Simple Summary
Salmonella typhimurium is one of the most widespread enteric pathogens causing enterocolitis in warm-blooded animals worldwide. It is frequently spread within pig production and threatens not only primary hosts but consumers of pork meat as well. Overall, finding novel non-antibiotic agents is desirable in preventing salmonellosis outbreaks or reducing ongoing illness, where multi-strain probiotics are promising adepts. We tested a multi-strain bacterial mixture in the gnotobiotic piglet model to verify potential probiotic and anti-Salmonella properties in vivo. The bacterial consortium colonized the gut successfully and protected piglet hosts against Salmonella translocation to the blood system. Thus, the assembled bacterial mix has a promising potential for probiotic intervention in pig production management.
Abstract
Probiotics are a potential strategy for salmonellosis control. A defined pig microbiota (DPM) mixture of nine bacterial strains previously exhibited probiotic and anti-Salmonella properties in vitro. Therefore, we evaluated its gut colonization ability and protection effect against S. typhimurium LT2-induced infection in the gnotobiotic piglet model. The DPM mixture successfully colonized the piglet gut and was stable and safe until the end of the experiment. The colon was inhabited by about 9 log CFU g−1 with a significant representation of bifidobacteria and lactobacilli compared to ileal levels around 7–8 log CFU g−1. Spore-forming clostridia and bacilli seemed to inhabit the environment only temporarily. The bacterial consortium contributed to the colonization of the gut at an entire length. The amplicon profile analysis supported the cultivation trend with a considerable representation of lactobacilli with bacilli in the ileum and bifidobacteria with clostridia in the colon. Although there was no significant Salmonella-positive elimination, it seems that the administered bacteria conferred the protection of infected piglets because of the slowed delayed infection manifestation without translocations of Salmonella cells to the blood circulation. Due to its colonization stability and potential protective anti-Salmonella traits, the DPM mixture has promising potential in pig production applications. However, advanced immunological tests are needed.
1. Introduction
The mammalian gut microbiome is considered a functional organ of the overall system of the host’s organism that is involved in substantial nutrient and drug metabolism, immunomodulation, stabilization of the gut mucosal barrier, and protection against pathogens [1,2,3]. Microbiome establishment with subsequent development is a critical phase of the young’s life [4,5] that is regulated by several factors, notably environmental [6]. If microbial balance is not preserved or is long-term disturbed by antibiotic effects, dysbiosis usually accompanied by a loss of microbial diversity can predispose to developing several unsolicited health problems, such as an onset of chronic inflammatory diseases [7]. Moreover, debilitated individuals may then be prone to outbreaks of fatal bacterial infections by pathogens to the detriment of beneficial microbes [8,9].
A dysbiotic gut could be a conducive environment for evolving Enterobacteriaceae members, which profit in these unfavorable conditions for other bacterial commensals [10,11,12]. For example, Salmonella spp. is a minacious pathogen entering the food chain, significantly threatening the overall health of the population, especially through the contamination of products of animal origin, including pork meat [13,14,15]. Specifically, non-typhoidal Salmonella enterica subsp. enterica serovar Typhimurium (S. typhimurium) is mostly dangerous for young or immunocompromised individuals, causing enterocolitis affecting the host’s distal ileum and colon [16,17]. Salmonella strains are commonly spread throughout the farm, and even suckling piglets are a risk source [18]. Stress and weaning, often accompanied by intestinal dysbiosis, are the ideal opportunities for the outbreak of salmonellosis in piglets [19,20].
There are several approaches to suppress salmonellosis infection in pigs. The probiotic intervention seems to be a promising supportive mitigation strategy [21,22], competing not only against pathogen attachment but also subsequent disease development [23]. Probiotics are life microorganisms whose administration in adequate amounts confers health benefits to the hosts [24]. In addition, thanks to all probiotic functional properties, they have the potential to replace some antibiotics [25,26], which are still widely administered, although there is a long-term approach to mitigate the spread of antibiotic resistance that has a far-reaching adverse effect on overall population health [27,28,29]. Furthermore, probiotics can exhibit antitoxin effects and contribute to an increase in growth performance [30]. Promisingly, the current trend of probiotics application in animal husbandry is still growing [31].
Recent studies pointed out several microbes with probiotic potential, promising for administration in swine production, such as lactobacilli [32], bifidobacteria [33], enterococci [34], Bacillus spp. [35], Clostridium spp. [36], and Saccharomyces cerevisiae [37]. Moreover, multi-strain probiotic mixtures could confer more complex effects with variable mechanisms, but it is crucial to focus on the evidence-based trials of probiotics efficacy [38]. Probiotic potential testing usually starts at the in vitro level with the subsequent implementation of in vivo assays to simulate the effect in complex host environments [39,40].
We previously developed a defined pig microbiota (DPM) mixture with in vitro-confirmed anti-Salmonella activity [41]. The aim of this work was to evaluate the colonization ability of this bacterial consortium and to verify its protective effect against salmonellosis induction, or the mitigation of induced infection, in vivo in gnotobiotic piglets by monitoring the clinical signs of infection and analyzing the ileal and colon contents by cultivation and microbiome analyses.
2. Materials and Methods
2.1. Ethical Approval
The work with animals was conducted according to the ethical standards defined by the EU legislation on the use of experimental animals (2010/63/EU) and approved by the Animal Care and Use Committee of the Czech Academy of Sciences (protocol 57/2021; 18 August 2021).
2.2. Design of In Vivo Experiment
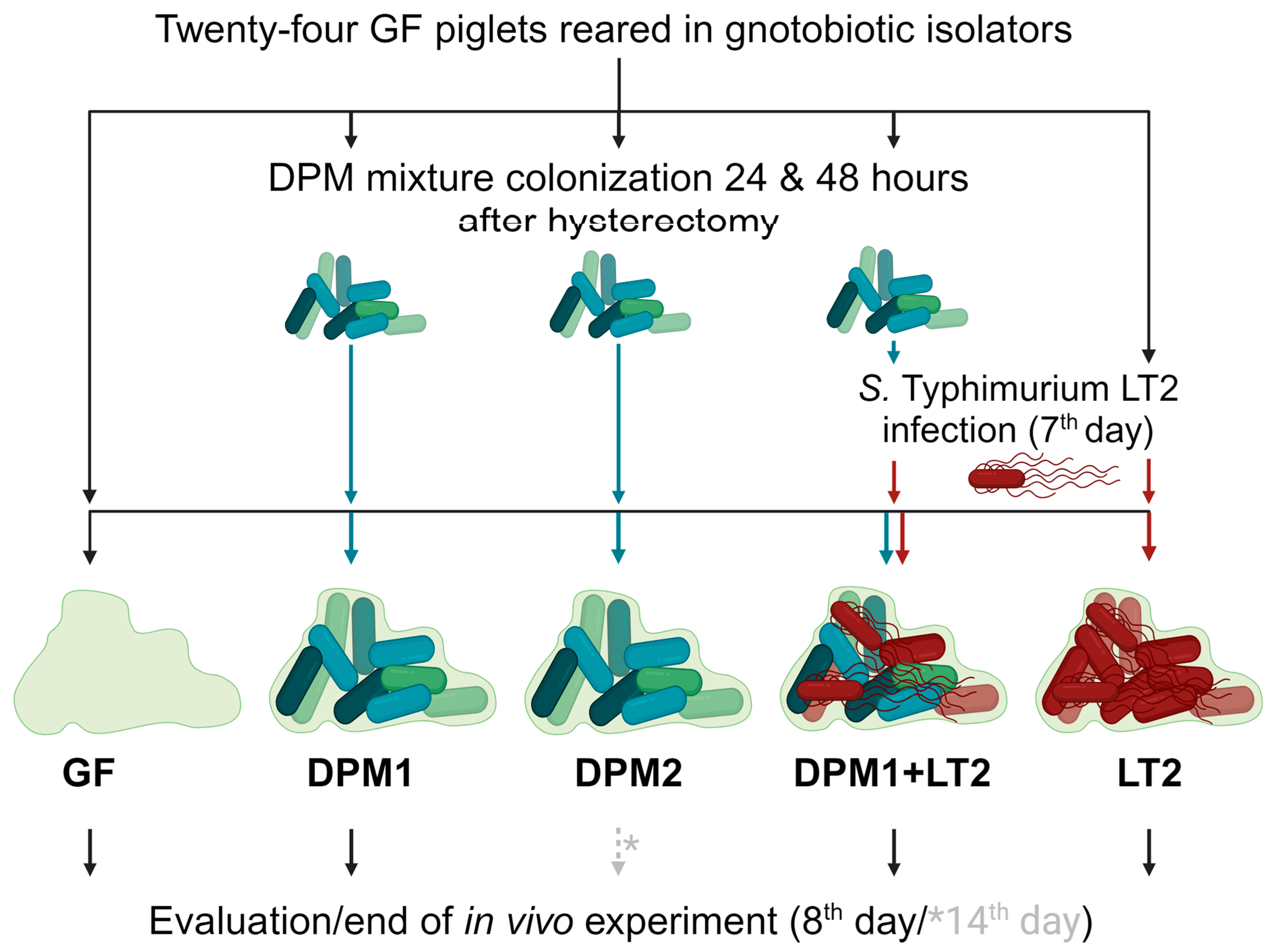
Germ-free (GF) piglets were obtained by the hysterectomy of pregnant miniature sows (Animal Research Institute, Kostelec nad Orlici, Czech Republic) under isoflurane anesthesia, and they were reared in gnotobiotic isolators and fed an autoclave-sterilized cow milk-based diet (CMD) with mineral and vitamin supplements [42]. Twenty-four gnotobiotic piglets were divided into five experimental groups: GF (GF piglets; negative control), LT2 (piglets infected with S. typhimurium LT2; positive control), DPM1 (piglets colonized by DPM mixture for 8 days), DPM2 (piglets colonized by DPM mixture for 14 days), and DPM1 + LT2 (piglets colonized by DPM mixture and then infected with S. typhimurium LT2) (Figure 1).
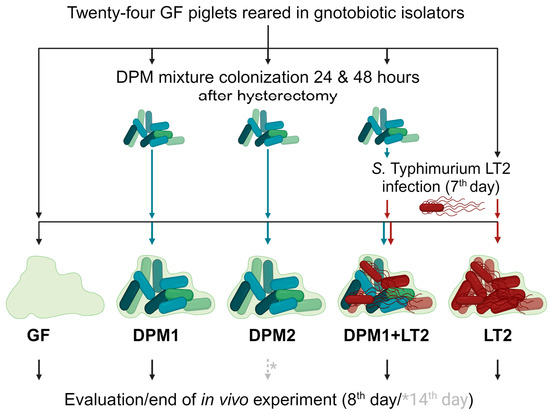
Figure 1.
Schema of in vivo experiment. Gnotobiotic piglets (n = 24) were divided into five experimental groups and were kept for two experimental time periods (8 days/14 days). After 24 and 48 h after hysterectomy, three groups (DPM1, DPM2, and DPM1 + LT2) were orally colonized by the defined pig microbiota mixture. On the seventh day of the piglets’ life, two groups (DPM1 + LT2 and LT2) were infected with S. typhimurium LT2. The eighth day was the end of the in vivo experiment for all groups with the exception of the DPM2 group, which ended on the fourteenth day. GF—germ-free; DPM—defined pig microbiota; DPM1—piglets associated with DPM mixture for 8 days; DPM2—piglets associated with DPM mixture for 14 days; DPM1 + LT2—piglets associated with DPM mixture and infected with S. typhimurium LT2; LT2—piglets infected with S. typhimurium LT2.
In general, GF piglets were repeatedly orally colonized with a single dose of DPM mixture (previously stored at −80 °C) in a CMD containing 6 × 108 CFU, 24 h and 48 h after hysterectomy. Then, seven-day-old piglets were orally infected by 1 × 106 CFU mL−1 of S. typhimurium LT2 in the CMD, as well [43]. Twenty-four hours after the Salmonella infection, the experiment was terminated for all groups except the DPM2 group, whose time was prolonged by the next six days (termination on their fourteenth day) for monitoring the DPM mixture’s safety over time and colonization stability. All animals were examined for clinical signs of enterocolitis (fever, anorexia, somnolence, and diarrhea) during the whole experiment. Finally, all subjects were exsanguinated via cardiac puncture under isoflurane anesthesia and samples, such as ileum and colon contents, mesenteric lymph nodes, and blood, were taken for cultivation and microbiome analysis at the end.
2.3. Bacteria Propagated for Association/Infection of Gnotobiotic Piglets
A DPM mixture consisting of nine bacterial strains, namely Bacillus sp. PG1, Bifidobacterium animalis subsp. lactis PG2, B. porcinum PG3, Clostridium sporogenes PG4, Lactobacillus amylovorus PG6, L. paracasei subsp. tolerans PG5, and three Limosilactobacillus reuteri strains (PG7, PG8, PG9), was prepared as described by Horvathova et al. [41]. These strains have previously exhibited anti-Salmonella activity, the ability to aggregate, adherence to epithelial cells, and bile and acid tolerance, and they have been without mutual inhibition and classified as safe without a pathogenic phenotype and resistance to antibiotics in vitro.
The S. typhimurium LT2 inoculum [44] was prepared and 1 × 106 CFU mL−1 per piglet was orally applied in 1 mL of CMD as described previously Splichal et al. [45]. The used dose was verified by cultivation overnight at 37 °C on Brilliant Green agar (Oxoid, Basingstoke, UK).
2.4. Cultivation Analysis
Ileum and colon content, mesenteric lymph nodes, and blood samples were analyzed for the presence of administered pig microbiota and S. typhimurium LT2 according to Modrackova et al. [46]. All samples were collected in tubes containing a dilution buffer consisting of 5 g L−1 of tryptone, 5 g L−1 of nutrient broth No. 2, 2.5 g L−1 of yeast extract (all Oxoid), 0.5 g L−1 of L-cysteine, 1 mL L−1 of Tween 80 (both Sigma-Aldrich, St. Louis, MO, USA), 30% glycerol (VWR, Radnor, PA, USA), and glass pearls for homogenization. All media were prepared in an oxygen-free carbon dioxide environment [47] and then sterilized. Immediately after dissection and sampling, all samples were frozen at −20 °C and transported to the analysis site within the same week. Then, decimal serial dilutions were spread on the following media. Wilkins-Chalgren Anaerobe Agar supplemented with 5 g L−1 of GMO-Free Soya Peptone (both Oxoid), 0.5 g L−1 of L-cysteine, and 1 mL L−1 of Tween 80 was used to determine the total counts of anaerobic bacteria (WSP). The same WSP medium was used for heat-resistant spore-forming bacteria (clostridia/bacilli group) as well; however, the inocula were previously treated with 15 min of pasteurization at 80 °C before plating. Then, the following two WSP medium variants were used for bifidobacteria: WSP supplemented with 100 mg L−1 of mupirocin (Oxoid) and 1 mL L−1 of acetic acid (Sigma-Aldrich) [48] (WSP-MUP), and WSP supplemented with 100 mg L−1 of mupirocin, 100 mg L−1 of norfloxacin (Oxoid), and 1 mL L−1 of acetic acid [49] (WSP-NORF). All the plates were incubated anaerobically using GENbag anaer (bioMérieux, Craponne, France) at 37 °C for 2 days. Then, Rogosa Agar (Oxoid) with 1.32 mL L−1 of acetic acid was used for the quantification of lactobacilli with subsequent cultivation under microaerophilic conditions at 37 °C for 3 days, and SS agar (Oxoid) for S. typhimurium LT2 under aerobic conditions at 37 °C for 24 h. An identity of determined bacteria was always verified with MALDI-TOF mass spectrometry (MALDI-TOF MS) using an extended direct transfer procedure with an HCCA matrix solution (α-cyano-4-hydroxycinnamic acid) according to the manufacturer’s instructions (Bruker Daltonik GmbH, Bremen, Germany) using Biotyper software (server distribution version 4.1.100 (PYTH), build 174; server module version 4.3.18, build 330).
Statistical Analyses of Cultivation Data
The bacterial mixture stability in vivo in time and its potential protective effect against S. typhimurium LT2 infection in gnotobiotic piglets (cultivation counts in log CFU g−1) were evaluated using StatSoft, Inc. (2013) (STATISTICA—data analysis software system, version 12, www.statsoft.com (accessed on 18 January 2024); Tulsa, OK, USA) and MS Excel 2013 (Redmond, WA, USA). The normality of the data was assessed by the Shapiro–Wilk W test (α = 0.05). Differences between the levels of lactobacilli, bifidobacteria, and clostridia/bacilli within the ileum and colon and two colonization time durations (DPM1, DPM2 animal groups) were evaluated using a t-test (α = 0.05). Then, differences in bacterial counts between DPM1 and DPM1 + LT2 groups, and similarly DPM1 + LT2 and LT2 groups, within the ileum and colon separately were assessed using a t-test (α = 0.05) and a Mann–Whitney U Test (α = 0.05).
2.5. 16S rRNA Gene Amplicon Sequencing
Total genomic DNA was extracted from 250 mg of ileum and colon fecal content using the QIAamp® PowerFecal® Pro DNA Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The extracted DNA was then used as a template for the preparation of amplicons from the V4 region of the 16S rRNA gene [50]. Purified amplicons were used to prepare libraries using the NEBNext Fast DNA Library Prep Set kit (New England Biolabs, Ipswich, MA, USA) according to Milani et al. [51]. The sequencing was then performed on the PGM sequencer of the Ion Torrent platform (Thermo Fisher Scientific, Waltham, MA, USA) as it was described previously by Mekadim et al. [52].
2.6. Microbiome Analyses
Analysis of the obtained sequences was carried out using the QIIME 2 version 2022.2 pipeline [53]. Raw sequences were filtered and trimmed using DADA2 [54]. Then, the obtained amplicon sequence variants (ASVs) were taxonomically classified using VSEARCH based on the SILVA database (release 138) with a 99% threshold [55]. The rarefaction was performed based on the sequence depth to normalize data. The alpha diversity of different animal groups was determined using Chao1, Shannon, and Simpson diversity indexes based on the Kruskal–Wallis test. Principal Coordinate Analysis (PCoA) was evaluated based on the weighted and unweighted unifrac distance (beta diversity). The boxplots for alpha diversity and the two-dimensional PCoA plots were generated in R-Studio (http://www.rstudio.com/, accessed on 16 October 2023) using qiime2R (https://github.com/jbisanz/qiime2R, accessed on 16 October 2023) and ggplot2 (https://ggplot2.tidyverse.org, accessed on 16 October 2023) packages. Permutational multivariate analysis of variance (PERMANOVA/Adonis) based on distance matrices was used to evaluate the dissimilarity among animal groups with the permutation set at 999. Linear discriminant analysis with the effect size (LEfSe) algorithm [56] was performed in the Galaxy module (http://huttenhower.sph.harvard.edu/galaxy (accessed on 18 October 2023) to identify bacterial genera with significant differential relative abundances between animal groups based on the Kruskal–Wallis test and the pairwise Wilcoxon test with an α value of 0.05 and threshold value at 2.0.
2.7. Data Accessibility
Sequences of raw data files were deposited in the NCBI database under Sequence Read Archive (SRA) accession numbers SUB13949577 and BioProject ID PRJNA1035042.
3. Results
To evaluate the ability of the DPM mixture to colonize the gut of piglets, namely the ileum and colon, and its possible protective effect against the infection caused by S. typhimurium LT2, the cultivation analysis and amplicon sequencing approaches were combined. In general, gnotobiotic piglets were divided into five monitored groups with different schemes of bacterial associations for the assessment of various DPM mixture’s abilities. The GF control animal group receiving no bacteria and only sterile CMD throughout the experiment confirmed the gnotobiotic model assay with both approaches.
3.1. Colonization Ability of DPM Mixture in Gnotobiotic Piglets
The colonization ability of the DPM mixture and its potential competitive capability against S. typhimurium LT2 were assessed in DPM1 + LT2, DPM1, and LT2 animal groups within two parts of the piglet gut, the ileum and colon. The detected high quantity of viable bacteria present in both parts of the gut confirmed the success of colonization of all bacteria from the administered bacterial consortium (Supplementary Material—Table S1). We detected no statistically significant differences in bacterial representation (lactobacilli, clostridia/bacilli, and S. typhimurium LT2) between the monitored experimental groups, except for bifidobacteria. Specifically, bifidobacterial counts on WSP-NORF were significantly higher in DPM1 (7.56 ± 0.14 log CFU g−1) compared to the DPM1 + LT2 (6.90 ± 0.63 log CFU g−1) group in the ileum, and on WSP-MUP in DPM1 (9.38 ± 0.23 log CFU g−1) compared to DPM1 + LT2 (8.43 ± 0.77 log CFU g−1) in the colon. However, based on the cultivation results, the infection caused by S. Typhimurium LT2 did not significantly influence other prior administered bacteria and vice versa.
Comparing the bacterial consortium colonization ability of two different parts of the piglets’ gut, our results showed that the DPM mixture successfully colonized both (Supplementary Material—Table S2). In general, the colon was considerably more colonized by administered bacteria in density more than 109 CFU g−1 of total counts of bacteria in all monitored experimental groups, while the bacteria present in the ileum reached lower concentrations of 107–108 CFU g−1. Specifically, the lactobacilli (9.13 ± 0.50 log CFU g−1) and bifidobacterial (9.38 ± 0.23 log CFU g−1 on WSP-MUP; 8.94 ± 0.13 log CFU g−1 on WSP-NORF) levels were significantly higher in the colon compared to a representation about an order of magnitude lower in the ileum within the DPM1 group. Similarly, the same significant trend was found for S. typhimurium LT2 within the DPM1 + LT2 (9.08 ± 0.48 compared to 8.27 ± 0.55 log CFU g−1) and LT2 (8.61 ± 0.05 compared to 7.73 ± 0.37 log CFU g−1) groups. Although not statistically significant, this difference was evident also for other bacterial groups.
3.2. Alpha and Beta Diversity of the Piglet’s Gut Microbiome
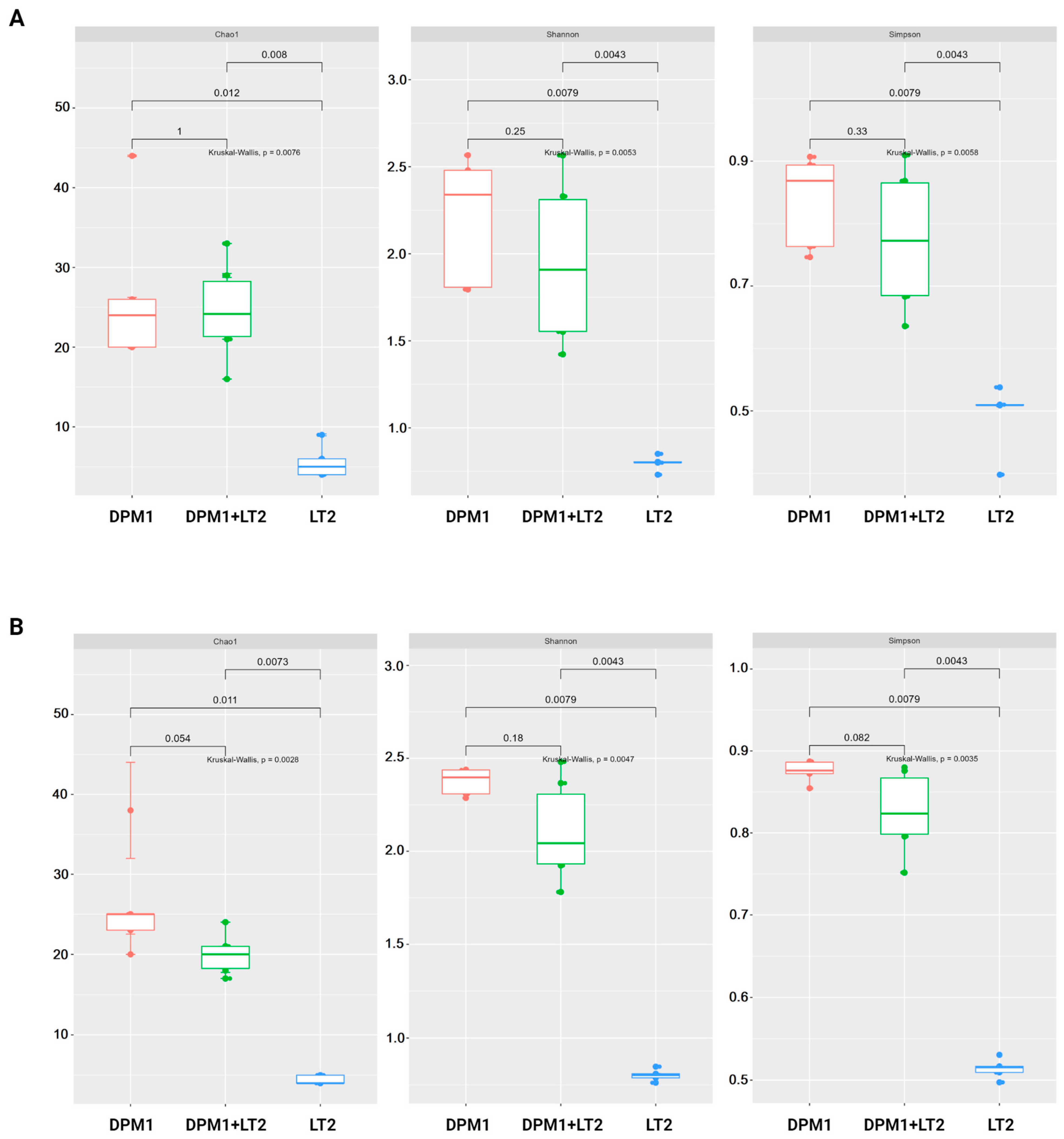
The results of alpha diversity using Chao1, Shannon, and Simpson diversity indexes are represented in the boxplot graph (Figure 2). In both the colon and ileum, bacterial diversity and richness were significantly higher in DPM1 and DPM1 + LT2 animal groups in comparison to the LT2 group (p ≤ 0.05). No significant difference was observed between DPM1 and DPM1 + LT2 groups in both intestinal parts (ileum and colon).
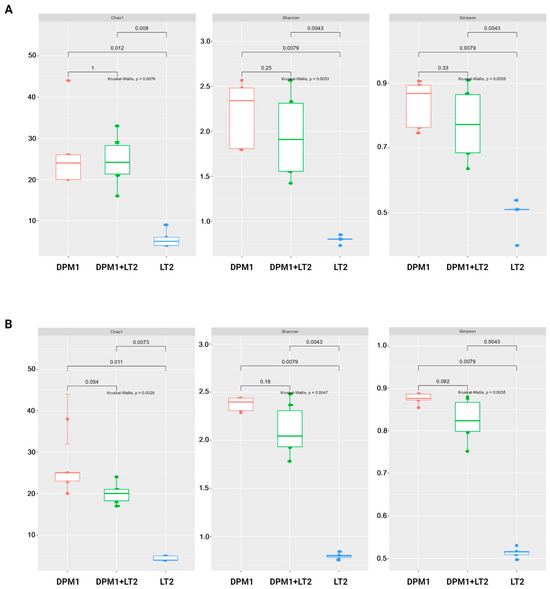
Figure 2.
Alpha diversity of the piglets’ gut microbiome. Boxplots illustrating alpha diversity using Chao1, Shannon, and Simpson diversity indices in the (A) ileum and (B) colon of DPM1, DPM1 + LT2, and LT2 animal groups. p ≤ 0.05 was considered statistically significant based on the Kruskal–Wallis test. DPM1—piglets associated with DPM mixture for 8 days; DPM1 + LT2—piglets associated with DPM mixture and infected with S. typhimurium LT2; LT2—piglets infected with S. typhimurium LT2.
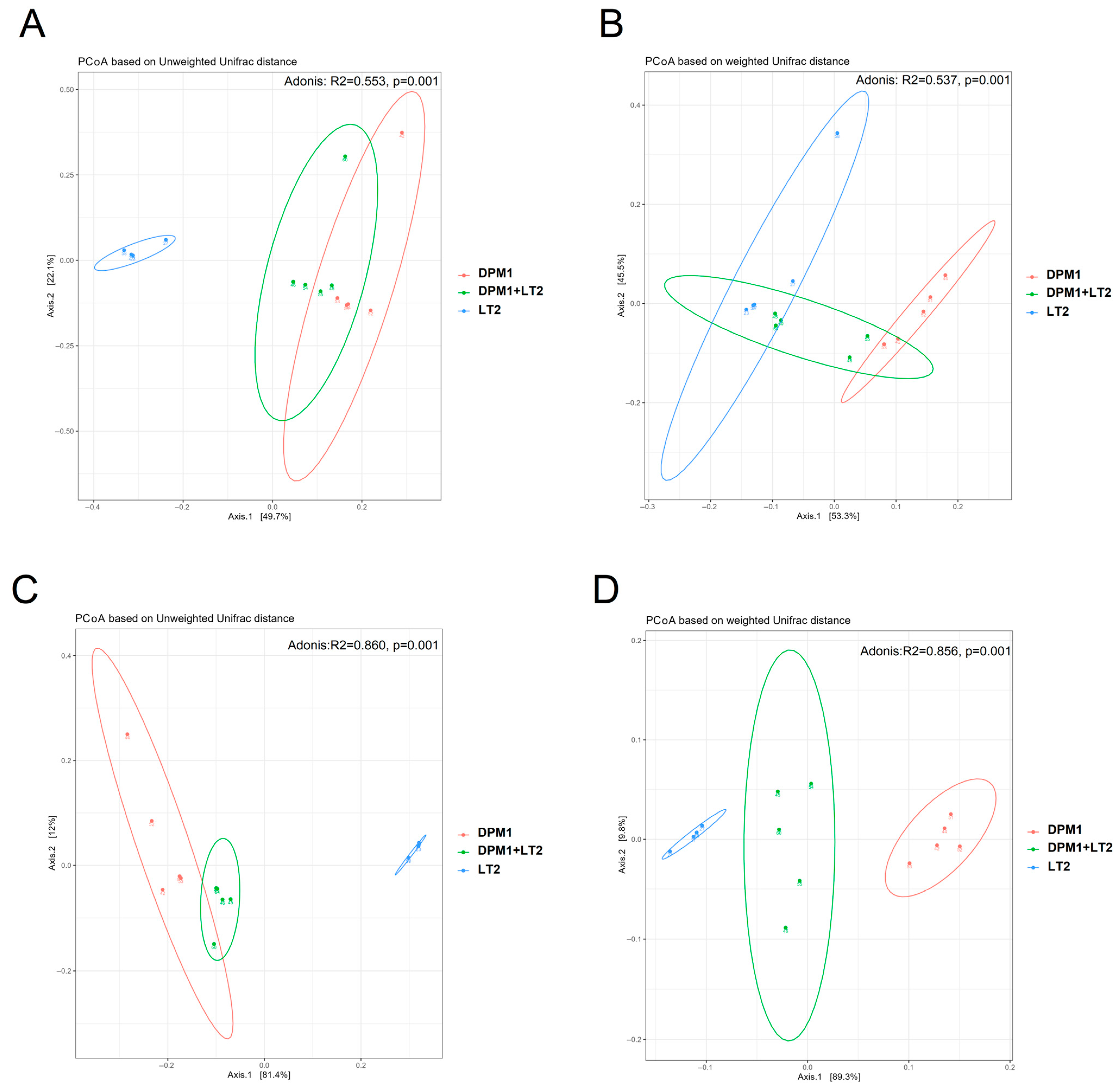
Principal Coordinate Analysis (PCoA) based on weighted and unweighted unifrac distances was performed to compare the bacterial diversity in the ileum and colon of DPM1, DPM1 + LT2, and LT2 animal groups (Figure 3). Both the colon and ileum presented distinguished and separated clusters showing a profound difference in microbiome diversity (p = 0.001) between different animal groups. Using the unweighted unifrac distance, the cluster of samples from LT2 animals was regrouped together and separated from the other clusters of DPM1 and DPM1 + LT2 groups that were clustered close.
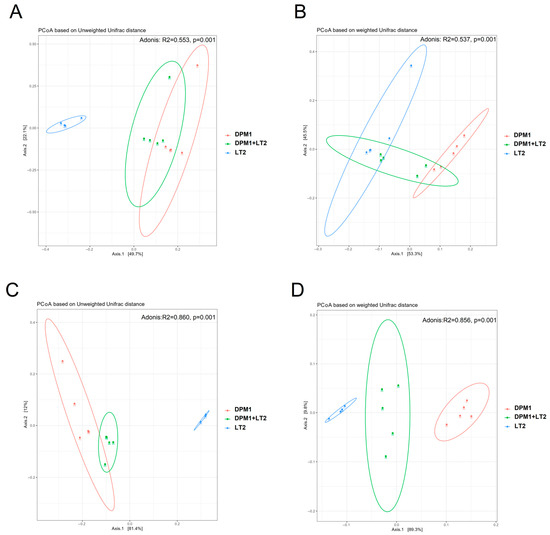
Figure 3.
Comparison of piglets’ microbiome beta bacterial diversity. The beta diversity using Principal Coordinate Analysis (PCoA) plots based on the unweighted (A,C) and weighted (B,D) unifrac distance showed distinct clusters of bacterial populations of the (A,B) ileum and (C,D) colon from DPM1, DPM1 + LT2, and LT2. p ≤ 0.05 was considered statistically significant. DPM1—piglets associated with DPM mixture for 8 days; DPM1 + LT2—piglets associated with DPM mixture and infected with S. typhimurium LT2; LT2—piglets infected with S. typhimurium LT2.
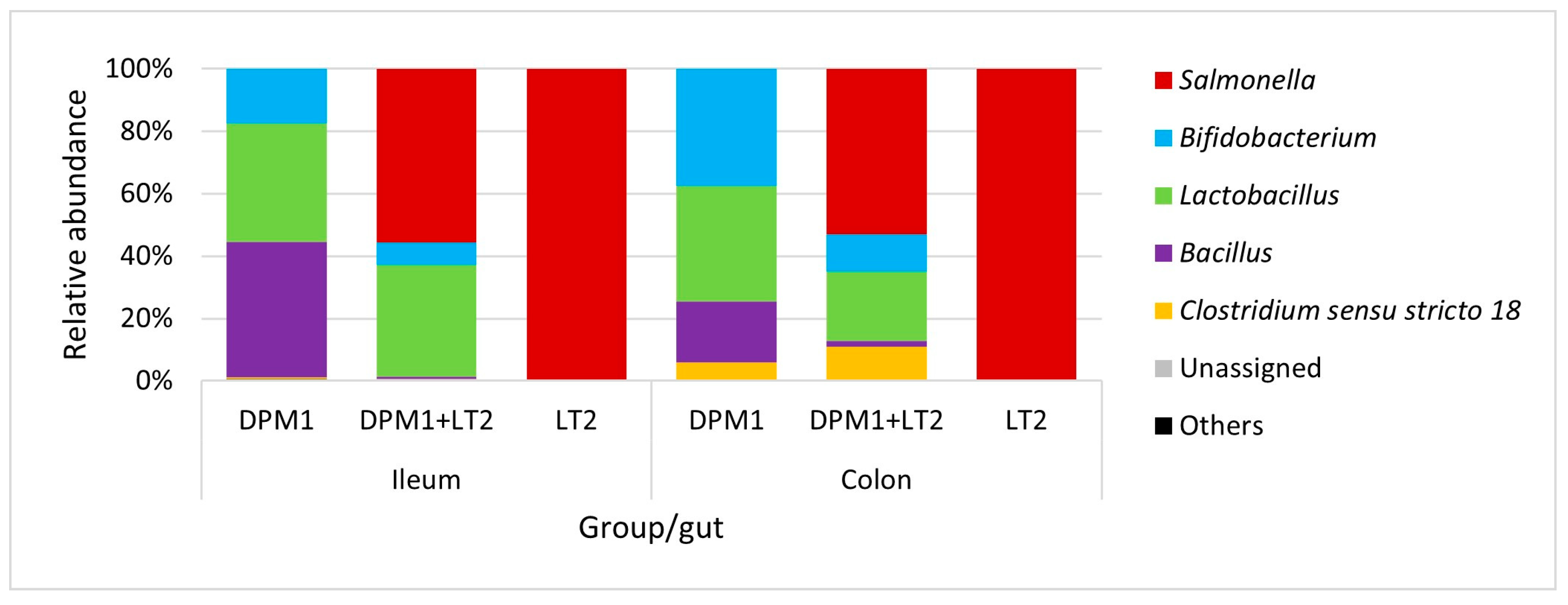
Bacillus, Lactobacillus, and Bifidobacterium were the most abundant genera in the ileum of DPM1 animals (Figure 4). We observed an increase in the abundance of Bifidobacterium spp. and Clostridium sensu stricto 18 and a decrease in the abundance of Bacillus spp. in the colon microbiota of the DPM1 animals. Salmonella was the dominant genus in the ileum microbiota of DPM1 + LT2. Lactobacillus spp. and Bifidobacterium spp. were also present in the ileum microbiota of the DPM1 + LT2 group. The abundance of Bifidobacterium spp. and Clostridium sensu stricto 18 was increased and the abundance of Lactobacillus spp. was decreased in the colon microbiota of the DPM1 + LT2 group with a dominance of Salmonella spp. Salmonella was the only bacterial genus present in both ileum and colon microbiota in the LT2 animal group.
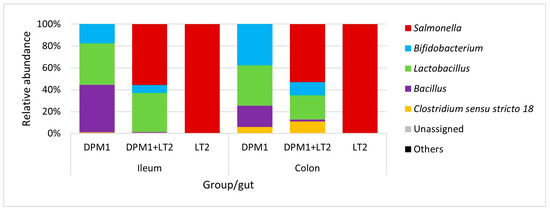
Figure 4.
Relative abundance of present bacteria in ileum and colon at the genus level. DPM1—piglets associated with DPM mixture for 8 days; DPM1 + LT2—piglets associated with DPM mixture and infected with S. typhimurium LT2; LT2—piglets infected with S. typhimurium LT2.
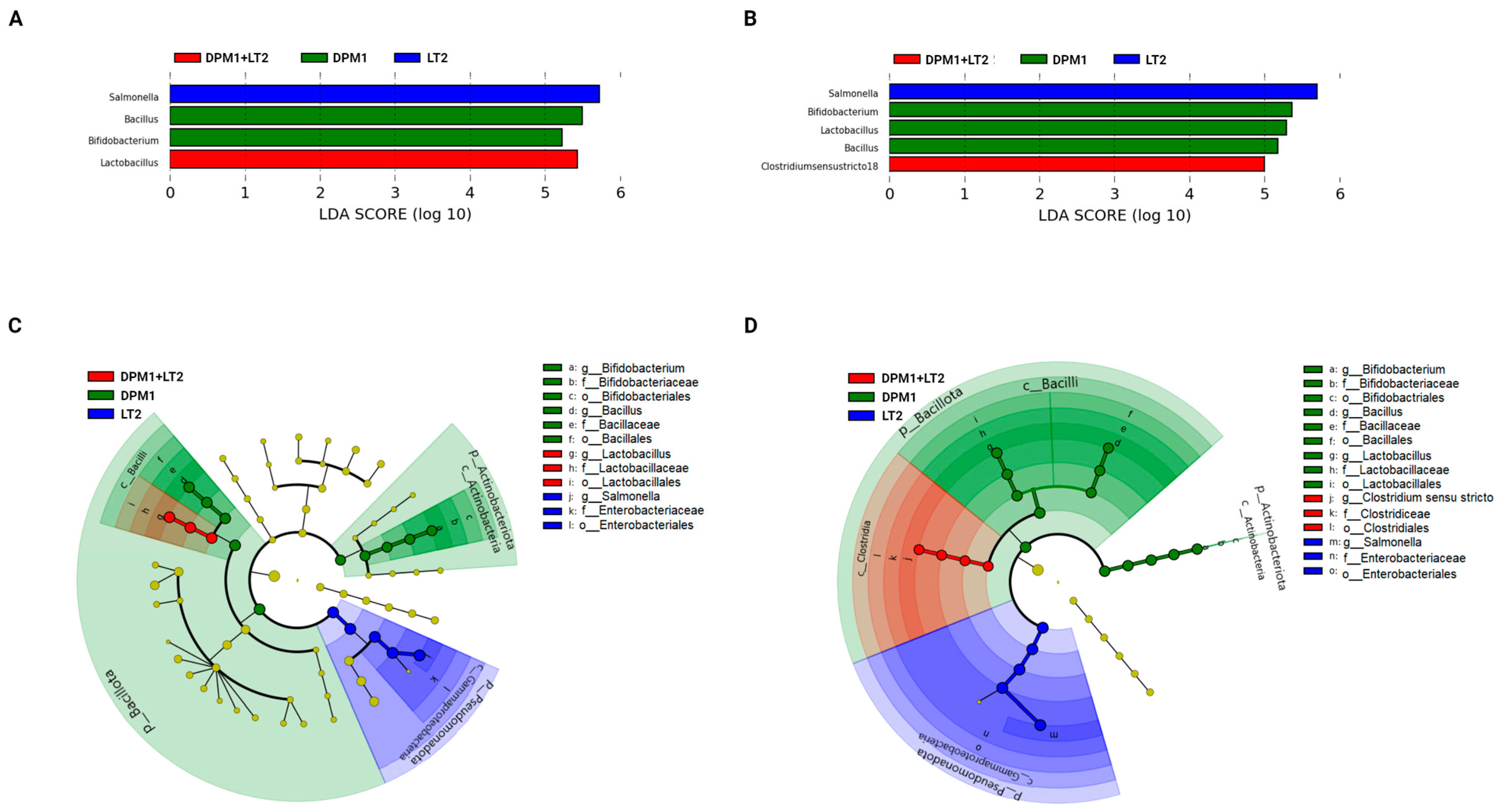
Linear discriminant analysis with effect size (LefSe) was used to detect bacterial genera (biomarkers) with significant differential relative abundances in ileum and colon microbiomes of animal groups (Figure 5). In the ileum, Bacillus and Bifidobacterium were the bacterial genera markers of the DPM1 animal groups. Lactobacillus was the only biomarker for DPM1 + LT2 pigs, while, in the colon, Bifidobacterium, Lactobacillus, and Bacillus were bacterial markers of the DPM1 animal groups. Clostridium sensu stricto 18 was the only bacterial genera marker of DPM1 + LT2 pigs. Salmonella was the unique biomarker of the infected LT2 animals in both ileum and colon microbiomes.
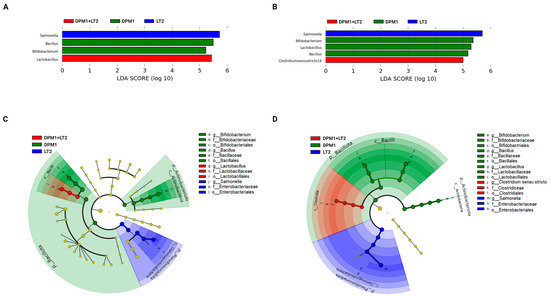
Figure 5.
Detected bacterial genera as biomarkers. Linear discriminant analysis effect size (LEfSe) of taxa at the genus level in the bacterial community of the (A) ileum and (B) colon of DPM1, DPM1 + LT2, and LT2 animal groups with alpha values of 0.05 and a threshold value of 2.0. [(C,D) are cladograms presenting a phylogenetic plot of LEfSe at different taxonomical levels of bacterial community in the (C) ileum and (D) colon of DPM1, DPM1 + LT2, and LT2 animal groups]. DPM1—piglets associated with DPM mixture for 8 days; DPM1 + LT2—piglets associated with DPM mixture and infected with S. typhimurium LT2; LT2—piglets infected with S. typhimurium LT2.
3.3. Assessment of a Possible Protective Effect of the Bacterial Mixture In Vivo
To assess the possible protection ability of the administered DPM mixture, all piglet groups were monitored for the clinical signs of infection. Piglet groups DPM1, DPM2, GF, and DPM1 + LT2 thrived for the whole experiment. Although DPM1 + LT2 individuals visually prospered, as well, there were detected typical clinical signs of the enteric infection such as fever, anorexia, sleepiness, and diarrhea; however, in decreased manifestations compared to the control LT2 group, in which the clinical signs initially appeared 6 h after infection. Moreover, cultivation analysis of blood samples showed the absence of bacteria within the DPM1, DPM2, and DPM1 + LT2 groups, while the presence of bacteria, specifically S. typhimurium LT2, in the blood was positive within the LT2 group (4.68 ± 0.68 CFU mL−1). Moreover, the bacterial presence in the mesenteric lymph nodes was confirmed, namely 4.86 ± 0.27 CFU g−1 for DPM1 + LT2 and 5.49 ± 0.43 CFU g−1 for LT2 groups.
3.4. Evaluation of Bacterial Consortium Stability and Safety in Time
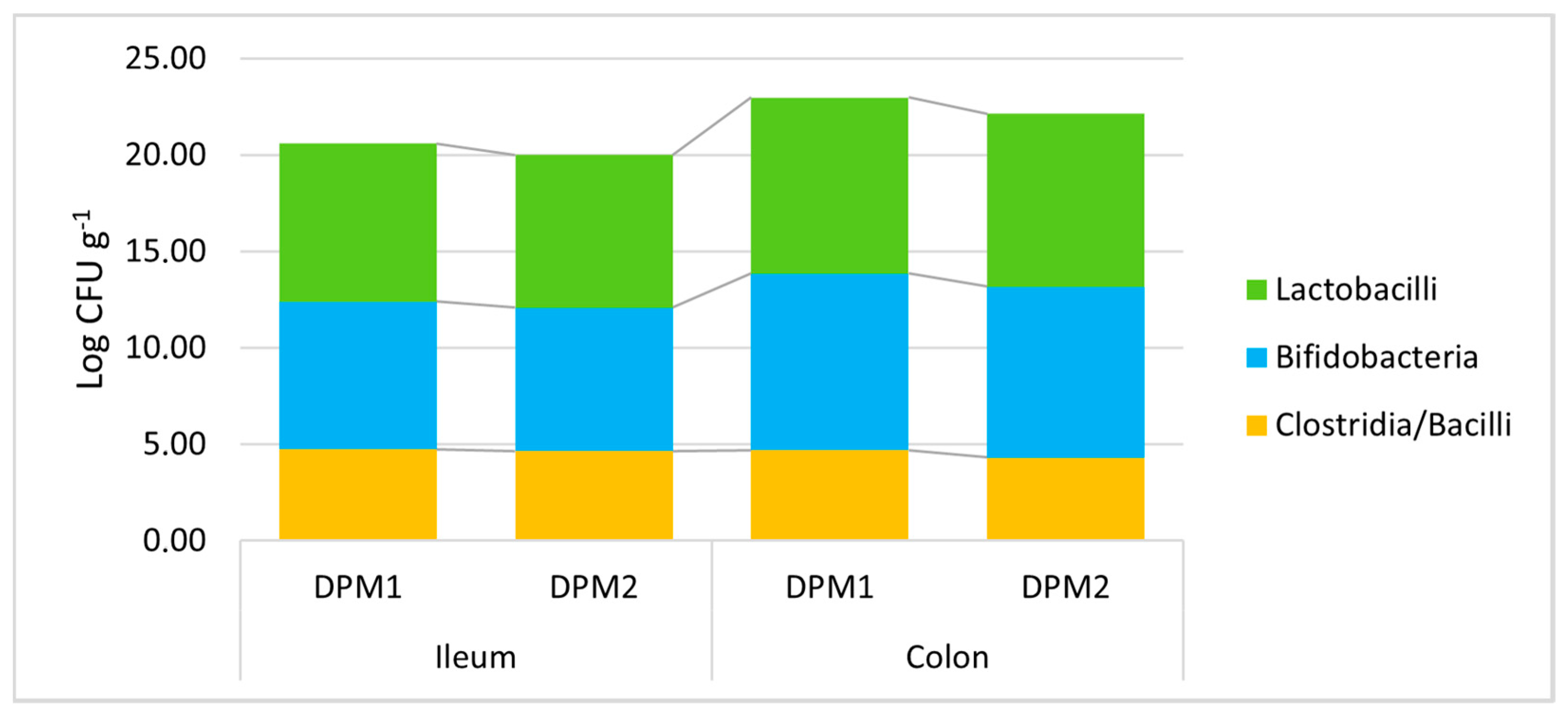
Additionally, the stability of the DPM mixture and the possible risk of pathobiont outbreak after a longer colonization time in vivo were assessed. Piglets in the DPM2 group were monitored under the same in vivo model conditions for 14 days and were compared to the DPM1 group. All determined viable bacterial levels did not significantly change after a prolongated time of the in vivo experiment in both parts of the gut (Figure 6, Supplementary Material—Table S3). In comparison with the concentration of approximately 5.50 × 107 CFU mL−1 of each bacterial strain in the administered mixture, we detected slightly higher numbers of lactobacilli and bifidobacteria compared to numbers of heat-resistant spore-forming bacteria about three orders of magnitude lower (clostridia and bacilli).
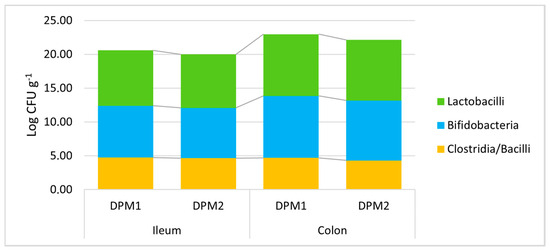
Figure 6.
Stability of DPM mixture in the piglets’ gut in time. DPM1—piglets associated with DPM mixture for 8 days; DPM2—piglets associated with DPM mixture for 14 days.
Furthermore, a slight non-significant trend of decreasing bacterial levels was found for longer periods of colonization. Then, there was a similar confirmation of the significant trend of higher bacterial levels in the colon environment compared to the ileum for lactobacilli and bifidobacteria (Supplementary Material—Table S4). Moreover, no pathobiont outbreak was detected; thus, the safety of the mixture described in vitro was also confirmed in vivo. Furthermore, a possible long-term colonization could be expected for the analyzed DPM mixture.
3.5. Piglets’ Gut Microbiome Alpha and Beta Diversity in Time
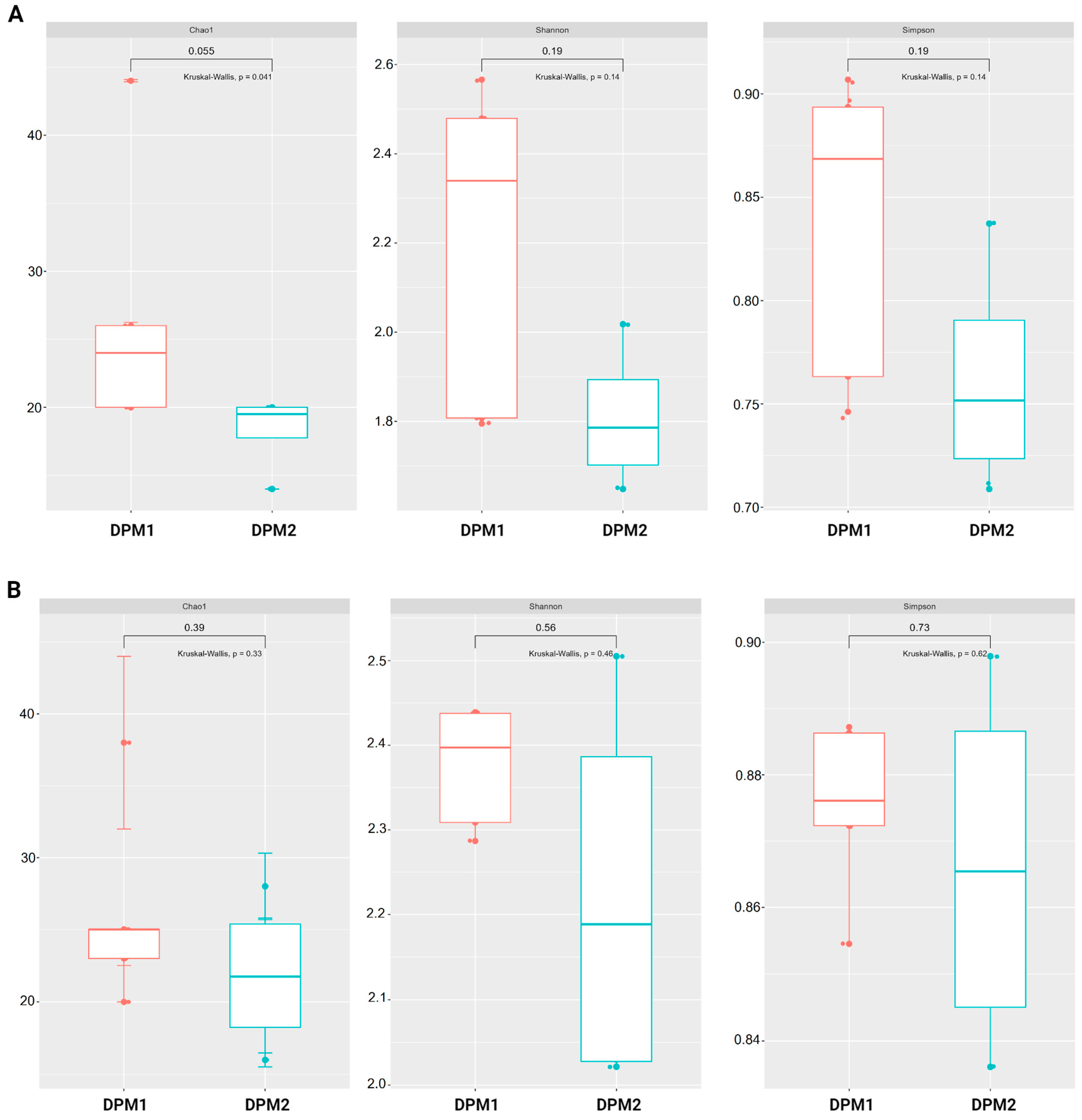
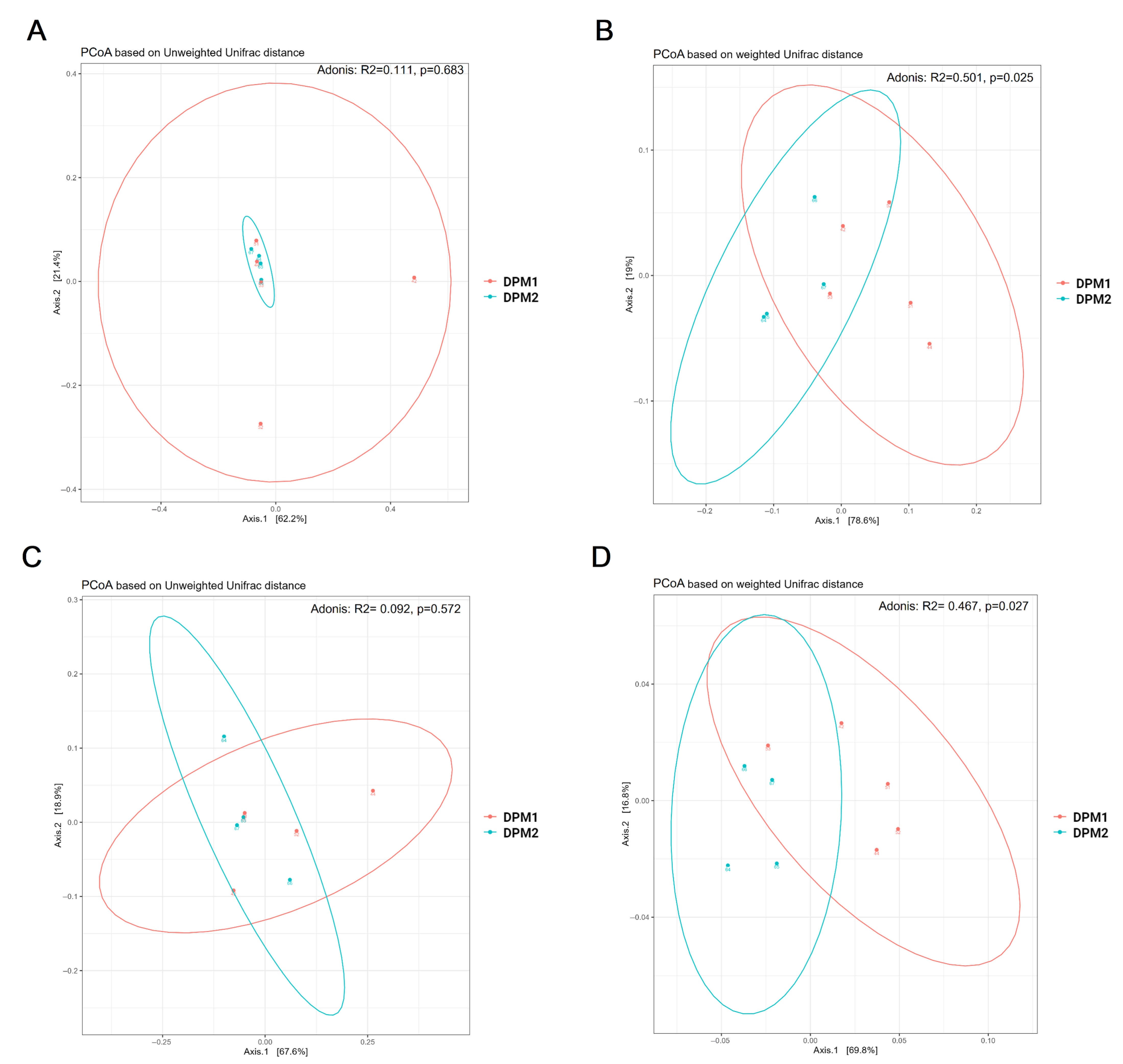
Using Chao1, Shannon, and Simpson diversity indexes, no significant difference in alpha diversity was observed between DPM1 and DPM2 animal groups in both the ileum and colon microbiomes (p > 0.05) (Figure 7). The beta diversity of both colon and ileum microbiomes showed no significant difference between DPM1 and DPM2 animal groups using unweighted unifrac distances (p > 0.05) (Figure 8). However, a significant difference was observed in both colon and ileum microbiomes using weighted unifrac distances (p ≤ 0.05). That means that the difference between DPM1 and DPM2 was in the abundance and not in the composition.
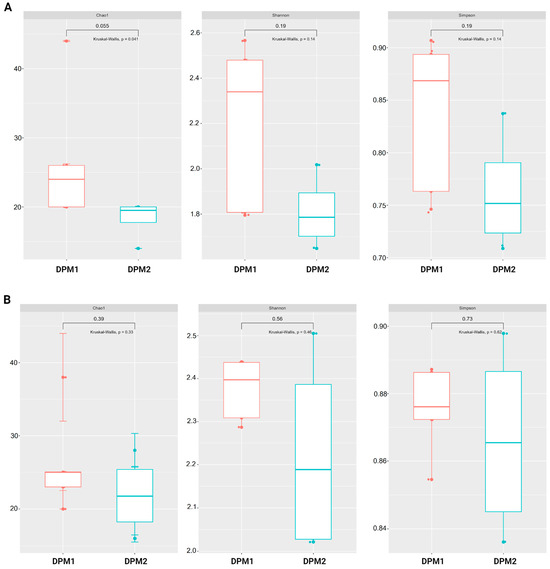
Figure 7.
Effect of the in vivo experimental time on alpha diversity of the gut microbiome. Boxplots illustrating alpha diversity using Chao1, Shannon, and Simpson diversity indices in the (A) ileum and (B) colon of DPM1 and DPM2 animal groups. p ≤ 0.05 was considered statistically significant based on the Kruskal–Wallis test. DPM1—piglets associated with DPM mixture for 8 days; DPM2—piglets associated with DPM mixture for 14 days.
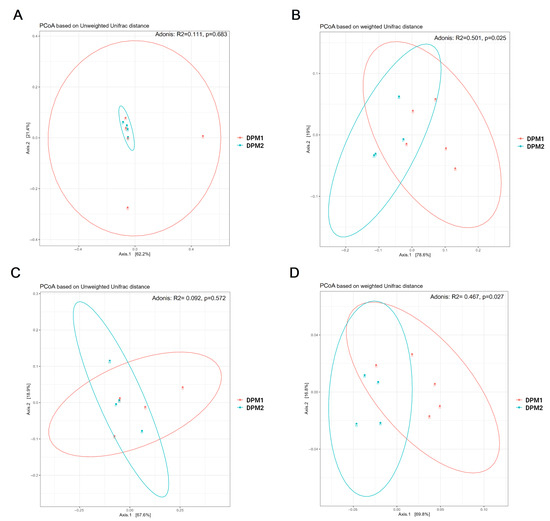
Figure 8.
Bacterial beta diversity of gut microbiome in time. Beta diversity using Principal Coordinate Analysis (PCoA) plots based on the unweighted (A,C) and weighted (B,D) unifrac distance in the (A,B) ileum and (C,D) colon from DPM1 and DPM2 animal groups. p ≤ 0.05 was considered statistically significant. DPM1—piglets associated with DPM mixture for 8 days; DPM2—piglets associated with DPM mixture for 14 days.
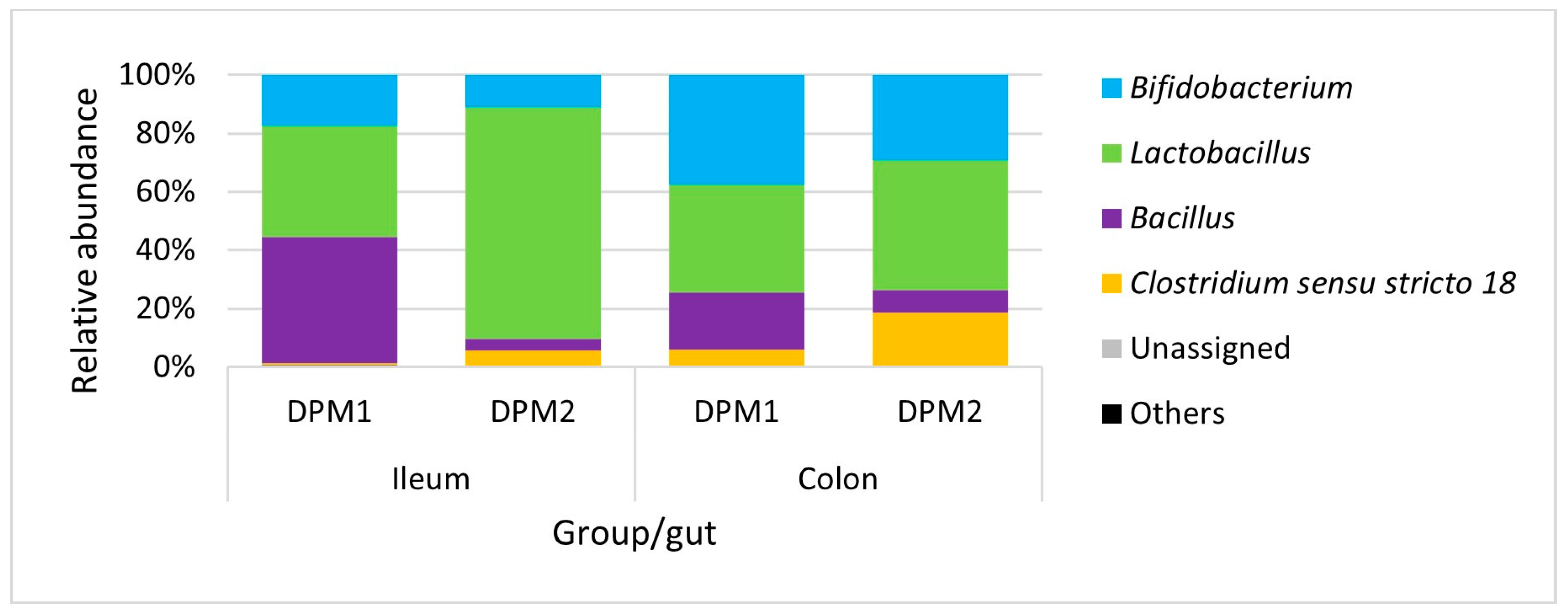
Analyzing microbiome composition (Figure 9), Lactobacillus was the dominant genus in the ileum of DPM2 animals. The proportion of Lactobacillus was higher in the ileum microbiota of DPM2 in comparison to the ileum microbiome of DPM1 animals. Bifidobacterium and Bacillus were higher in the ileum microbiome of DPM1 animals. Bifidobacterium, Bacillus, and Clostridium sensu stricto 18 were also the identified abundant genera in the ileum microbiota of DPM2 animals. The abundance of Bifidobacterium and Clostridium sensu stricto 18 was increased in the colon microbiota of the DPM1 and DPM2 animals. The abundance of Lactobacillus was decreased only in the colon microbiota of the DPM2 animals.
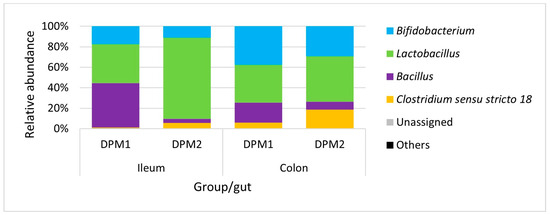
Figure 9.
Relative abundance of present bacteria in time at the genus level. DPM1—piglets associated with DPM mixture for 8 days; DPM2—piglets associated with DPM mixture for 14 days.
Using LefSe analysis, no significant difference in relative abundance was observed between DPM1 and DPM2 in both ileum and colon microbiomes.
4. Discussion
Microbes inhabiting the pigs’ gut form a complex ecosystem conferring pivotal nutritional, physiological, and immunological functions for the host [57], such as the utilization of undigested substrates with various metabolic regulations [58], bioactive compound production [59], protection against pathogens [60], and immunostimulation [61]. Its diversity increases from birth to weaning and is significantly influenced by the environment and contact with sows [62]. Unfortunately, the pig farm environment is closely related to bacterial pathogens as well, specifically S. typhimurium which is the most frequent causative agent of infection development [63].
Salmonellosis outbreak significantly disrupts the autochthonous microbiota and affects the immune response [64]. Moreover, it threatens not only the life of the host but there is a significant risk of developing the disease in pork consumers, notably menacing the health of the global population [65] as a significant food safety hazard. Thus, it is desirable to increase the detection of S. typhimurium originating from farms in pork meat and ensure biosecurity precautions [66]. Positively, some countries have already developed several confident control measures for the management of the reduction in or even elimination of Salmonella exposition in the pig farms and pork production chain, e.g., the monitoring of animal movement and possible contacts; the treatment of feed and water; regular sampling at the animal, feed, and environment level; and thorough cleaning and disinfection [14,67,68], to avoid the recycling of this zoonotic hazard at the farm level.
In the era of antibiotic resistance spread in animal husbandry, there is a need to search for novel non-antibiotic alternatives to keep individuals in good health shape, where one of the promising strategies for pigs is a probiotic intervention [69,70,71]. Probiotic supplements in animal feed have already been used not only to improve the health parameters and welfare of the hosts, but also to reduce the action of alimentary pathogens as well [21]. Popularly, probiotics consisting of more bacterial strains could synergistically enhance the targeted effect [72], but it is important to perform strain selection based on the function and efficacy [38]. Moreover, there used to be a recommendation to use microbial strains of the same origin as the targeted host [73]. Thus, the pig origin of our administered multi-strain bacterial consortium, consisting of B. animalis subsp. lactis PG2, B. porcinum PG3, L. amylovorus PG6, L. paracasei subsp. tolerans PG5, L. reuteri PG7, PG8, PG9, C. sporogenes PG4, and Bacillus sp. PG1, probably contributed to the detected colonization success in the gnotobiotic piglets. Moreover, this combination of various bacterial species in one administered consortium conferred integral colonization of the gut, showing amplicon profiling results—specifically, a more considerable representation of lactobacilli and bacilli levels in the ileum and bifidobacterial with clostridial concentrations in the colon. In addition, the DPM mixture, assessed as safe, seems to have long-term colonization capacity.
In general, the pigs’ gut microbiota is subjected to dynamic shifts and is being changed during the life of the pig hosts, but Bacteroides spp., Prevotella spp., and Lactobacillus could be identified as their core bacterial genera [74]. Furthermore, bifidobacteria are considered highly abundant commensals in the gut of conventional pigs, and wild boars as well [75,76]. Similarly, clostridia [77,78] and bacilli [79] considerably occur in this complex environment. When focusing on the mentioned bacterial groups in the pig fecal microbiota, we previously confirmed their common occurrence [41]. Thanks to the fact that these commensals could be easily cultivated under the laboratory conditions and acquired selected isolates exhibited anti-Salmonella activity and probiotic potential in vitro, we combined the cultivation and amplicon sequencing approaches for in vivo assessment of the DPM mixture colonization ability in the gnotobiotic piglets’ model. Our results showed that the DPM mixture commendably colonized the gut of piglets with a significantly more inhabited colon compared to the ileum. It is generally recognized that colon microbiota is a mostly populated ecosystem within mammalian individuals with a detrimental impact on the host’s health and confers significant functions [80]. This bilateral, dynamic, and symbiotic relationship is enabled by the gut providing a favorable environment full of energy supplies and thriving conditions for microorganisms, securing eubiosis and creating the host–gut microbiota axis [81].
As follows, to verify the DPM mixture’s protective effect against S. typhimurium LT2-induced infection, we used the gnotobiotic piglet model [42]. Although the Salmonella load was not significantly decreased by supplementation with a consortium of nine bacterial strains in the gut of DPM1 + LT2, milder and slowed infection manifestations were detected in comparison with the LT2 control group. Moreover, administered commensals seem to interact with S. Typhimurium LT2, causing a protective barrier function against its entrance into the blood circulation, because no bacteria were detected in blood samples of the DPM1 + LT2 group compared to their presence in the LT2 group, where the pathogen translocation into the blood circulation was detected. Although there was a bacterial absence in the blood of the DPM1 + LT2 group, microbes were detected in their mesenteric lymph nodes and further interactions with S. typhimurium LT2 could be expected. These findings suggest the DPM mixture’s possible protective character that could probably confer to piglets’ immune stimulation. Interestingly, the in vivo amelioration of non-typhoidal Salmonella infection by supplementation with single probiotic strain L. rhamnosus GG has already been described before [43], as well as by L. reuteri KUB-AC5 [82], B. animalis subsp. lactis NFBAL23 [83], B. thermophilum RBL67 [84], and E. coli Nissle 1917 [45] or the in vitro anti-Salmonella activity of L. amylovorus SLZX20-1 [85] and L. paracasei DUP-13076 [86]. However, no anti-inflammatory reaction was revealed for single commensal lactobacilli strains, namely L. amylovorus P1 and L. mucosae P5. Interestingly, the authors highlighted a better protective potential when using multiple strains in a mixture [45]. Myhill et al. [87] support this suggestion showing two probiotic mixtures as well, namely Bac. amyloliquefaciens 516, Bac. subtilis 541 with Enterococcus faecium 669, and B. animalis subsp. lactis BB-12 with L. rhamnosus LGG, affecting the host’s immune homeostasis desirably.
Although bifidobacteria and lactobacilli are considered safe and are widely used as probiotics in variable food- and feedstuffs [88], there is a growing trend for looking for other applicable bacterial species for probiotic interventions in the form of vegetative cells or spores. In general, some species of spore-forming bacteria, such as clostridia and bacilli, are significant parts of the commensal gut microbiota and could confer several probiotic and technological benefits, e.g., anti-inflammation effects, sporulation behavior with thermostability, and the production of desired metabolites [89,90,91,92]. However, some of them remain controversial [93] and further research is necessary. Deng et al. [94] emphasize the necessity of thorough testing, as they detected hemolytic activity, carried enterotoxin and antibiotic resistance genes, and cytotoxicity in several analyzed probiotic supplements containing bacterial spores. However, based on our in vitro testing [41], we found clostridial and Bacilli spore-forming strains lacking these traits and showing promising probiotic character. Although their presence in the gut content from both parts was the lowest (103–105 CFU g−1) compared to other administered bacterial groups at the end of the in vivo experiment, it seems that they are part of the transient gut microbiota as previously described by Zhang et al. [95], but they probably contributed to the hosts’ well-being as well. The probiotic effect of several Clostridium and Bacillus strains has already been described before, e.g., belonging to species of C. butyricum [96], Bac. subtilis [97], Bac. licheniformis and Bac. pumilus [98], and Bac. coagulans [99].
The DPM mixture including viable bifidobacteria and lactobacilli and the heat-resistant C. sporogenes PG4 and Bacillus sp. PG1 have a promising probiotic potential for administration to pigs verified at the gnotobiotic piglet model in vivo thanks to its colonization stability, safety, and possible protective traits such as alleviating infection manifestation and preventing the translocation of Salmonella cells to the blood circulation. Nevertheless, further advanced tests are needed for the verification of its suitability for application in pig production.
5. Conclusions
Our results confirmed the colonization ability and potential protective effect of the administered multi-strain DPM mixture against S. typhimurium LT2. However, there is a need to carry out further tests, especially immunological ones. If the potential protective effect is confirmed, it is necessary to continue with in vivo testing with conventional pig hosts for observing the DPM mixture supplementation effects in natural husbandry conditions.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ani14121779/s1. Table S1: Comparison of determined cultivation counts (log CFU g−1) of administered bacteria in the piglets’ ileum or colon between the monitored experimental animal groups; Table S2: Comparison of determined cultivation counts (log CFU g−1) of administered bacteria present in the ileum and colon for each tested animal group; Table S3: Comparison of determined cultivation counts (log CFU g−1) of administered bacteria present in the ileum and colon in time; Table S4: Comparison of determined cultivation counts (log CFU g−1) of administered bacteria present in the ileum and colon per DPM1 and DPM2 animal groups.
Author Contributions
Conceptualization, E.V. and I.S.; methodology, I.S., A.S. and E.V.; validation, V.N.-B.; formal analysis, N.M. and C.M.; investigation, K.H., C.M., I.S., A.S. and A.A.; resources, E.V., I.S. and J.M.; software, C.M.; data curation, V.N.-B.; writing—original draft preparation, N.M.; writing—review and editing, V.N.-B., E.V., I.S. and A.S.; visualization, N.M. and C.M.; supervision, I.S., J.M. and E.V.; funding acquisition, E.V.; project administration, E.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grant 21-15621S of the Czech Science Foundation.
Institutional Review Board Statement
The work with animals was conducted according to the ethical standards defined by the EU legislation on the use of experimental animals (2010/63/EU) and approved by the Animal Care and Use Committee of the Czech Academy of Sciences (protocol 57/2021; 18 August 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, and further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank Jana Machova and Hana Sychrovska for taking care of the animals.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, J.L.; Xu, J.; Leip, D.D.; Chen, C.-H.; Westover, B.P.; Weatherford, J.; Buhler, J.D.; Gordon, J.I. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science 2005, 307, 1955–1959. [Google Scholar] [CrossRef] [PubMed]
- Pant, A.; Maiti, T.K.; Mahajan, D.; Das, B. Human gut microbiota and drug metabolism. Microb. Ecol. 2023, 86, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Gensollen, T.; Iyer, S.S.; Kasper, D.L.; Blumberg, R.S. How colonization by microbiota in early life shapes the immune system. Science 2016, 352, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Enav, H.; Bäckhed, F.; Ley, R.E. The developing infant gut microbiome: A strain-level view. Cell Host Microbe 2022, 30, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, L.J.; Monga, M.; Miller, A.W. Defining dysbiosis for a cluster of chronic diseases. Sci. Rep. 2019, 9, 12918. [Google Scholar] [CrossRef]
- Karin, M.; Lawrence, T.; Nizet, V. Innate immunity gone awry: Linking microbial infections to chronic inflammation and cancer. Cell 2006, 124, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.Y.; Inohara, N.; Nuñez, G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017, 10, 18–26. [Google Scholar] [CrossRef]
- Stecher, B.; Robbiani, R.; Walker, A.W.; Westendorf, A.M.; Barthel, M.; Kremer, M.; Chaffron, S.; Macpherson, A.J.; Buer, J.; Parkhill, J. Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 2007, 5, e244. [Google Scholar]
- Lupp, C.; Robertson, M.L.; Wickham, M.E.; Sekirov, I.; Champion, O.L.; Gaynor, E.C.; Finlay, B.B. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2007, 2, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Baldelli, V.; Scaldaferri, F.; Putignani, L.; Del Chierico, F. The role of Enterobacteriaceae in gut microbiota dysbiosis in inflammatory bowel diseases. Microorganisms 2021, 9, 697. [Google Scholar] [CrossRef] [PubMed]
- Swart, A.N.; Evers, E.G.; Simons, R.L.L.; Swanenburg, M. Modeling of Salmonella contamination in the pig slaughterhouse. Risk Anal. 2016, 36, 498–515. [Google Scholar] [CrossRef] [PubMed]
- Correia-Gomes, C.; Leonard, F.; Graham, D. Description of control programmes for Salmonella in pigs in Europe. Progress to date? J. Food Saf. 2021, 41, e12916. [Google Scholar] [CrossRef]
- Smith, R.P.; May, H.E.; Burow, E.; Meester, M.; Tobias, T.J.; Sassu, E.-L.; Pavoni, E.; Di Bartolo, I.; Prigge, C.; Wasyl, D. Assessing pig farm biosecurity measures for the control of Salmonella on European farms. Epidemiol. Infect. 2023, 151, e130. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, A.K. Foodborne Microbial Pathogens: Mechanisms and Pathogenesis; Springer: Berlin/Heidelberg, Germany, 2018; pp. 271–287. [Google Scholar] [CrossRef]
- Campos, J.; Mourão, J.; Peixe, L.; Antunes, P. Non-typhoidal Salmonella in the pig production chain: A comprehensive analysis of its impact on human health. Pathogens 2019, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Casanova-Higes, A.; Marín-Alcalá, C.M.; Andrés-Barranco, S.; Cebollada-Solanas, A.; Alvarez, J.; Mainar-Jaime, R.C. Weaned piglets: Another factor to be considered for the control of Salmonella infection in breeding pig farms. Vet. Res. 2019, 50, 45. [Google Scholar] [CrossRef] [PubMed]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut microbiota dysbiosis in postweaning piglets: Understanding the keys to health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef] [PubMed]
- Callaway, T.R.; Morrow, J.L.; Edrington, T.S.; Genovese, K.J.; Dowd, S.; Carroll, J.; Dailey, J.W.; Harvey, R.B.; Poole, T.L.; Anderson, R.C. Social stress increases fecal shedding of Salmonella typhimurium by early weaned piglets. Curr. Issues Intest. Microbiol. 2006, 7, 65–72. [Google Scholar]
- Pereira, W.A.; Franco, S.M.; Reis, I.L.; Mendonça, C.M.N.; Piazentin, A.C.M.; Azevedo, P.O.S.; Marcos, L.P.; De Martinis, E.C.P.; Gierus, M.; Oliveira, R.P.S. Beneficial effects of probiotics on the pig production cycle: An overview of clinical impacts and performance. Vet. Microbiol. 2022, 269, 109431. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Liu, F.; Mao, Y.; Zhang, Y.; Zeng, H.; Ren, S.; Guo, L.; Chen, Z.; Hrabchenko, N. Mechanisms and applications of probiotics in prevention and treatment of swine diseases. Porc. Health Manag. 2023, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.S.; Amalaradjou, M.A.; Venkitanarayanan, K. Antivirulence properties of probiotics in combating microbial pathogenesis. Adv. Appl. Microbiol. 2017, 98, 1–29. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Rabetafika, H.N.; Razafindralambo, A.; Ebenso, B.; Razafindralambo, H.L. Probiotics as Antibiotic Alternatives for Human and Animal Applications. Encyclopedia 2023, 3, 561–581. [Google Scholar] [CrossRef]
- Barba-Vidal, E.; Martín-Orúe, S.M.; Castillejos, L. Practical aspects of the use of probiotics in pig production: A review. Livest. Sci. 2019, 223, 84–96. [Google Scholar] [CrossRef]
- Lekagul, A.; Tangcharoensathien, V.; Yeung, S. Patterns of antibiotic use in global pig production: A systematic review. Vet. Anim. Sci. 2019, 7, 100058. [Google Scholar] [CrossRef] [PubMed]
- Barton, M.D. Impact of antibiotic use in the swine industry. Curr. Opin. Microbiol. 2014, 19, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Paruch, L.; Chen, X.; Van Eerde, A.; Skomedal, H.; Wang, Y.; Liu, D.; Liu Clarke, J. Antibiotic application and resistance in swine production in China: Current situation and future perspectives. Front. Vet. Sci. 2019, 6, 136. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.F.; Nyachoti, M. Using probiotics to improve swine gut health and nutrient utilization. Anim. Nutr. 2017, 3, 331–343. [Google Scholar] [CrossRef]
- Lambo, M.T.; Chang, X.; Liu, D. The recent trend in the use of multistrain probiotics in livestock production: An overview. Animals 2021, 11, 2805. [Google Scholar] [CrossRef]
- Valeriano, V.D.V.; Balolong, M.P.; Kang, D.K. Probiotic roles of Lactobacillus sp. in swine: Insights from gut microbiota. J. Appl. Microbiol. 2017, 122, 554–567. [Google Scholar] [CrossRef]
- Barba-Vidal, E.; Castillejos, L.; Roll, V.F.B.; Cifuentes-Orjuela, G.; Moreno Muñoz, J.A.; Martín-Orúe, S.M. The probiotic combination of Bifidobacterium longum subsp. infantis CECT 7210 and Bifidobacterium animalis subsp. lactis BPL6 reduces pathogen loads and improves gut health of weaned piglets orally challenged with Salmonella typhimurium. Front. Microbiol. 2017, 8, 1570. [Google Scholar]
- Lan, R.; Kim, I. Enterococcus faecium supplementation in sows during gestation and lactation improves the performance of sucking piglets. Vet. Med. Sci. 2020, 6, 92–99. [Google Scholar] [CrossRef]
- Luise, D.; Bosi, P.; Raff, L.; Amatucci, L.; Virdis, S.; Trevisi, P. Bacillus spp. probiotic strains as a potential tool for limiting the use of antibiotics, and improving the growth and health of pigs and chickens. Front. Microbiol. 2022, 13, 801827. [Google Scholar] [PubMed]
- Cao, G.; Tao, F.; Hu, Y.; Li, Z.; Zhang, Y.; Deng, B. Positive effects of a Clostridium butyricum-based compound probiotic on growth performance, immune responses, intestinal morphology, hypothalamic neurotransmitters, and colonic microbiota in weaned piglets. Food Funct. 2019, 10, 2926–2934. [Google Scholar] [CrossRef] [PubMed]
- García, G.R.; Dogi, C.A.; Poloni, V.L.; Fochesato, A.S.; De Moreno de Leblanc, A.; Cossalter, A.M.; Payros, D.; Oswald, I.P.; Cavaglieri, L.R. Beneficial effects of Saccharomyces cerevisiae RC016 in weaned piglets: In vivo and ex vivo analysis. Benef. Microbes 2019, 10, 33–42. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L.V. Efficacy of single-strain probiotics versus multi-strain mixtures: Systematic review of strain and disease specificity. Dig. Dis. Sci. 2021, 66, 694–704. [Google Scholar] [CrossRef]
- Vinderola, G.; Gueimonde, M.; Gomez-Gallego, C.; Delfederico, L.; Salminen, S. Correlation between in vitro and in vivo assays in selection of probiotics from traditional species of bacteria. Trends Food Sci. Technol. 2017, 68, 83–90. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Zoumpopoulou, G.; Foligné, B.; Alexandraki, V.; Kazou, M.; Pot, B.; Tsakalidou, E. Discovering probiotic microorganisms: In vitro, in vivo, genetic and omics approaches. Front. Microbiol. 2015, 6, 58. [Google Scholar] [CrossRef]
- Horvathova, K.; Modrackova, N.; Splichal, I.; Splichalova, A.; Amin, A.; Ingribelli, E.; Killer, J.; Doskocil, I.; Pechar, R.; Kodesova, T. Defined Pig Microbiota with a Potential Protective Effect against Infection with Salmonella typhimurium. Microorganisms 2023, 11, 1007. [Google Scholar] [CrossRef]
- Splichalova, A.; Slavikova, V.; Splichalova, Z.; Splichal, I. Preterm life in sterile conditions: A study on preterm, germ-free piglets. Front. Immunol. 2018, 9, 220. [Google Scholar] [CrossRef] [PubMed]
- Splichalova, A.; Jenistova, V.; Splichalova, Z.; Splichal, I. Colonization of preterm gnotobiotic piglets with probiotic Lactobacillus rhamnosus GG and its interference with Salmonella typhimurium. Clin. Exp. Immunol. 2019, 195, 381–394. [Google Scholar] [CrossRef]
- Trebichavský, I.; Dlabac, V.; Reháková, Z.; Zahradnícková, M.; Splichal, I. Cellular changes and cytokine expression in the ilea of gnotobiotic piglets resulting from peroral Salmonella typhimurium challenge. Infect. Immun. 1997, 65, 5244–5249. [Google Scholar] [CrossRef] [PubMed]
- Splichal, I.; Donovan, S.M.; Splichalova, Z.; Neuzil Bunesova, V.; Vlkova, E.; Jenistova, V.; Killer, J.; Svejstil, R.; Skrivanova, E.; Splichalova, A. Colonization of germ-free piglets with commensal Lactobacillus amylovorus, Lactobacillus mucosae, and probiotic E. coli Nissle 1917 and their interference with Salmonella typhimurium. Microorganisms 2019, 7, 273. [Google Scholar] [CrossRef] [PubMed]
- Modrackova, N.; Stovicek, A.; Burtscher, J.; Bolechova, P.; Killer, J.; Domig, K.J.; Neuzil-Bunesova, V. The bifidobacterial distribution in the microbiome of captive primates reflects parvorder and feed specialization of the host. Sci. Rep. 2021, 11, 1–13. [Google Scholar]
- Hungate, R.E.; Macy, J. The roll-tube method for cultivation of strict anaerobes. Bull. Ecol. Res. Comm. 1973, 17, 123–126. [Google Scholar]
- Rada, V.; Petr, J. A new selective medium for the isolation of glucose non-fermenting bifidobacteria from hen caeca. J. Microbiol. Methods 2000, 43, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Vlková, E.; Salmonová, H.; Bunešová, V.; Geigerová, M.; Rada, V.; Musilová, Š. A new medium containing mupirocin, acetic acid, and norfloxacin for the selective cultivation of bifidobacteria. Anaerobe 2015, 34, 27–33. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef]
- Milani, C.; Hevia, A.; Foroni, E.; Duranti, S.; Turroni, F.; Lugli, G.A.; Sanchez, B.; Martin, R.; Gueimonde, M.; van Sinderen, D. Assessing the fecal microbiota: An optimized ion torrent 16S rRNA gene-based analysis protocol. PLoS ONE 2013, 8, e68739. [Google Scholar] [CrossRef]
- Mekadim, C.; Skalnikova, H.K.; Cizkova, J.; Cizkova, V.; Palanova, A.; Horak, V.; Mrazek, J. Dysbiosis of skin microbiome and gut microbiome in melanoma progression. BMC Microbiol. 2022, 22, 63. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Fouhse, J.M.; Zijlstra, R.T.; Willing, B.P. The role of gut microbiota in the health and disease of pigs. Anim. Front. 2016, 6, 30–36. [Google Scholar] [CrossRef]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; De Los Reyes-gavilán, C.G.; Salazar, N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 185. [Google Scholar]
- Mamo, G. Anaerobes as sources of bioactive compounds and health promoting tools. Anaerobes Biotechnol. 2016, 433–464. [Google Scholar] [CrossRef]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef]
- Wampach, L.; Heintz-Buschart, A.; Fritz, J.V.; Ramiro-Garcia, J.; Habier, J.; Herold, M.; Narayanasamy, S.; Kaysen, A.; Hogan, A.H.; Bindl, L. Birth mode is associated with earliest strain-conferred gut microbiome functions and immunostimulatory potential. Nat. Commun. 2018, 9, 5091. [Google Scholar] [CrossRef]
- Chen, X.; Xu, J.; Ren, E.; Su, Y.; Zhu, W. Co-occurrence of early gut colonization in neonatal piglets with microbiota in the maternal and surrounding delivery environments. Anaerobe 2018, 49, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.P.; Kingsley, R.A. Salmonella pathogenesis and host-adaptation in farmed animals. Curr. Opin. Microbiol. 2021, 63, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Bescucci, D.M.; Moote, P.E.; Ortega Polo, R.; Uwiera, R.R.E.; Inglis, G.D. Salmonella enterica serovar Typhimurium temporally modulates the enteric microbiota and host responses to overcome colonization resistance in swine. Appl. Environ. Microbiol. 2020, 86, e01569-20. [Google Scholar] [PubMed]
- Popa, G.L.; Papa, M.I. Salmonella spp. infection-a continuous threat worldwide. Germs 2021, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Marin, C.; Chinillac, M.C.; Cerdà-Cuéllar, M.; Montoro-Dasi, L.; Sevilla-Navarro, S.; Ayats, T.; Marco-Jimenez, F.; Vega, S. Contamination of pig carcass with Salmonella enterica serovar Typhimurium monophasic variant 1, 4 [5], 12:i:-originates mainly in live animals. Sci. Total Environ. 2020, 703, 134609. [Google Scholar] [CrossRef]
- Roasto, M.; Bonardi, S.; Mäesaar, M.; Alban, L.; Gomes-Neves, E.; Vieira-Pinto, M.; Vågsholm, I.; Elias, T.; Lindegaard, L.L.; Blagojevic, B. Salmonella enterica prevalence, serotype diversity, antimicrobial resistance and control in the European pork production chain. Trends Food Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Blagojevic, B.; Nesbakken, T.; Alvseike, O.; Vågsholm, I.; Antic, D.; Johler, S.; Houf, K.; Meemken, D.; Nastasijevic, I.; Pinto, M.V. Drivers, opportunities, and challenges of the European risk-based meat safety assurance system. Food Control 2021, 124, 107870. [Google Scholar] [CrossRef]
- Low, C.X.; Tan, L.T.-H.; Ab Mutalib, N.-S.; Pusparajah, P.; Goh, B.-H.; Chan, K.-G.; Letchumanan, V.; Lee, L.-H. Unveiling the impact of antibiotics and alternative methods for animal husbandry: A review. Antibiotics 2021, 10, 578. [Google Scholar] [CrossRef] [PubMed]
- Monger, X.C.; Gilbert, A.-A.; Saucier, L.; Vincent, A.T. Antibiotic resistance: From pig to meat. Antibiotics 2021, 10, 1209. [Google Scholar] [CrossRef]
- Vt Nair, D.; Venkitanarayanan, K.; Kollanoor Johny, A. Antibiotic-resistant Salmonella in the food supply and the potential role of antibiotic alternatives for control. Foods 2018, 7, 167. [Google Scholar] [CrossRef]
- Kwoji, I.D.; Aiyegoro, O.A.; Okpeku, M.; Adeleke, M.A. Multi-strain probiotics: Synergy among isolates enhances biological activities. Biology 2021, 10, 322. [Google Scholar] [CrossRef] [PubMed]
- Shokryazdan, P.; Faseleh Jahromi, M.; Liang, J.B.; Ho, Y.W. Probiotics: From isolation to application. J. Am. Coll. Nutr. 2017, 36, 666–676. [Google Scholar] [CrossRef]
- Luo, Y.; Ren, W.; Smidt, H.; Wright, A.-D.G.; Yu, B.; Schyns, G.; McCormack, U.M.; Cowieson, A.J.; Yu, J.; He, J. Dynamic distribution of gut microbiota in pigs at different growth stages: Composition and contribution. Microbiol. Spectr. 2022, 10, e00688-21. [Google Scholar] [CrossRef] [PubMed]
- Pechar, R.; Killer, J.; Mekadim, C.; Geigerová, M.; Rada, V. Classification of culturable bifidobacterial population from colonic samples of wild pigs (Sus scrofa) based on three molecular genetic methods. Curr. Microbiol. 2017, 74, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Pechar, R.; Killer, J.; Salmonová, H.; Geigerová, M.; Švejstil, R.; Švec, P.; Sedláček, I.; Rada, V.; Benada, O. Bifidobacterium apri sp. nov., a thermophilic actinobacterium isolated from the digestive tract of wild pigs (Sus scrofa). Int. J. Syst. Evol. Microbiol. 2017, 67, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Piazuelo, D.; Estellé, J.; Revilla, M.; Criado-Mesas, L.; Ramayo-Caldas, Y.; Óvilo, C.; Fernández, A.I.; Ballester, M.; Folch, J.M. Characterization of bacterial microbiota compositions along the intestinal tract in pigs and their interactions and functions. Sci. Rep. 2018, 8, 12727. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Højberg, O.; Canibe, N.; Jensen, B.B. Phylogenetic diversity of cultivable butyrate-producing bacteria from pig gut content and feces. J. Anim. Sci. 2016, 94, 377–381. [Google Scholar] [CrossRef]
- Larsen, N.; Thorsen, L.; Kpikpi, E.N.; Stuer-Lauridsen, B.; Cantor, M.D.; Nielsen, B.; Brockmann, E.; Derkx, P.M.F.; Jespersen, L. Characterization of Bacillus spp. strains for use as probiotic additives in pig feed. Appl. Microbiol. Biotechnol. 2014, 98, 1105–1118. [Google Scholar] [CrossRef]
- Álvarez, J.; Real, J.M.F.; Guarner, F.; Gueimonde, M.; Rodríguez, J.M.; de Pipaon, M.S.; Sanz, Y. Gut microbes and health. Gastroenterol. Hepatol. 2021, 44, 519–535. [Google Scholar] [CrossRef]
- Colella, M.; Charitos, I.A.; Ballini, A.; Cafiero, C.; Topi, S.; Palmirotta, R.; Santacroce, L. Microbiota revolution: How gut microbes regulate our lives. World J. Gastroenterol. 2023, 29, 4368. [Google Scholar] [CrossRef]
- Buddhasiri, S.; Sukjoi, C.; Kaewsakhorn, T.; Nambunmee, K.; Nakphaichit, M.; Nitisinprasert, S.; Thiennimitr, P. Anti-inflammatory effect of probiotic Limosilactobacillus reuteri KUB-AC5 against Salmonella infection in a mouse colitis model. Front. Microbiol. 2021, 12, 716761. [Google Scholar] [PubMed]
- Lin, Y.; Xie, Z.; Li, Z.; Yuan, C.; Zhang, C.; Li, Y.; Xie, K.; Wang, K. Assessment of the role and mechanism of Bifidobacterium animalis subsp. lactis isolated from neonates’ feces in protecting neonatal rats from Salmonella infection. Microb. Pathog. 2023, 174, 105935. [Google Scholar]
- Tanner, S.A.; Chassard, C.; Rigozzi, E.; Lacroix, C.; Stevens, M.J.A. Bifidobacterium thermophilum RBL67 impacts on growth and virulence gene expression of Salmonella enterica subsp. enterica serovar Typhimurium. BMC Microbiol. 2016, 16, 46. [Google Scholar]
- Shen, J.; Zhang, J.; Zhao, Y.; Lin, Z.; Ji, L.; Ma, X. Tibetan pig-derived probiotic Lactobacillus amylovorus SLZX20-1 improved intestinal function via producing enzymes and regulating intestinal microflora. Front. Nutr. 2022, 9, 846991. [Google Scholar] [CrossRef]
- Muyyarikkandy, M.S.; Amalaradjou, M.A. Lactobacillus bulgaricus, Lactobacillus rhamnosus and Lactobacillus paracasei attenuate Salmonella enteritidis, Salmonella heidelberg and Salmonella typhimurium colonization and virulence gene expression in vitro. Int. J. Mol. Sci. 2017, 18, 2381. [Google Scholar] [CrossRef]
- Myhill, L.J.; Stolzenbach, S.; Mejer, H.; Krych, L.; Jakobsen, S.R.; Kot, W.; Skovgaard, K.; Canibe, N.; Nejsum, P.; Nielsen, D.S. Parasite-probiotic interactions in the gut: Bacillus sp. and Enterococcus faecium regulate type-2 inflammatory responses and modify the gut microbiota of pigs during helminth infection. Front. Immunol. 2022, 12, 793260. [Google Scholar] [CrossRef]
- Ailioaie, L.M.; Litscher, G. Probiotics, photobiomodulation, and disease management: Controversies and challenges. Int. J. Mol. Sci. 2021, 22, 4942. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Zhang, K.; Ma, X.; He, P. Clostridium species as probiotics: Potentials and challenges. J. Anim. Sci. Biotechnol. 2020, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Mun, D.; Kyoung, H.; Kong, M.; Ryu, S.; Jang, K.B.; Baek, J.; Park, K., II; Song, M.; Kim, Y. Effects of Bacillus-based probiotics on growth performance, nutrient digestibility, and intestinal health of weaned pigs. J. Anim. Sci. Technol. 2021, 63, 1314. [Google Scholar] [CrossRef]
- He, Y.; Jinno, C.; Kim, K.; Wu, Z.; Tan, B.; Li, X.; Whelan, R.; Liu, Y. Dietary Bacillus spp. enhanced growth and disease resistance of weaned pigs by modulating intestinal microbiota and systemic immunity. J. Anim. Sci. Biotechnol. 2020, 11, 101. [Google Scholar] [CrossRef]
- Soares, M.B.; Almada, C.N.; Pereira, E.P.R.; Ferreira, B.M.; Balthazar, C.F.; Khorshidian, N.; Rocha, R.S.; Xavier-Santos, D.; Cruz, A.G.; Ranadheera, C.S. Sporeforming probiotic bacteria: Characteristics, health benefits, and technological aspects for their applications in foods and beverages. Trends Food Sci. Technol. 2023. [Google Scholar] [CrossRef]
- Todorov, S.D.; Ivanova, I.V.; Popov, I.; Weeks, R.; Chikindas, M.L. Bacillus spore-forming probiotics: Benefits with concerns? Crit. Rev. Microbiol. 2022, 48, 513–530. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Chen, Y.; Sun, T.; Wu, Y.; Su, Y.; Liu, C.; Zhou, J.; Deng, Y.; Wen, J. Antimicrobial resistance, virulence characteristics and genotypes of Bacillus spp. from probiotic products of diverse origins. Food Res. Int. 2021, 139, 109949. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Derrien, M.; Levenez, F.; Brazeilles, R.; Ballal, S.A.; Kim, J.; Degivry, M.-C.; Quéré, G.; Garault, P.; van Hylckama Vlieg, J.E.T. Ecological robustness of the gut microbiota in response to ingestion of transient food-borne microbes. ISME J. 2016, 10, 2235–2245. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Jin, D.; Huang, S.; Wu, J.; Xu, M.; Liu, T.; Dong, W.; Liu, X.; Wang, S.; Zhong, W. Clostridium butyricum, a butyrate-producing probiotic, inhibits intestinal tumor development through modulating Wnt signaling and gut microbiota. Cancer Lett. 2020, 469, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, M.; Racedo, S.M.; Denayrolles, M.; Ripert, G.; Desfougères, T.; Lobach, A.R.; Simon, R.; Pélerin, F.; Jüsten, P.; Urdaci, M.C. Safety assessment of Bacillus subtilis CU1 for use as a probiotic in humans. Regul. Toxicol. Pharmacol. 2017, 83, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Zhang, J.; Li, Y.; Li, K.; Gong, S.; Li, F.; Wang, P.; Iqbal, M.; Kulyar, M.F.-e.-A.; Li, J. Probiotic potential of bacillus licheniformis and bacillus pumilus isolated from Tibetan yaks, China. Probiotics Antimicrob. Proteins 2022, 14, 579–594. [Google Scholar] [CrossRef]
- Altun, G.K.; Erginkaya, Z. Identification and characterization of Bacillus coagulans strains for probiotic activity and safety. LWT 2021, 151, 112233. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).