Effects of Two Different Straw Pellets on Yak Growth Performance and Ruminal Microbiota during Cold Season
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Experimental Design, and Feeding
2.2. Assessment Growth Performance
2.3. Sample Collection
2.4. Feed Analysis
2.5. Determination of Rumen Fermentation Parameters
2.6. DNA Extraction and Analysis of Bacterial Community in Rumen
2.7. Bioinformatic Analysis
2.8. Statistical Analyses
3. Results
3.1. Growth Performance of Yaks
3.2. Parameters of Rumen Fermentation
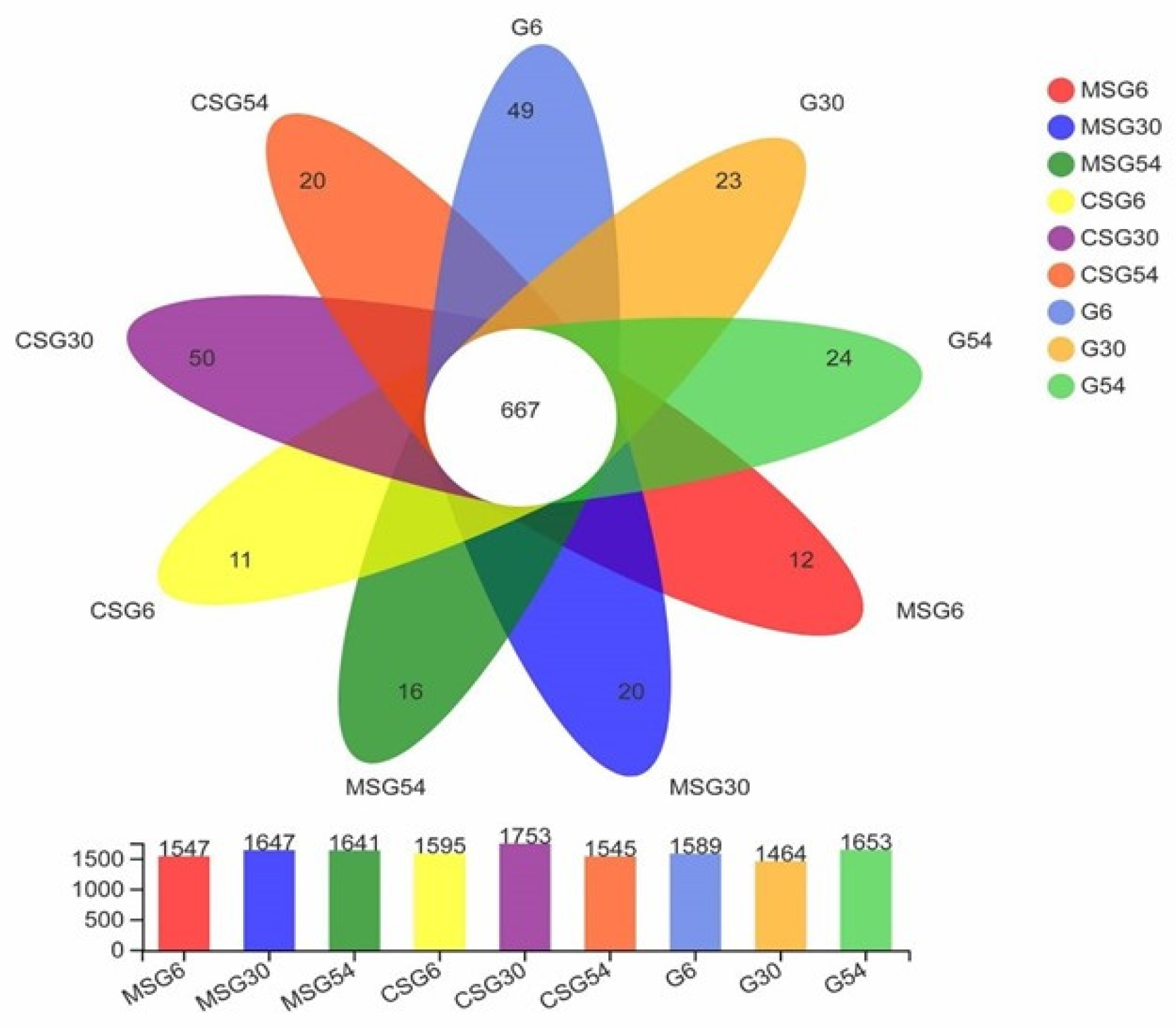
3.3. Sequencing of the Ruminal Microbiota
3.4. Alpha Diversity of Rumen Microbiota
3.5. Beta Diversity of Rumen Microbiota
3.6. The Variation in the Rumen Microbiota
3.7. Correlations of Microbial Communities with VFAs and ADG
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Long, L.; Zhu, Y.; Li, Z.; Zhang, H.; Liu, L.; Bai, J. Differential expression of skeletal muscle mitochondrial proteins in yak, dzo, and cattle: A proteomics-based study. J. Vet. Med. Sci. 2020, 82, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, K.; Wang, L.; Yang, Y.; Ni, Z.; Xie, X.; Shao, X.; Han, J.; Wan, D.; Qiu, Q. Genome-wide patterns of copy number variation in the Chinese yak genome. BMC Genom. 2016, 17, 379. [Google Scholar] [CrossRef]
- Li, K.; Mehmood, K.; Zhang, H.; Jiang, X.; Shahzad, M.; Dong, X.; Li, J. Characterization of fungus microbial diversity in healthy and diarrheal yaks in Gannan region of Tibet Autonomous Prefecture. Acta Trop. 2018, 182, 14–26. [Google Scholar] [CrossRef]
- Lang, Y.; Sha, K.; Zhang, R.; Xie, P.; Luo, X.; Sun, B.; Li, H.; Zhang, L.; Zhang, S.; Liu, X. Effect of electrical stimulation and hot boning on the eating quality of Gannan yak longissimus lumborum. Meat Sci. 2016, 112, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.A.; Zhang, J.B.; Liang, Z.; Yang, C.; Kalwar, Q.; Shah, T.; Du, M.; Muhammad, I.; Zheng, J.; Yan, P.; et al. Dynamics of rumen bacterial composition of yak (Bos grunniens) in response to dietary supplements during the cold season. PeerJ 2021, 9, e11520. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Fang, L.; Meng, Q.; Li, S.; Chai, S.; Liu, S.; Schonewille, J.T. Assessment of Ruminal Bacterial and Archaeal Community Structure in Yak (Bos grunniens). Front. Microbiol. 2017, 8, 179. [Google Scholar] [CrossRef]
- Bergmann, G.T. Microbial community composition along the digestive tract in forage- and grain-fed bison. BMC Vet. Res. 2017, 13, 253. [Google Scholar] [CrossRef]
- Morgavi, D.P.; Rathahao-Paris, E.; Popova, M.; Boccard, J.; Nielsen, K.F.; Boudra, H. Rumen microbial communities influence metabolic phenotypes in lambs. Front. Microbiol. 2015, 6, 1060. [Google Scholar] [CrossRef]
- Fan, Q.; Wanapat, M.; Hou, F. Chemical Composition of Milk and Rumen Microbiome Diversity of Yak, Impacting by Herbage Grown at Different Phenological Periods on the Qinghai-Tibet Plateau. Animals 2020, 10, 1030. [Google Scholar] [CrossRef]
- Liu, H.; Xu, T.; Xu, S.; Ma, L.; Han, X.; Wang, X.; Zhang, X.; Hu, L.; Zhao, N.; Chen, Y.; et al. Effect of dietary concentrate to forage ratio on growth performance, rumen fermentation and bacterial diversity of Tibetan sheep under barn feeding on the Qinghai-Tibetan plateau. PeerJ 2019, 7, e7462. [Google Scholar] [CrossRef]
- Dai, D.; Pang, K.; Liu, S.; Wang, X.; Yang, Y.; Chai, S.; Wang, S. Effects of Concentrate Supplementation on Growth Performance, Rumen Fermentation, and Bacterial Community Composition in Grazing Yaks during the Warm Season. Animals 2022, 12, 1398. [Google Scholar] [CrossRef]
- Sha, Y.; Hu, J.; Shi, B.; Dingkao, R.; Wang, J.; Li, S.; Zhang, W.; Luo, Y.; Liu, X. Supplementary feeding of cattle-yak in the cold season alters rumen microbes, volatile fatty acids, and expression of SGLT1 in the rumen epithelium. PeerJ 2021, 9, e11048. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.N.; Nguyen, A.Q.; Johir, M.A.H.; Guo, W.; Ngo, H.H.; Chaves, A.V.; Nghiem, L.D. Application of rumen and anaerobic sludge microbes for bio harvesting from lignocellulosic biomass. Chemosphere 2019, 228, 702–708. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, H.; Zhang, P.; Zhang, G.; Cai, Y.; Wang, Q.; Zhou, Z.; Ding, Y.; Zubair, M. Effect of substrate load on anaerobic fermentation of rice straw with rumen liquid as inoculum: Hydrolysis and acidogenesis efficiency, enzymatic activities and rumen bacterial community structure. Waste Manag. 2021, 124, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, I.; Wallace, R.J.; Morais, S. The rumen microbiome: Balancing food security and environmental impacts. Nat. Rev. Microbiol. 2021, 19, 553–566. [Google Scholar] [CrossRef]
- Grilli, D.J.; Fliegerova, K.; Kopecny, J.; Lama, S.P.; Egea, V.; Sohaefer, N.; Pereyra, C.; Ruiz, M.S.; Sosa, M.A.; Arenas, G.N.; et al. Analysis of the rumen bacterial diversity of goats during shift from forage to concentrate diet. Anaerobe 2016, 42, 17–26. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Buchanan-Smith, J.G. Effects of dietary neutral detergent fiber concentration and supplementary long hay on chewing activities and milk production of dairy cows. J. Dairy Sci. 1989, 72, 2288–2300. [Google Scholar] [CrossRef]
- Malik, M.I.; Rashid, M.A.; Yousaf, M.S.; Naveed, S.; Javed, K.; Rehman, H. Effect of Physical Form and Level of Wheat Straw Inclusion on Growth Performance and Blood Metabolites of Fattening Goat. Animals 2020, 10, 1861. [Google Scholar] [CrossRef]
- Gasiorek, M.; Stefanska, B.; Pruszynska-Oszmalek, E.; Komisarek, J.; Nowak, W. Effects of the straw inclusion in the diet of dairy calves on growth performance, rumen fermentation, and blood metabolites during pre- and post-weaning periods. J. Anim. Physiol. Anim. Nutr. 2022, 106, 33–44. [Google Scholar] [CrossRef]
- Matar, A.M.; Abdelrahman, M.M.; Alhidary, I.A.; Ayadi, M.A.; Alobre, M.M.; Aljumaah, R.S. Effects of Roughage Quality and Particle Size on Rumen Parameters and Fatty Acid Profiles of Longissimus Dorsi Fat of Lambs Fed Complete Feed. Animals 2020, 10, 2182. [Google Scholar] [CrossRef] [PubMed]
- Terre, M.; Castells, L.; Khan, M.A.; Bach, A. Interaction between the physical form of the starter feed and straw provision on growth performance of Holstein calves. J. Dairy Sci. 2015, 98, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Henderson, G.; Cox, F.; Ganesh, S.; Jonker, A.; Young, W.; Global Rumen Census, C.; Janssen, P.H. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci. Rep. 2015, 5, 14567. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods ofAnalysis, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Zhang, Q.; Degen, A.; Hao, L.; Huang, Y.; Niu, J.; Wang, X.; Chai, S.; Liu, S. An increase in dietary lipid content from different forms of double-low rapeseed reduces enteric methane emission in Datong yaks on the Qinghai-Tibetan Plateau. Anim. Sci. J. 2020, 91, e13489. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Huang, L.; Li, N.; Ke, W.; Xiang, Y.; Ma, Y. Auxiliary rapid identification of pathogenic and antagonistic microorganisms associated with Coptis chinensis root rot by high-throughput sequencing. Sci. Rep. 2021, 11, 11141. [Google Scholar] [CrossRef] [PubMed]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Cole, J.R.; Wang, Q.; Cardenas, E.; Fish, J.; Chai, B.; Farris, R.J.; Kulam-Syed-Mohideen, A.S.; McGarrell, D.M.; Marsh, T.; Garrity, G.M.; et al. The Ribosomal Database Project: Improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009, 37, D141–D145. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Wang, Y.; Sheng, H.F.; He, Y.; Wu, J.Y.; Jiang, Y.X.; Tam, N.F.; Zhou, H.W. Comparison of the levels of bacterial diversity in freshwater, intertidal wetland, and marine sediments by using millions of illumina tags. Appl. Environ. Microbiol. 2012, 78, 8264–8271. [Google Scholar] [CrossRef]
- Liu, X.; Sha, Y.Z.; Dingkao, R.; Zhang, W.; Lv, W.B.; Wei, H.; Shi, H.; Hu, J.; Wang, J.Q.; Li, S.B.; et al. Interactions between Rumen Microbes, VFAs, and Host Genes Regulate Nutrient Absorption and Epithelial Barrier Function during Cold Season Nutritional Stress in Tibetan Sheep. Front. Microbiol. 2020, 11, 593062. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Wu, F.; Qiu, X.; Yu, Z.; Niu, W.; He, Y.; Su, H.; Cao, B. Effects of Dietary Energy on Growth Performance, Rumen Fermentation and Bacterial Community, and Meat Quality of Holstein-Friesians Bulls Slaughtered at Different Ages. Animals 2019, 9, 1123. [Google Scholar] [CrossRef]
- Russell, J.B.; Rychlik, J.L. Factors that alter rumen microbial ecology. Science 2001, 292, 1119–1122. [Google Scholar] [CrossRef] [PubMed]
- Malmuthuge, N.; Griebel, P.J.; Guan, L. The gut microbiome and its potential role in the development and function of newborn calf gastrointestinal tract. Front. Vet. Sci. 2015, 2, 36. [Google Scholar] [CrossRef] [PubMed]
- Grandl, F.; Zeitz, J.O.; Clauss, M.; Furger, M.; Kreuzer, M.; Schwarm, A. Evidence for increasing digestive and metabolic efficiency of energy utilization with age of dairy cattle as determined in two feeding regimes. Animal 2018, 12, 515–527. [Google Scholar] [CrossRef]
- Colomban, A.; Roger, L.; Boyaval, P. Production of propionic acid from whey permeate by sequential fermentation, ultrafiltration, and cell recycling. Biotechnol. Bioeng. 1993, 42, 1091–1098. [Google Scholar] [CrossRef]
- Gomez, E.; Canela, N.; Herrero, P.; Cereto, A.; Gimeno, I.; Carrocera, S.; Martin-Gonzalez, D.; Murillo, A.; Munoz, M. Metabolites Secreted by Bovine Embryos In Vitro Predict Pregnancies That the Recipient Plasma Metabolome Cannot, and Vice Versa. Metabolites 2021, 11, 162. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, D.; Wang, L.; Hao, J.; Wang, J.; Zhou, X.; Wang, W.; Qiu, Q.; Huang, X.; Zhou, J.; et al. Convergent Evolution of Rumen Microbiomes in High-Altitude Mammals. Curr. Biol. 2016, 26, 1873–1879. [Google Scholar] [CrossRef] [PubMed]
- Dominianni, C.; Sinha, R.; Goedert, J.J.; Pei, Z.; Yang, L.; Hayes, R.B.; Ahn, J. Sex, body mass index, and dietary fiber intake influence the human gut microbiome. PLoS ONE 2015, 10, e0124599. [Google Scholar] [CrossRef]
- Fernandes, K.A.; Kittelmann, S.; Rogers, C.W.; Gee, E.K.; Bolwell, C.F.; Bermingham, E.N.; Thomas, D.G. Faecal microbiota of forage-fed horses in New Zealand and the population dynamics of microbial communities following dietary change. PLoS ONE 2014, 9, e112846. [Google Scholar] [CrossRef]
- de Menezes, A.B.; Lewis, E.; O’Donovan, M.; O’Neill, B.F.; Clipson, N.; Doyle, E.M. Microbiome analysis of dairy cows fed pasture or total mixed ration diets. FEMS Microbiol. Ecol. 2011, 78, 256–265. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.A.; Yang, C.; Zhang, J.; Kalwar, Q.; Liang, Z.; Li, C.; Du, M.; Yan, P.; Long, R.; Han, J.; et al. Effects of Dietary Energy Levels on Rumen Fermentation, Microbial Diversity, and Feed Efficiency of Yaks (Bos grunniens). Front. Microbiol. 2020, 11, 625. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Kaakoush, N.O. Insights into the Role of Erysipelotrichaceae in the Human Host. Front. Cell. Infect. Microbiol. 2015, 5, 84. [Google Scholar] [CrossRef]
- Jami, E.; White, B.A.; Mizrahi, I. Potential Role of the Bovine Rumen Microbiome in Modulating Milk Composition and Feed Efficiency. PLoS ONE 2014, 9, e85423. [Google Scholar] [CrossRef]
- Murphy, E.F.; Cotter, P.D.; Healy, S.; Marques, T.M.; O’Sullivan, O.; Fouhy, F.; Clarke, S.F.; O’Toole, P.W.; Quigley, E.M.; Stanton, C.; et al. Composition and energy harvesting capacity of the gut microbiota: Relationship to diet, obesity and time in mouse models. Gut 2010, 59, 1635–1642. [Google Scholar] [CrossRef]
- Myer, P.R.; Smith, T.P.L.; Wells, J.E.; Kuehn, L.A.; Freetly, H.C. Rumen Microbiome from Steers Differing in Feed Efficiency. PLoS ONE 2015, 10, e0129174. [Google Scholar] [CrossRef]
- Fernando, S.C.; Purvis, H.T., 2nd; Najar, F.Z.; Sukharnikov, L.O.; Krehbiel, C.R.; Nagaraja, T.G.; Roe, B.A.; Desilva, U. Rumen microbial population dynamics during adaptation to a high-grain diet. Appl. Environ. Microbiol. 2010, 76, 7482–7490. [Google Scholar] [CrossRef]
- Xue, D.; Chen, H.; Luo, X.; Guan, J.; He, Y.; Zhao, X. Microbial diversity in the rumen, reticulum, omasum, and abomasum of yak on a rapid fattening regime in an agro-pastoral transition zone. J. Microbiol. 2018, 56, 734–743. [Google Scholar] [CrossRef]
- Hu, R.; Zou, H.W.; Wang, Z.S.; Cao, B.H.; Peng, Q.H.; Jing, X.P.; Wang, Y.X.; Shao, Y.Q.; Pei, Z.X.; Zhang, X.F.; et al. Nutritional Interventions Improved Rumen Functions and Promoted Compensatory Growth of Growth-Retarded Yaks as Revealed by Integrated Transcripts and Microbiome Analyses. Front. Microbiol. 2019, 10, 318. [Google Scholar] [CrossRef]
- Perea, K.; Perz, K.; Olivo, S.K.; Williams, A.; Lachman, M.; Ishaq, S.L.; Thomson, J.; Yeoman, C.J. Feed efficiency phenotypes in lambs involve changes in ruminal, colonic, and small-intestine-located microbiota. J. Anim. Sci. 2017, 95, 2585–2592. [Google Scholar] [CrossRef]
- Dai, X.; Tian, Y.; Li, J.; Luo, Y.; Liu, D.; Zheng, H.; Wang, J.; Dong, Z.; Hu, S.; Huang, L. Metatranscriptomic analyses of plant cell wall polysaccharide degradation by microorganisms in the cow rumen. Appl. Environ. Microbiol. 2015, 81, 1375–1386. [Google Scholar] [CrossRef]
- Waters, J.L.; Ley, R.E. The human gut bacteria Christensenellaceae are widespread, heritable, and associated with health. BMC Biol. 2019, 17, 83. [Google Scholar] [CrossRef]
- Vital, M.; Howe, A.C.; Tiedje, J.M. Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. mBio 2014, 5, e00889. [Google Scholar] [CrossRef]
- Haas, K.N.; Blanchard, J.L. Kineothrix alysoides, gen. nov., sp nov., a saccharolytic butyrate-producer within the family Lachnospiraceae. Int. J. Syst. Evol. Microbiol. 2017, 67, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ma, M.P.; Diao, Q.Y.; Tu, Y. Saponin-Induced Shifts in the Rumen Microbiome and Metabolome of Young Cattle. Front. Microbiol. 2019, 10, 356. [Google Scholar] [CrossRef]
- Kong, F.L.; Hua, Y.T.; Zeng, B.; Ning, R.H.; Li, Y.; Zhao, J.C. Gut microbiota signatures of longevity. Curr. Biol. 2016, 26, R832–R833. [Google Scholar] [CrossRef]
- Pacifico, C.; Petri, R.M.; Ricci, S.; Mickdam, E.; Wetzels, S.U.; Neubauer, V.; Zebeli, Q. Unveiling the Bovine Epimural Microbiota Composition and Putative Function. Microorganisms 2021, 9, 342. [Google Scholar] [CrossRef]
- Des Rosiers, C.; David, F.; Garneau, M.; Brunengraber, H. Nonhomogeneous labeling of liver mitochondrial acetyl-CoA. J. Biol. Chem. 1991, 266, 1574–1578. [Google Scholar] [CrossRef]
- Miller, T.L.; Wolin, M.J. Bioconversion of Cellulose to Acetate with Pure Cultures of Ruminococcus albus and a Hydrogen-Using Acetogen. Appl. Environ. Microbiol. 1995, 61, 3832–3835. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Tsedan, G.; Liu, Y.; Hou, F. Shrub coverage alters the rumen bacterial community of yaks (Bos grunniens) grazing in alpine meadows. J. Anim. Sci. Technol. 2020, 62, 504–520. [Google Scholar] [CrossRef] [PubMed]
- Huws, S.A.; Lee, M.R.; Muetzel, S.M.; Scott, M.B.; Wallace, R.J.; Scollan, N.D. Forage type and fish oil cause shifts in rumen bacterial diversity. FEMS Microbiol. Ecol. 2010, 73, 396–407. [Google Scholar] [CrossRef]
- Ellekilde, M.; Krych, L.; Hansen, C.H.; Hufeldt, M.R.; Dahl, K.; Hansen, L.H.; Sorensen, S.J.; Vogensen, F.K.; Nielsen, D.S.; Hansen, A.K. Characterization of the gut microbiota in leptin deficient obese mice—Correlation to inflammatory and diabetic parameters. Res. Vet. Sci. 2014, 96, 241–250. [Google Scholar] [CrossRef]
- Xin, J.; Chai, Z.; Zhang, C.; Zhang, Q.; Zhu, Y.; Cao, H.; Zhong, J.; Ji, Q. Comparing the Microbial Community in Four Stomach of Dairy Cattle, Yellow Cattle and Three Yak Herds in Qinghai-Tibetan Plateau. Front. Microbiol. 2019, 10, 1547. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.N.; Pan, J.Y.; Chen, H.; Chen, X.; Ye, Z.Q.; Yuan, H.B.; Sun, H.L.; Wan, P. Oyster polysaccharides ameliorate intestinal mucositis and improve metabolism in 5-fluorouracil-treated S180 tumour-bearing mice. Carbohyd. Polym. 2021, 256, 117545. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, W.; Li, B.; Qian, S.; Wei, B.; Gong, S.; Wang, J.; Liu, M.; Wei, M. Nuciferine modulates the gut microbiota and prevents obesity in high-fat diet-fed rats. Exp. Mol. Med. 2020, 52, 1959–1975. [Google Scholar] [CrossRef]






| Diet Treatment | Dry Matter (%) | Crude Protein (%) | Neutral Detergent Fiber (%) | Acid Detergent Fiber (%) |
|---|---|---|---|---|
| Corn straw | 91.57 | 3.30 | 63.55 | 41.98 |
| Mixed straw | 94.76 | 7.09 | 58.78 | 38.79 |
| Month | Gender | Number | Growth Properties | Group | |||
|---|---|---|---|---|---|---|---|
| MSG | CSG | G | p | ||||
| 6M | Male | N = 5 (MSG) | IBW (kg) | 51.90 ± 2.76 | 48.50 ± 3.76 | 45.00 ± 1.22 | 0.207 |
| N = 4 (CSG) | FBW (kg) | 59.60 ± 1.72 a | 51.50 ± 2.73 b | 44.95 ± 1.64 c | 0.001 | ||
| N = 4 (G) | ADG (kg/d) | 0.13 ± 0.02 a | 0.05 ± 0.01 b | 0.00 ± 0.01 b | 0.005 | ||
| Female | N = 4 (MSG) | IBW (kg) | 45.87 ± 3.47 | 42.66 ± 1.33 | 45.62 ± 1.66 | 0.628 | |
| N = 3 (CSG) | FBW (kg) | 54.37 ± 4.98 a | 45.16 ± 0.16 ab | 40.75 ± 0.75 b | 0.038 | ||
| N = 4 (G) | ADG (kg/d) | 0.09 ± 0.02 a | 0.03 ± 0.01 b | −0.01 ± 0.00 c | 0.002 | ||
| 18M | Male | N = 5 (MSG) | IBW (kg) | 109.26 ± 2.05 | 106.83 ± 0.44 | 106.50 ± 1.05 | 0.369 |
| N = 3 (CSG) | FBW (kg) | 122.80 ± 1.39 a | 113.00 ± 2.51 b | 104.28 ± 1.89 c | <0.001 | ||
| N = 7 (G) | ADG (kg/d) | 0.15 ± 0.02 a | 0.07 ± 0.02 a | −0.02 ± 0.02 b | 0.001 | ||
| Female | N = 3 (MSG) | IBW (kg) FBW (kg) ADG (kg/d) | 111.50 ± 6.33 125.33 ± 6.88 a 0.15 ± 0.03 a | 108.83 ± 3.63 114.66 ± 2.18 ab 0.06 ± 0.02 b | 104.20 ± 1.71 106.20 ± 2.76 b −0.02 ± 0.02 b | 0.367 0.010 0.004 | |
| N = 3 (CSG) | |||||||
| N = 5 (G) | |||||||
| 30M | Male | N = 4 (MSG) | IBW (kg) | 153.25 ± 8.95 | 144.0 ± 7.11 | 124.62 ± 8.21 | 0.092 |
| N = 3 (CSG) | FBW (kg) | 165.00 ± 7.93 a | 150.00 ± 8.24 a | 122.87 ± 8.25 b | 0.015 | ||
| N = 3 (G) | ADG (kg/d) | 0.21 ± 0.03 a | 0.14 ± 0.02 a | −0.03 ± 0.01 b | 0.001 | ||
| Female | N = 4 (MSG) | IBW (kg) FBW (kg) ADG (kg/d) | 159.87 ± 9.64 177.50 ± 7.59 a 0.20 ± 0.03 a | 147.50 ± 7.64 158.66 ± 7.31 ab 0.12 ± 0.04 a | 134.00 ± 10.88 131.00 ± 10.54 b −0.03 ± 0.01 b | 0.193 0.015 0.002 | |
| N = 3 (CSG) | |||||||
| N = 3 (G) | |||||||
| 42M | Male | N = 3 (MSG) | IBW (kg) | 203.16 ± 2.08 | 202.66 ± 4.60 | 198.33 ± 4.25 | 0.638 |
| N = 3 (CSG) | FBW (kg) | 229.66 ± 0.66 a | 216.66 ± 4.05 b | 197.33 ± 4.80 c | 0.002 | ||
| N = 3 (G) | ADG (kg/d) | 0.30 ± 0.03 a | 0.16 ± 0.02 b | −0.01 ± 0.00 c | <0.001 | ||
| Female | N = 4 (MSG) | IBW (kg) FBW (kg) ADG (kg/d) | 198.75 ± 12.19 218.75 ± 13.40 a 0.22 ± 0.01 a | 192.00 ± 3.04 205.33 ± 2.40 ab 0.15 ± 0.05 b | 191.87 ± 2.70 190.37 ± 2.85 b −0.01 ± 0.00 c | 0.701 0.026 <0.001 | |
| N = 3 (CSG) | |||||||
| N = 8 (G) | |||||||
| 54M | Male | N = 4 (MSG) | IBW (kg) | 239.62 ± 7.08 | 231.66 ± 3.00 | 221.83 ± 1.42 | 0.125 |
| N = 3 (CSG) | FBW (kg) | 259.75 ± 9.47 a | 243.16 ± 1.01 ab | 221.33 ± 1.76 a | 0.015 | ||
| N = 3 (G) | ADG (kg/d) | 0.36 ± 0.08 a | 0.23 ± 0.07 ab | −0.01 ± 0.01 b | 0.026 | ||
| Female | N = 4 (MSG) | IBW (kg) | 219.87 ± 2.97 238.00 ± 7.57 a 0.20 ± 0.06 a | 212.50 ± 3.43 222.50 ± 3.79 ab 0.11 ± 0.01 ab | 207.50 ± 9.18 206.25 ± 8.87 b 0.01 ± 0.02 b | 0.372 0.034 0.025 | |
| N = 4 (CSG) | FBW (kg) | ||||||
| N = 4 (G) | ADG (kg/d) | ||||||
| Diet Treatment | Growth Properties | Gender | Month | |||||
|---|---|---|---|---|---|---|---|---|
| 6M | 18M | 30M | 42M | 54M | p | |||
| MSG | ADG (kg/day) | Male (n = 21) | 0.13 ± 0.12 c | 0.15 ± 0.02 bc | 0.21 ± 0.03 bc | 0.30 ± 0.03 ab | 0.36 ± 0.08 a | 0.013 |
| Female (n = 19) | 0.09 ± 0.02 | 0.15 ± 0.03 | 0.20 ± 0.03 | 0.22 ± 0.01 | 0.20 ± 0.06 | 0.166 | ||
| CSG | ADG (kg/day) | Male (n = 16) | 0.05 ± 0.01 b | 0.07 ± 0.02 b | 0.14 ± 0.02 ab | 0.16 ± 0.02 ab | 0.23 ± 0.07 a | 0.024 |
| Female (n = 16) | 0.03 ± 0.01 | 0.06 ± 0.02 | 0.12 ± 0.04 | 0.15 ± 0.05 | 0.11 ± 0.01 | 0.131 | ||
| G | ADG (kg/day) | Male (n = 20) | 0.00 ± 0.01 | −0.02 ± 0.02 | −0.03 ± 0.02 | −0.01 ± 0.00 | −0.01 ± 0.01 | 0.862 |
| Female (n = 24) | −0.01 ± 0.00 | −0.02 ± 0.02 | −0.04 ± 0.02 | −0.01 ± 0.00 | 0.01 ± 0.02 | 0.294 | ||
| Growth Properties | Diet Treatment | Gender | Month | ||||
|---|---|---|---|---|---|---|---|
| 6M | 18M | 30M | 42M | 54M | |||
| MSG | ADG (kg/day) | Male (n = 21) | 0.13 ± 0.02 | 0.15 ± 0.02 | 0.21 ± 0.03 | 0.30 ± 0.03 | 0.36 ± 0.08 |
| Female (n = 19) | 0.09 ± 0.02 | 0.15 ± 0.03 | 0.20 ± 0.03 | 0.22 ± 0.01 | 0.20 ± 0.06 | ||
| p | 0.432 | 0.948 | 0.072 | 0.072 | 0.204 | ||
| CSG | ADG (kg/day) | Male (n = 16) | 0.05 ± 0.01 | 0.07 ± 0.02 | 0.14 ± 0.02 | 0.16 ± 0.02 | 0.23 ± 0.07 |
| Female (n = 16) | 0.03 ± 0.01 | 0.06 ± 0.02 | 0.12 ± 0.04 | 0.15 ± 0.05 | 0.11 ± 0.01 | ||
| p | 0.274 | 0.845 | 0.818 | 0.868 | 0.115 | ||
| G | ADG (kg/day) | Male (n = 20) | 0.00 ± 0.01 a | −0.02 ± 0.02 | −0.03 ± 0.02 | −0.01 ± 0.00 | −0.01 ± 0.01 |
| Female (n = 24) | −0.01 ± 0.00 b | −0.02 ± 0.02 | −0.04 ± 0.02 | −0.01 ± 0.00 | 0.01 ± 0.02 | ||
| p | 0.420 | 0.852 | 0.860 | 0.353 | 0.503 | ||
| Item | ADG | |
|---|---|---|
| Gender | male | 0.11 ± 0.01 |
| female | 0.07 ± 0.01 | |
| Age | 6M | 0.04 ± 0.01 a |
| 18M | 0.05 ± 0.01 a | |
| 30M | 0.10 ± 0.02 ab | |
| 42M | 0.11 ± 0.02 ab | |
| 54M | 0.15 ± 0.03 b | |
| Diets | MSG | 0.20 ± 0.01a |
| CSG | 0.11 ± 0.01 b | |
| G | −0.01 ± 0.01 c | |
| p | ||
| Gender | 0.129 | |
| Age | 0.010 | |
| Diets | <0.001 | |
| Age × gender | 0.252 | |
| Gender × diets | 0.300 | |
| Diets × age | 0.047 | |
| Gender × age × diets | 0.129 |
| Month | Diet Treatment | Acetate (%) | Propionate (%) | Butyrate (%) | Isobutyrate (%) | Isovalerate (%) | Valerate (%) | TVFA (mmol/L) | A/P |
|---|---|---|---|---|---|---|---|---|---|
| 6M | MSG (n = 3) | 55.58 ± 1.45 a | 26.42 ± 0.39 a | 11.33 ± 0.10 | 2.11 ± 0.11 a | 2.37 ± 0.12 a | 2.18 ± 0.11 a | 27.28 ± 1.41 a | 2.10 ± 0.08 a |
| CSG (n = 3) | 49.13 ± 0.77 b | 31.12 ± 0.07 b | 12.39 ± 0.06 | 2.35 ± 0.07 a | 2.56 ± 0.07 ab | 2.45 ± 0.09 a | 21.69 ± 1.11 b | 1.58 ± 0.06 b | |
| G (n = 3) | 45.49 ± 2.24 b | 33.55 ± 1.15 b | 12.48 ± 0.14 | 2.72 ± 0.11 b | 2.90 ± 0.13 b | 2.87 ± 0.10 b | 18.80 ± 0.90 b | 1.36 ± 0.11 b | |
| p | 0.012 | 0.012 | 0.710 | 0.016 | 0.041 | 0.005 | 0.006 | 0.003 | |
| 30M | MSG (n = 3) | 55.16 ± 0.84 a | 27.62 ± 1.00 a | 10.03 ± 0.95 a | 2.14 ± 0.13 | 2.84 ± 0.29 | 2.22 ± 0.07 a | 29.44 ± 4.40 | 2.00 ± 0.08 a |
| CSG (n = 3) | 54.47 ± 1.38 a | 29.44 ± 0.83 ab | 9.52 ± 0.48 a | 2.15 ± 0.04 | 2.10 ± 0.09 | 2.31 ± 0.02 a | 21.37 ± 0.61 | 1.85 ± 0.09 a | |
| G (n = 3) | 43.16 ± 4.84 b | 32.41 ± 1.54 b | 15.97 ± 2.74 b | 2.42 ± 0.14 | 3.11 ± 0.68 | 2.89 ± 0.02 b | 23.90 ± 1.50 | 1.35 ± 0.20 b | |
| p | 0.049 | 0.049 | 0.066 | 0.195 | 0.300 | 0.020 | 0.180 | 0.038 | |
| 54M | MSG (n = 3) | 53.49 ± 0.54 a | 28.43 ± 1.78 | 13.63 ± 0.21 a | 1.93 ± 0.20 | 2.39 ± 0.14 | 2.06 ± 0.17 a | 25.87 ± 0.11 a | 1.89 ± 0.10 |
| CSG (n = 3) | 49.86 ± 0.80 ab | 30.84 ± 0.55 | 10.37 ± 0.24 b | 2.40 ± 0.07 | 2.58 ± 0.01 | 2.54 ± 0.05 ab | 20.92 ± 0.12 b | 1.61 ± 0.03 | |
| G (n = 3) | 51.18 ± 0.83 b | 26.72 ± 1.71 | 12.24 ± 1.26 a | 2.40 ± 0.08 | 2.82 ± 0.01 | 2.72 ± 0.18 b | 21.30 ± 0.11 b | 1.93 ± 0.13 | |
| p | 0.035 | 0.217 | 0.023 | 0.078 | 0.148 | 0.049 | < 0.001 | 0.125 |
| Diet Treatment | Month | Acetate (%) | Propionate (%) | Butyrate (%) | Isobutyrate (%) | Isovalerate (%) | Valerate (%) | TVFA (mmol/L) | A/P |
|---|---|---|---|---|---|---|---|---|---|
| MSG | 6M (n = 3) | 55.58 ± 1.45 | 26.42 ± 0.39 | 11.33 ± 1.01 ab | 2.11 ± 0.11 | 2.37 ± 0.12 | 2.18 ± 0.06 | 27.28 ± 1.43 | 2.10 ± 0.08 |
| 30M (n = 3) | 55.16 ± 0.84 | 27.62 ± 1.00 | 10.03 ± 0.95 a | 2.14 ± 0.13 | 2.84 ± 0.29 | 2.22 ± 0.07 | 29.44 ± 4.40 | 2.00 ± 0.08 | |
| 54M (n = 3) | 51.56 ± 0.54 | 28.43 ± 1.78 | 13.63 ± 0.21 b | 1.93 ± 0.20 | 2.391 ± 0.14 | 2.06 ± 0.17 | 25.87 ± 0.11 | 1.83 ± 0.10 | |
| p | 0.371 | 0.531 | 0.054 | 0.627 | 0.250 | 0.616 | 0.658 | 0.331 | |
| CSG | 6M (n = 3) | 49.13 ± 0.77 a | 31.12 ± 0.73 | 12.39 ± 0.60 a | 2.35 ± 0.07 ab | 2.56 ± 0.07 a | 2.45 ± 0.09 | 21.69 ± 0.90 | 1.58 ± 0.06 a |
| 30M (n = 3) | 54.47 ± 1.38 b | 29.44 ± 0.83 | 9.52 ± 0.48 b | 2.15 ± 0.04 a | 2.10 ± 0.09 b | 2.31 ± 0.22 | 21.37 ± 1.11 | 1.85 ± 0.09 b | |
| 54M (n = 3) | 49.86 ± 0.80 a | 30.84 ± 0.55 | 10.37 ± 0.24 b | 2.40 ± 0.07 b | 2.58 ± 0.01 a | 2.54 ± 0.05 | 20.92 ± 0.12 | 1.61 ± 0.03 ab | |
| p | 0.020 | 0.282 | 0.013 | 0.075 | 0.004 | 0.126 | 0.769 | 0.060 | |
| G | 6M (n = 3) | 45.49 ± 2.24 | 33.55 ± 1.55 a | 12.48 ± 1.40 | 2.72 ± 0.11 | 2.90 ± 0.13 | 2.87 ± 0.10 | 18.80 ± 0.90 a | 1.36 ± 0.11 |
| 30M (n = 3) | 43.16 ± 4.84 | 32.41 ± 1.54 a | 15.97 ± 2.74 | 2.42 ± 0.13 | 3.11 ± 0.68 | 2.93 ± 0.22 | 23.90 ± 1.50 b | 1.38 ± 0.17 | |
| 54M (n = 3) | 51.18 ± 0.83 | 26.72 ± 1.71 b | 12.24 ± 1.26 | 2.40 ± 0.08 | 2.82 ± 0.01 | 2.72 ± 0.18 | 21.30 ± 0.11 ab | 1.93 ± 0.13 | |
| p | 0.237 | 0.028 | 0.508 | 0.096 | 0.909 | 0.758 | 0.035 | 0.053 |
| Month | Indexes | MSG | CSG | G | p |
|---|---|---|---|---|---|
| 6M | Shannon | 5.27 ± 0.19 | 5.26 ± 0.06 | 5.01 ± 0.51 | 0.553 |
| Simpson | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.01 | 0.750 | |
| Ace | 1306.62 ± 50.02 | 1323.93 ± 110.85 | 1167.95 ± 271.84 | 0.515 | |
| Chao | 1324.44 ± 33.49 | 1355.71 ± 133.66 | 1198.87 ± 284.54 | 0.569 | |
| 30M | Shannon | 5.30 ± 0.11 | 5.12 ± 0.37 | 4.78 ± 0.49 | 0.284 |
| Simpson | 0.01 ± 0.00 | 0.03 ± 0.01 | 0.04 ± 0.04 | 0.432 | |
| Ace | 1383.76 ± 55.99 a | 1368.35 ± 129.77 a | 1093.15 ± 10.45 b | 0.008 | |
| Chao | 1409.77 ± 45.01 a | 1417.73 ± 116.85 a | 1094.16 ± 7.17 b | 0.002 | |
| 54M | Shannon | 5.19 ± 0.35 | 5.08 ± 0.03 | 5.10 ± 0.24 | 0.858 |
| Simpson | 0.02 ± 0.01 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.933 | |
| Ace | 1287.73 ± 90.01 | 1254.94 ± 15.99 | 1279.90 ± 114.13 | 0.886 | |
| Chao | 1324.84 ± 91.54 | 1278.72 ± 44.80 | 1308.16 ± 116.34 | 0.820 |
| Diet Treatment | Indexes | 6M | 30M | 54M | p |
|---|---|---|---|---|---|
| MSG | Shannon | 5.27 ± 0.18 | 5.29 ± 0.10 | 5.18 ± 0.35 | 0.850 |
| Simpson | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.01 | 0.540 | |
| Ace | 1306.61 ± 50.01 | 1383.76 ± 55.99 | 1287.72 ± 99.00 | 0.261 | |
| Chao | 1324.44 ± 33.99 | 1409.76 ± 45.01 | 1324.83 ± 91.54 | 0.231 | |
| CSG | Shannon | 5.25 ± 0.06 | 5.12 ± 0.37 | 5.08 ± 0.03 | 0.625 |
| Simpson | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.104 | |
| Ace | 1323.92 ± 110.84 | 1368.34 ± 129.77 | 1254.94 ± 15.99 | 0.422 | |
| Chao | 1355.70 ± 133.66 | 1417.72 ± 116.84 | 1278.72 ± 44.79 | 0.339 | |
| G | Shannon | 5.01 ± 0.51 | 4.78 ± 0.49 | 5.10 ± 0.24 | 0.666 |
| Simpson | 0.02 ± 0.01 | 0.04 ± 0.04 | 0.02 ± 0.00 | 0.589 | |
| Ace | 1167.95 ± 271.84 | 1093.15 ± 10.45 | 1279.90 ± 114.13 | 0.450 | |
| Chao | 1198.87 ± 284.54 | 1094.16 ± 7.17 | 1308.16 ± 116.34 | 0.395 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Shi, B.; Zuo, Z.; Qi, Y.; Zhao, S.; Zhang, X.; Lan, L.; Shi, Y.; Liu, X.; Li, S.; et al. Effects of Two Different Straw Pellets on Yak Growth Performance and Ruminal Microbiota during Cold Season. Animals 2023, 13, 335. https://doi.org/10.3390/ani13030335
Wang X, Shi B, Zuo Z, Qi Y, Zhao S, Zhang X, Lan L, Shi Y, Liu X, Li S, et al. Effects of Two Different Straw Pellets on Yak Growth Performance and Ruminal Microbiota during Cold Season. Animals. 2023; 13(3):335. https://doi.org/10.3390/ani13030335
Chicago/Turabian StyleWang, Xiangyan, Bingang Shi, Zhi Zuo, Youpeng Qi, Shijie Zhao, Xueping Zhang, Lijuan Lan, Yu Shi, Xiu Liu, Shaobin Li, and et al. 2023. "Effects of Two Different Straw Pellets on Yak Growth Performance and Ruminal Microbiota during Cold Season" Animals 13, no. 3: 335. https://doi.org/10.3390/ani13030335
APA StyleWang, X., Shi, B., Zuo, Z., Qi, Y., Zhao, S., Zhang, X., Lan, L., Shi, Y., Liu, X., Li, S., Wang, J., & Hu, J. (2023). Effects of Two Different Straw Pellets on Yak Growth Performance and Ruminal Microbiota during Cold Season. Animals, 13(3), 335. https://doi.org/10.3390/ani13030335








