Object Play as a Positive Emotional State Indicator for Farmed Spotted Paca (Cuniculus paca)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Note
2.2. Subjects and Housing Conditions
2.3. Data Collection
2.4. Acoustic Parameters of Bark Calls
2.5. Data Analysis and Statistics
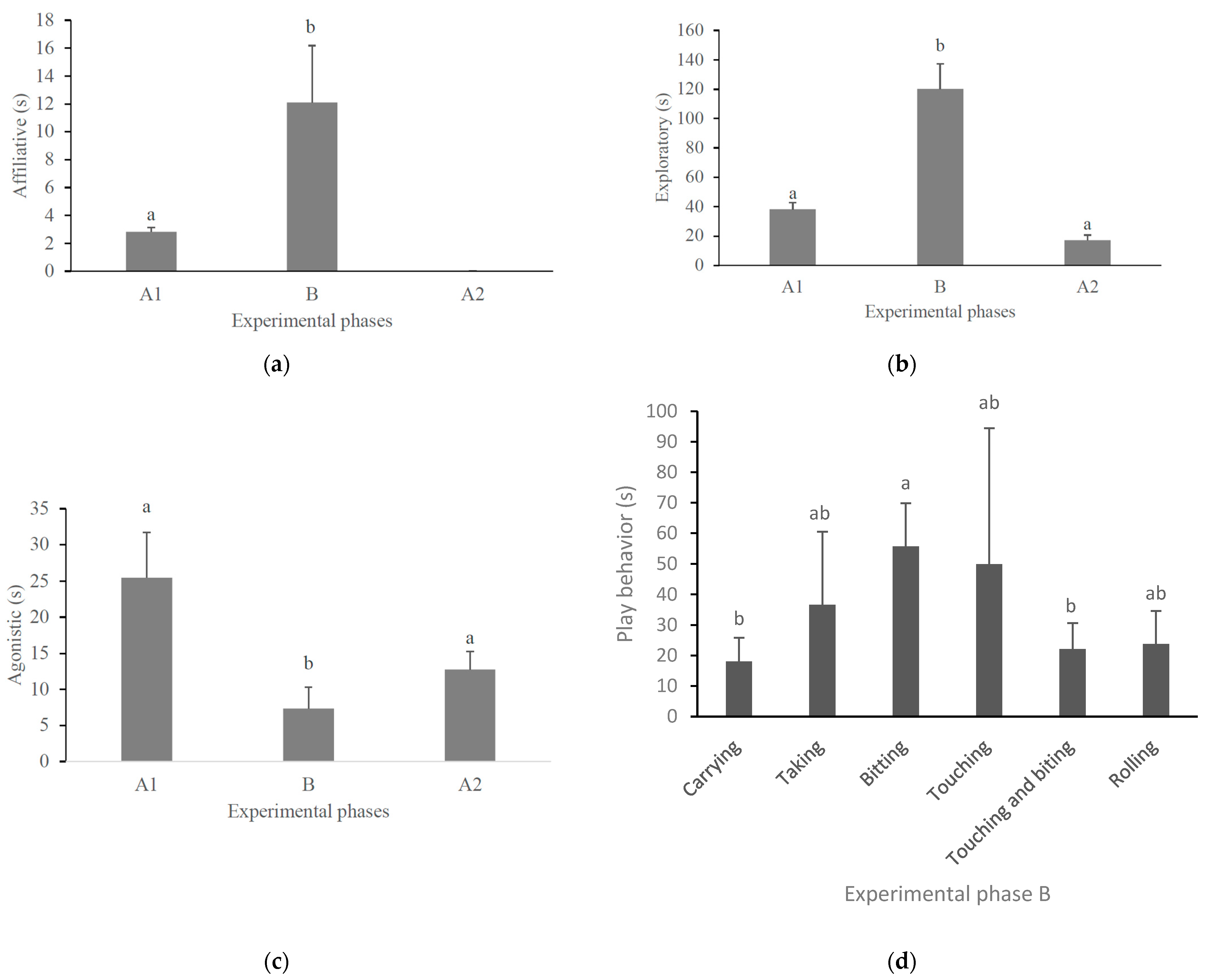
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newberry, R.C. Environmental enrichment: Increasing the biological relevance of captive environments. Appl. Anim. Behav. Sci. 1995, 44, 229–243. [Google Scholar] [CrossRef]
- Burghardt, G.M. The Genesis of Animal Play: Testing the Limits; Bradford Books; MIT Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Fagen, R. Animal Play Behavior; Oxford University Press: New York, NY, USA, 1981. [Google Scholar]
- Boissy, A.; Manteuffel, G.; Jensen, M.B.; Moe, R.O.; Spruijt, B.; Keeling, L.J.; Winckler, C.; Forkman, B.; Dimitrov, I.; Langbein, J.; et al. Assessment of positive emotions in animals to improve their welfare. Physiol. Behav. 2007, 92, 375–397. [Google Scholar] [CrossRef] [PubMed]
- Held, S.D.; Špinka, M. Animal play and animal welfare. Anim. Behav. 2011, 81, 891–899. [Google Scholar] [CrossRef]
- Ahloy-Dallaire, J.; Espinosa, J.; Mason, G. Play and optimal welfare: Does play indicate the presence of positive affective states? Behav. Process. 2018, 156, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Keeling, L.J.; Winckler, C.; Hintze, S.; Forkman, B. Towards a positive welfare protocol for cattle: A critical review of indicators and suggestion of how we might proceed. Front. Anim. Sci. 2021, 2, 70. [Google Scholar] [CrossRef]
- Jones, N.; Sherwen, S.; Robbins, R.; McLelland, D.; Whittaker, A. Welfare assessment tools in zoos: From theory to practice. Vet. Sci. 2021, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- Reimert, I.; Bolhuis, J.E.; Kemp, B.; Rodenburg, T.B. Indicators of positive and negative emotions and emotional contagion in pigs. Physiol. Behav. 2013, 109, 42–50. [Google Scholar] [CrossRef]
- Schwing, R.; Nelson, X.J.; Wein, A.; Parsons, S. Positive emotional contagion in a New Zealand parrot. Curr. Biol. 2017, 27, R213–R214. [Google Scholar] [CrossRef]
- Nogueira, S.S.C.; Soledade, J.P.; Pompéia, S.; Nogueira-Filho, S.L.G. The effect of environmental enrichment on play behaviour in white-lipped peccaries (Tayassu pecari). Anim. Welf. 2011, 20, 505–514. [Google Scholar] [CrossRef]
- Godyń, D.; Nowicki, J.; Herbut, P. Effects of environmental enrichment on pig welfare—A review. Animals 2019, 9, 383. [Google Scholar] [CrossRef]
- Borges, M.P.; Byk, J.; Del-Claro, K. Influência de técnicas de enriquecimento ambiental no aumento do bem-estar de Callithrix penicillata (E. Geoffroy, 1812) (Primates: Callitrichidae). [Influence of environmental enrichment techniques on increasing the well-being of Callithrix penicillata (E. Geoffroy, 1812) (Primates: Callitrichidae). Biotemas 2011, 24, 83–94. [Google Scholar]
- Mitani, J.C.; Merriwether, D.A.; Zhang, C. Male affiliation, cooperation and kinship in wild chimpanzees. Anim. Behav. 2000, 59, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Miranda-de La Lama, G.C.; Villarroel, M.; María, G.A. Behavioural and physiological profiles following exposure to novel environment and social mixing in lambs. Small Rumin. Res. 2012, 103, 158–163. [Google Scholar] [CrossRef]
- Burgdorf, J.; Kroes, R.A.; Moskal, J.R.; Pfaus, J.G.; Brudzynski, S.M.; Panksepp, J. Ultrasonic vocalizations of rats (Rattus norvegicus) during mating, play, and aggression: Behavioral concomitants, relationship to reward, and self-administration of playback. J. Comp. Psychol. 2008, 122, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.A.; Bonvicino, C.R. Paca. In Mamíferos do Brasil [Brazilian Mammals]; Reis, N.R., Peracchi, A.L., Pedro, W.A., Lima, I.P., Eds.; Nelio R. dos Reis Editora: Londrina, Brazil, 2011; pp. 358–406. [Google Scholar]
- Eisenberg, J.F.; Redford, K.H. Mammals of the Neotropics. The Central Neotropics; The University of Chicago Press: Chicago, IL, USA, 1999. [Google Scholar]
- Smythe, N.; Brown Guanti, O.D. La Domesticación y Cría de la Paca (Agouti paca) [Domestication and Breeding of the Paca (Cuniculus paca)]; (No. L01/1667); FAO: Rome, Italy, 1995. [Google Scholar]
- Emmons, L. Cuniculus paca. The IUCN Red List of Threatened Species 2016: E.T699A22197347. 2016. Available online: https://dx.doi.org/10.2305/IUCN.UK.2016-2.RLTS.T699A22197347.en (accessed on 3 October 2023).
- Emmons, L.; Feer, F. Neotropical Rainforest Mammals: A Field Guide, 2nd ed.; University of Chicago Press: Chicago, IL, USA, 1997. [Google Scholar]
- Beck–King, H.; Helversen, O.V.; Beck–King, R. Home range, population density, and food resources of Agouti paca (Rodentia: Agoutidae) in Costa Rica: A study using alternative methods 1. Biotropica 1999, 31, 675–685. [Google Scholar] [CrossRef]
- Harmsen, B.J.; Wooldridge, R.L.; Gutierrez, S.M.; Doncaster, C.P.; Foster, R.J. Spatial and temporal interactions of free-ranging pacas (Cuniculus paca). Mammal Res. 2018, 63, 161–172. [Google Scholar] [CrossRef]
- Lima, S.G.; Sousa-Lima, R.S.; Tokumaru, R.S.; Nogueira-Filho, S.L.G.; Nogueira, S.S.C. Vocal complexity and sociality in spotted paca (Cuniculus paca). PLoS ONE 2018, 13, e0190961. [Google Scholar] [CrossRef]
- Mattos, A.; Silva, V.J. Viabilidade econômica da criação de pacas (Cuniculus paca L.) em Presidente Tancredo Neves, Bahia. [Economic viability of breeding pacas (Cuniculus paca L.) in Presidente Tancredo Neves, Bahia]. Rev. iPecege 2016, 2, 56–79. [Google Scholar] [CrossRef]
- Hosken, F.M.; Oliveira, M.H.V.; Malheiros, J.M.; Martins, E.H.; Ferreira, F.N.A.; Ferreira, W.M.; Mota, K.C.d.N.; Lara, L.B. Experimental ethology of intensively reared lowland pacas (Cuniculus paca). Trop. Anim. Health Prod. 2021, 53, 367. [Google Scholar] [CrossRef]
- Correia, F.C.S.; Silva, F.R.; Souza, V.T.; Ribeiro, V.M.F.; Gomes, F.A. Criação de pacas (Cuniculus paca) como alternativa de diversificação de produção e renda em Rio Branco-Acre. [The captive breeding of Cuniculus paca as an alternative for production diversification]. Arq. Ciênc. Vet. Zool. UNIPAR 2016, 19, 81–89. [Google Scholar]
- Sabatini, V.; Paranhos da Costa, M.J.R. Etograma da paca (Agouti paca, Linnaeus, 1766) em cativeiro. [The ethogram of captive paca (Agouti paca, Linnaeus, 1766)]. Rev. Etol. 2001, 3, 3–14. [Google Scholar]
- Nogueira, S.S.C.; Nogueira-Filho, S.L.G.; Duarte, J.; Mendl, M. Temperament, plasticity, and emotions in defensive behaviour of paca (Mammalia, Hystricognatha). Animals 2021, 11, 293. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.F.; Lima, S.G.; Nogueira-Filho, S.L.G.; Held, S.; Paul, E.; Mendl, M.; Nogueira, S.S.C. Vocal expression of emotions in farmed spotted paca (Cuniculus paca). Appl. Anim. Behav. Sci. 2022, 256, 105753. [Google Scholar] [CrossRef]
- Altino, V.S.; Rezende, D.C.; Nogueira, S.S.C.; Aldrigui, L.G.; Roldan, M.; Duarte, J.M.; Fureix, C.; Mendel, M.; Nogueira-Filho, S.L.G. Validation of complementary non-invasive tools for stress assessment in spotted paca (Cuniculus paca). Anim. Welf. 2023, 32, e54. [Google Scholar] [CrossRef]
- Mason, G.J. Species differences in responses to captivity: Stress, welfare, and the comparative method. Trends Ecol. Evol. 2010, 25, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Waiblinger, S.; Boivin, X.; Pedersen, V.; Tosi, M.V.; Janczak, A.M.; Visser, E.K.; Jones, R.B. Assessing the human–animal relationship in farmed species: A critical review. Appl. Anim. Behav. Sci. 2006, 101, 185–242. [Google Scholar] [CrossRef]
- Zulkifli, I. Review of human-animal interactions and their impact on animal productivity and welfare. J. Anim. Sci. Biotechnol. 2013, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, A. Consumer demand theory and the assessment of animal welfare. Anim. Behav. 1987, 35, 293–295. [Google Scholar] [CrossRef]
- Dudink, S.; Simonse, H.; Marks, I.; de Jonge, F.H.; Spruijt, B.M. Announcing the arrival of enrichment increases play behaviour and reduces weaning-stress-induced behaviours of piglets directly after weaning. Appl. Anim. Behav. Sci. 2006, 101, 86–101. [Google Scholar] [CrossRef]
- Altmann, J. Observational study of behavior: Sampling methods. Behaviour 1974, 49, 227–266. [Google Scholar] [CrossRef]
- Cordoni, G. Social play in captive wolves (Canis lupus): Not only an immature affair. Behaviour 2009, 146, 1363–1385. [Google Scholar] [CrossRef]
- Lee, P.C.; Moss, C.J. African elephant play, competence, and social complexity. Anim. Behav. Cogn. 2014, 1, 144–156. [Google Scholar] [CrossRef]
- Martin, J.E.; Ison, S.H.; Baxter, E.M. The influence of neonatal environment on piglet play behaviour and post-weaning social and cognitive development. Appl. Anim. Behav. Sci. 2015, 163, 69–79. [Google Scholar] [CrossRef]
- Yang, C.H.; Ko, H.L.; Salazar, L.C.; Llonch, L.; Manteca, X.; Camerlink, I.; Llonch, P. Pre-weaning environmental enrichment increases piglets’ object play behaviour on a large-scale commercial pig farm. Appl. Anim. Behav. Sci. 2018, 202, 7–12. [Google Scholar] [CrossRef]
- Horback, K. Nosing around: Play in pigs. Anim. Behav. Cogn. 2022, 2, 186. [Google Scholar] [CrossRef]
- Heffner, C.L. Research Methods for Education, Psychology and the Social Sciences. 2004. Available online: https://allpsych.com/research-methods (accessed on 12 September 2022).
- Hänninen, L.; Pastell, M. CowLog: Open-source software for coding behaviors from digital video. Behav. Res. Methods 2009, 41, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Charif, R.A.; Waack, A.M.; Strickman, L.M. Raven Pro 1.4 User’s Manual; Cornell Lab of Ornithology: Ithaca, NY, USA, 2010. [Google Scholar]
- Boersma, P.; Weenink, D. Praat: Doing Phonetics by Computer, version 5.3.06; Computer Software; Institute of Phonetic Sciences: Amsterdam, The Netherlands, 2022; Available online: http://www.praat.org (accessed on 3 December 2022).
- Briefer, E.F. Vocal expression of emotions in mammals: Mechanisms of production and evidence. J. Zool. 2012, 288, 1–20. [Google Scholar] [CrossRef]
- Pelletier, A.N.; Kaufmann, T.; Mohak, S.; Milan, R.; Nahallage, C.A.D.; Agung, I.G. Behavior systems approach to object play: Stone handling repertoire as a measure of propensity for complex foraging and percussive tool use in the genus Macaca. Anim. Behav. Cogn. 2017, 4, 455–473. [Google Scholar] [CrossRef]
- Greene, W.E.; Melillo-Sweeting, K.; Dudzinski, K.M. Comparing object play in captive and wild dolphins. Int. J. Comp. Psychol. 2011, 24, 292–306. [Google Scholar] [CrossRef]
- Cooper, M.A.; Grizzell, J.A.; Whitten, C.J.; Burghardt, G.M. Comparing the ontogeny, neurobiology, and function of social play in hamsters and rats. Neurosci. Biobehav. Rev. 2023, 147, 105102. [Google Scholar] [CrossRef]
- Smythe, N.P. Paca. In Little Know Small Animals with Promising Economic Future. Microlivestock; Robinson, J.G., Redford, K.H., Eds.; National Academy: Washington, DC, USA, 1991; pp. 263–269. [Google Scholar]
- Bonatelli, M. A Subplacenta da Paca (Agouti paca, Linnaeus 1766). Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2005. [Google Scholar]
- Gifford, A.K.; Cloutier, S.; Newberry, R.C. Objects as enrichment: Effects of object exposure time and delay interval on object recognition memory of the domestic pig. Appl. Anim. Behav. Sci. 2007, 107, 206–217. [Google Scholar] [CrossRef]
- Fureix, C.; Meagher, R.K. What can inactivity (in its various forms) reveal about affective states in non-human animals? A review. Appl. Anim. Behav. Sci. 2015, 171, 8–24. [Google Scholar] [CrossRef]
- Young, R. Environmental Enrichment; Blackwell Science: Oxford, UK, 2003. [Google Scholar]
- Palagi, E. Social play in bonobos (Pan paniscus) and chimpanzees (Pan troglodytes): Implications for natural social systems and interindividual relationships. Am. J. Phys. Anthropol. 2006, 129, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Serres, A.; Hao, Y.; Wang, D. Body contacts and social interactions in captive odontocetes are influenced by the context: An implication for welfare assessment. Animals 2020, 10, 924. [Google Scholar] [CrossRef] [PubMed]
- Yamanashi, Y.; Nogami, E.; Teramoto, M. Adult-adult social play in captive chimpanzees: Is it indicative of positive animal welfare? Appl. Anim. Behav. Sci. 2018, 199, 75–83. [Google Scholar] [CrossRef]
- Cordoni, G.; Pirarba, L.; Elies, S.; Demuru, E.; Guéry, J.P.; Norscia, I. Adult–adult play in captive lowland gorillas (Gorilla gorilla gorilla). Primates 2022, 63, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Machado, S.P.; Caldara, F.R.; Foppa, L.; de Moura, R.; Gonçalves, L.M.P.; Garcia, R.G.; Nääs, I.d.A.; Nieto, V.M.O.d.S.; de Oliveira, G.F. Behavior of pigs reared in enriched environment: Alternatives to extend pigs attention. PLoS ONE 2017, 12, e0168427. [Google Scholar] [CrossRef]
- Spinka, M.; Newberry, R.C.; Bekoff, M. Mammalian play: Training for the unexpected. Q. Rev. Biol. 2001, 76, 141–168. [Google Scholar] [CrossRef]
- Bekoff, M.; Byers, J.A. Animal Play: Evolutionary, Comparative and Ecological Approaches; Cambridge University Press: New York, NY, USA, 1998. [Google Scholar]
- Meagher, R.K.; Mason, G.J. Environmental enrichment reduces signs of boredom in caged mink. PLoS ONE 2012, 7, e49180. [Google Scholar] [CrossRef]
- van der Harst, J.E.; Baars, A.M.; Spruijt, B.M. Standard housed rats are more sensitive to rewards than enriched housed rats as reflected by their anticipatory behaviour. Behav. Brain Res. 2003, 142, 151–156. [Google Scholar] [CrossRef]
- Kuczaj, S.; Lacinak, T.; Fad, O.; Trone, M.; Solangi, M.; Ramos, J. Keeping environmental enrichment enriching. Int. J. Comp. Psychol. 2002, 15, 127–137. [Google Scholar] [CrossRef]


| Behavior * | Description | Reference |
|---|---|---|
| Affiliative | The individual touch with its snout the snout of another paca and/or may lie side by side. | [28] |
| Agonistic | The individual attacks another that does not respond to the aggression; the individual attacks another that responds aggressively, moving forward with fur raised and sometimes vocalizing. | [26,28] |
| Object play (with boomer ball) ** | The individual picks up the ball and then moves around the pen with it (carrying); or it can take the ball to the shelter (taking); or it can bite the ball repeatedly (biting) and then touch the ball with one of its paws (touching) or it can do both movements (touching and biting); it can also roll the ball with its paw (rolling). | [38,39,40,41,42] |
| Exploratory | The individual sniffs the air with its head up. It may also sniff the ground or objects in the pen, except for the balls, with its head down. | [28] |
| Bark call | A call produced alone or in sequences of two to ten short elements (notes). *** | [24,30] |
| Play Behavior | Sex | Occurrence | Mean (s) |
|---|---|---|---|
| Carrying | Female | 3 | 16.3 (9.1) |
| Male | 2 | 20.6 (18.5) | |
| Taking | Female | 1 | 60.5 (-) |
| Male | 1 | 12.7 (-) | |
| Biting | Female | 7 | 55.5 (21.2) |
| Male | 5 | 55.9 (19.2) | |
| Touching | Female | 1 | 5.5 (-) |
| Male | 5 | 94.5 (-) | |
| Touching and biting | Female | 2 | 25.3 (11.8) |
| Male | 6 | 14.2 (4.7) | |
| Rolling | Female | 2 | 23.8 (14.8) |
| Behavior | Factor | F-Value | p-Value |
|---|---|---|---|
| Affiliative | Sex | F1, 8.28 = 2.16 | 0.179 |
| Phase | F1, 7.83 = 23.49 | 0.001 | |
| Sex × Phase | F1, 7.83 = 4.94 | 0.058 | |
| Exploratory | Sex | F1, 92 = 0.76 | 0.386 |
| Phase | F1, 92 = 18.48 | <0.001 | |
| Sex × Phase | F1, 92 = 1.38 | 0.257 | |
| Agonistic | Sex | F1, 24 = 0.03 | 0.386 |
| Phase | F2, 24 = 3.93 | 0.033 | |
| Sex × Phase | F2, 24 = 0.42 | 0.660 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, A.F.d.; Lima, S.G.C.; Nogueira-Filho, S.L.G.; Held, S.D.E.; Mendl, M.; Nogueira, S.S.C. Object Play as a Positive Emotional State Indicator for Farmed Spotted Paca (Cuniculus paca). Animals 2024, 14, 78. https://doi.org/10.3390/ani14010078
Lima AFd, Lima SGC, Nogueira-Filho SLG, Held SDE, Mendl M, Nogueira SSC. Object Play as a Positive Emotional State Indicator for Farmed Spotted Paca (Cuniculus paca). Animals. 2024; 14(1):78. https://doi.org/10.3390/ani14010078
Chicago/Turabian StyleLima, Allison F. de, Stella G. C. Lima, Sérgio L. G. Nogueira-Filho, Suzanne D. E. Held, Michael Mendl, and Selene S. C. Nogueira. 2024. "Object Play as a Positive Emotional State Indicator for Farmed Spotted Paca (Cuniculus paca)" Animals 14, no. 1: 78. https://doi.org/10.3390/ani14010078
APA StyleLima, A. F. d., Lima, S. G. C., Nogueira-Filho, S. L. G., Held, S. D. E., Mendl, M., & Nogueira, S. S. C. (2024). Object Play as a Positive Emotional State Indicator for Farmed Spotted Paca (Cuniculus paca). Animals, 14(1), 78. https://doi.org/10.3390/ani14010078







