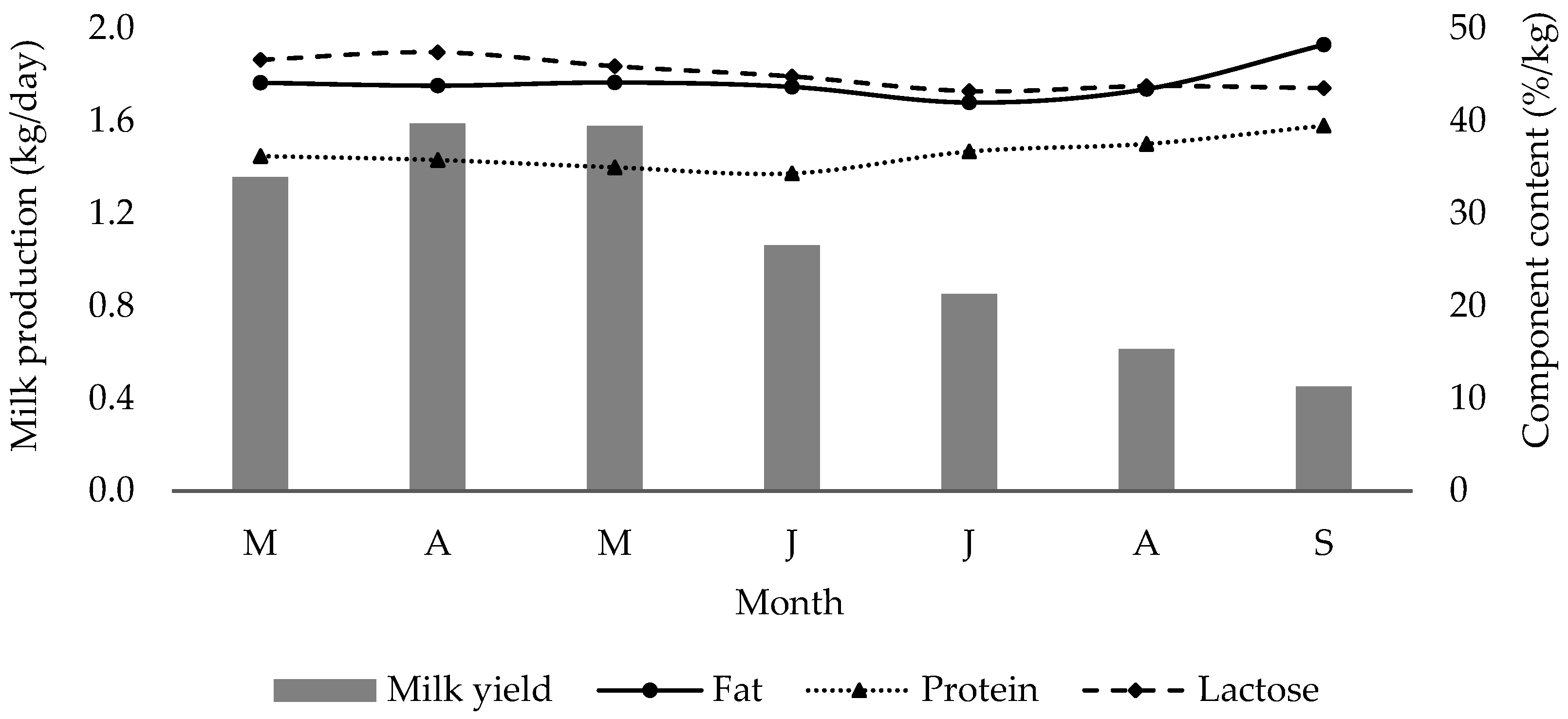
| Farm milk yield (kg/day) | 211.00 b | 246.10 b,c | 248.90 c | 163.50 | 130.50 a,b | 92.50 a | 68.57 a | 308.260 | *** |
| Fat (%) | 4.28 a | 4.25 a | 4.28 a | 4.21 a | 4.05 a | 4.18 a | 4.64 b | 0.714 | *** |
| Protein (%) | 3.51 a,b | 3.47 a,b | 3.39 a,b | 3.31 a | 3.54 | 3.61 b | 3.80 c | 4.602 | *** |
| Lactose (%) | 4.52 c,d | 4.60 c,d | 4.45 c | 4.32 b,c | 4.17 a,b | 4.21 a,b | 4.19 a,b | 0.755 | *** |
| Total solids (%) | 13.23 a | 13.15 a | 13.38 a,b | 14.70 c | 14.31 c | 15.18 c | 15.73 c | 4.305 | *** |
| Solid non-fat (%) | 9.10 c | 8.82 b | 8.74 a,b | 8.52 a | 8.59 a,b | 8.69 a,b | 8.77 a,b | 0.846 | *** |
| Fat: protein ratio | 1.22 | 1.23 | 1.26 c | 1.27 c | 1.16 a | 1.16 a,b | 1.23 | 0.206 | *** |
| TVC (log cfu/mL) | 2.44 b | 2.41 | 2.37 | 2.32 | 2.34 | 2.26 | 2.13 a | 0.418 | NS |
| Variable | Sampling Month | SEM | Significance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| March (n = 20) | April (n = 20) | May (n = 20) | June (n = 20) | July (n = 20) | August (n = 20) | September (n = 14) | |||
| Fatty acid | |||||||||
| C4:0 | 1.16 | 1.23 | 1.25 | 1.25 | 1.33 | 1.29 | 1.17 | 0.272 | NS |
| C6:0 | 1.57 | 1.68 | 1.63 | 1.58 | 1.61 | 1.62 | 1.52 | 0.209 | NS |
| C8:0 | 2.29 a,b | 2.42 b | 2.32 a,b | 2.17 a | 2.14 a | 2.18 a | 2.15 a | 0.458 | *** |
| C10:0 | 9.32 b | 9.12 b | 8.63 | 8.07 a | 8.03 a | 8.19 a | 8.52 | 2.264 | *** |
| C11:0 | 0.17 | 0.19 | 0.19 | 0.33 | 0.17 | 0.29 | 0.12 | 0.310 | NS |
| C12:0 | 4.84 c | 4.03 b | 3.68 a,b | 3.20 a | 3.41 a,b | 3.51 a,b | 4.12 b | 2.438 | *** |
| C13:0 | 1.22 | 1.24 | 1.16 | 1.57 | 1.06 | 1.39 | 0.84 | 0.958 | NS |
| C14:0 | 10.66 b | 9.09 a | 8.95 a | 8.37 a | 9.23 a | 9.06 a | 9.91 a,b | 3.257 | *** |
| C14:1 | 0.40 b | 0.30 a | 0.27 a | 0.31 a | 0.29 a | 0.33 a,b | 0.33 a,b | 0.190 | *** |
| C15:0 | 0.93 | 0.84 | 0.88 | 0.90 | 0.93 | 0.88 | 0.87 | 0.143 | NS |
| C15:1 | 0.27 | 0.25 | 0.24 | 1.60 | 0.23 | 1.44 | 0.19 | 2.743 | NS |
| C16:0 | 28.13 | 25.62 | 25.33 | 25.10 | 26.92 | 25.59 | 27.10 | 4.980 | NS |
| C16:1 | 0.41 c | 0.40 b,c | 0.38 | 0.38 | 0.34 | 0.34 a,b | 0.31 a | 0.154 | ** |
| C17:0 | 0.68 a | 0.70 a | 0.78 | 0.84 b | 0.86 b | 0.77 | 0.75 | 0.295 | *** |
| C17:1 | 0.30 | 0.25 | 0.24 | 1.00 | 0.27 | 0.92 | 0.25 | 1.509 | NS |
| C18:0 | 9.02 a | 11.95 b,c | 13.90 b,c | 13.57 b,c | 13.58 b,c | 12.44 c | 12.12 c | 7.439 | *** |
| C18:1 trans | 0.89 | 1.09 | 0.94 | 0.96 | 1.04 | 0.89 | 0.90 | 0.340 | NS |
| C18:1 trans-11 (VA) | 0.71 | 1.15 | 1.12 | 2.21 | 1.08 | 2.17 | 0.95 | 2.623 | NS |
| C18:1 cis-9 | 23.51 | 23.99 | 22.86 | 21.42 | 22.94 | 22.23 | 23.41 | 3.785 | NS |
| C18:2 n-6 trans | 0.55 | 0.90 | 0.92 | 0.96 | 0.67 | 0.77 | 0.64 | 0.694 | NS |
| C18:2 n-6 cis | 1.92 | 1.99 | 2.31 b | 2.20 | 2.09 | 1.94 | 1.80 a | 0.738 | ** |
| C18:3 n-3 | 0.64 | 0.84 | 1.23 | 1.28 | 1.12 | 0.94 | 1.09 | 0.991 | *** |
| C18:2 cis-9 trans-11 (CLA) | 0.79 | 0.94 | 0.99 | 0.97 | 0.84 | 1.00 | 0.99 | 0.311 | NS |
| Lipid class | |||||||||
| SFA 1 | 69.99 | 68.09 | 68.70 | 66.94 | 69.29 | 67.21 | 69.19 | 4.945 | NS |
| MUFA 2 | 26.49 | 27.42 | 26.04 | 27.87 | 26.19 | 28.32 | 26.34 | 3.989 | NS |
| PUFA 3 | 3.52 a | 4.49 b | 5.26 d | 5.19 c,d | 4.52 b,c | 4.47 b | 4.47 b,c | 2.559 | *** |
| UFA 4 | 30.01 | 31.91 | 31.30 | 33.06 | 30.71 | 32.79 | 30.81 | 4.945 | NS |
| OCFA 5 | 3.40 | 3.28 | 3.30 | 5.91 | 3.35 | 5.41 | 2.90 | 5.193 | NS |
| n-3 | 0.57 a | 0.84 b | 1.23 c | 1.28 c | 1.12 c | 0.94 b | 1.09 b,c | 1.097 | *** |
| n-6 | 2.47 a | 2.89 | 3.23 c | 3.04 b,c | 2.77 | 2.62 a,b | 2.46 a | 1.251 | *** |
| Δ9-desaturase activity | |||||||||
| DI14 | 0.04 | 0.03 | 0.03 | 0.07 | 0.03 | 0.07 | 0.03 | 0.078 | NS |
| DI16 | 0.01 | 0.02 | 0.01 | 0.05 | 0.01 | 0.04 | 0.01 | 0.066 | NS |
| DI18 | 0.72 c | 0.67 b,c | 0.62 a,b | 0.60 a | 0.63 a,b | 0.63 a,b | 0.66 b | 0.180 | *** |
| Index | Sampling Month | SEM | Significance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| March (n = 20) | April (n = 20) | May (n = 20) | June (n = 20) | July (n = 20) | August (n = 20) | September (n = 14) | |||
| AI 1 | 2.60 b | 2.13 a | 2.14 a | 2.06 | 2.25 | 2.22 | 2.41 | 0.815 | ** |
| TI 2 | 2.95 | 2.61 | 2.59 | 2.52 | 2.74 | 2.70 | 2.74 | 0.621 | NS |
| h/H 3 | 0.69 | 0.80 | 0.80 | 1.19 | 0.75 | 0.96 | 0.73 | 0.751 | NS |
| PUFA/SFA 4 | 0.05 a | 0.07 b | 0.08 b | 0.08 c | 0.07 b | 0.07 b | 0.06 b | 0.041 | *** |
| Variable | Sampling Month | SEM | Significance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| March (n = 20) | April (n = 20) | May (n = 20) | June (n = 20) | July (n = 20) | August (n = 20) | September (n = 14) | |||
| TPC (mg GAE/mL) 1 | 1.10 a | 1.20 a | 1.15 a | 1.10 a | 1.18 a | 1.24 | 1.39 b | 0.406 | *** |
| DPPH (μM TE/mL) 2 | 21.10 | 23.54 b,c | 22.99 | 20.15 a,b | 19.41 a | 20.89 | 24.68 c | 8.142 | ** |
| FRAP (μM TE/mL) 2 | 34.42 a | 38.43 b | 45.98 | 37.38 | 39.24 | 37.97 | 34.63 | 16.850 | * |
| ABTS (μM TE/mL) 2 | 586.95 b,c | 490.77 a | 505.19 a,b | 530.93 | 507.93 a,b | 503.39 a,b | 625.86 c | 208.197 | *** |
| Variable | Sampling Month | SEM | Significance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| March (n = 20) | April (n = 20) | May (n = 20) | June (n = 20) | July (n = 20) | August (n = 20) | September (n = 14) | |||
| pH | 6.75 b | 6.72 | 6.68 a | 6.68 a | 6.70 | 6.72 | 6.69 a | 0.113 | *** |
| Electrical conductivity (mS/cm) | 5.22 a | 5.18 a | 5.28 a | 5.58 b | 5.81 b | 5.75 b | 5.78 b | 1.222 | *** |
| Refractive index | 1.3479 | 1.3478 | 1.3481 | 1.3481 | 1.3476 a | 1.3482 | 1.3487 b | 0.001 | * |
| Brix (°Bx) | 10.06 | 9.98 | 10.18 | 10.21 | 9.84 a | 10.28 | 10.54 b | 0.936 | * |
| Density (g/mL) | 1.035 | 1.035 | 1.035 | 1.034 | 1.035 | 1.035 | 1.038 | 0.005 | NS |
| FDP (−°C) | 0.551 | 0.548 | 0.543 | 0.543 | 0.547 | 0.551 | 0.553 | 0.017 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasapidou, E.; Iliadis, I.-V.; Mitlianga, P.; Papatzimos, G.; Karatzia, M.-A.; Papadopoulos, V.; Amanatidis, M.; Tortoka, V.; Tsiftsi, E.; Aggou, A.; et al. Variations in Composition, Antioxidant Profile, and Physical Traits of Goat Milk within the Semi-Intensive Production System in Mountainous Areas during the Post-Weaning to End-of-Lactation Period. Animals 2023, 13, 3505. https://doi.org/10.3390/ani13223505
Kasapidou E, Iliadis I-V, Mitlianga P, Papatzimos G, Karatzia M-A, Papadopoulos V, Amanatidis M, Tortoka V, Tsiftsi E, Aggou A, et al. Variations in Composition, Antioxidant Profile, and Physical Traits of Goat Milk within the Semi-Intensive Production System in Mountainous Areas during the Post-Weaning to End-of-Lactation Period. Animals. 2023; 13(22):3505. https://doi.org/10.3390/ani13223505
Chicago/Turabian StyleKasapidou, Eleni, Iraklis-Vasileios Iliadis, Paraskevi Mitlianga, Georgios Papatzimos, Maria-Anastasia Karatzia, Vasileios Papadopoulos, Michail Amanatidis, Vasiliki Tortoka, Ekaterini Tsiftsi, Antonia Aggou, and et al. 2023. "Variations in Composition, Antioxidant Profile, and Physical Traits of Goat Milk within the Semi-Intensive Production System in Mountainous Areas during the Post-Weaning to End-of-Lactation Period" Animals 13, no. 22: 3505. https://doi.org/10.3390/ani13223505
APA StyleKasapidou, E., Iliadis, I.-V., Mitlianga, P., Papatzimos, G., Karatzia, M.-A., Papadopoulos, V., Amanatidis, M., Tortoka, V., Tsiftsi, E., Aggou, A., & Basdagianni, Z. (2023). Variations in Composition, Antioxidant Profile, and Physical Traits of Goat Milk within the Semi-Intensive Production System in Mountainous Areas during the Post-Weaning to End-of-Lactation Period. Animals, 13(22), 3505. https://doi.org/10.3390/ani13223505








