Adaptive Expression and ncRNA Regulation of Genes Related to Digestion and Metabolism in Stomach of Red Pandas during Suckling and Adult Periods
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. RNA Extraction and Library Preparation for Sequencing
2.3. Differentially Expressed lncRNAs, miRNAs, mRNAs, and Enrichment Analyses
2.4. Constructions of Regulatory Networks among lncRNAs, miRNAs, and mRNAs
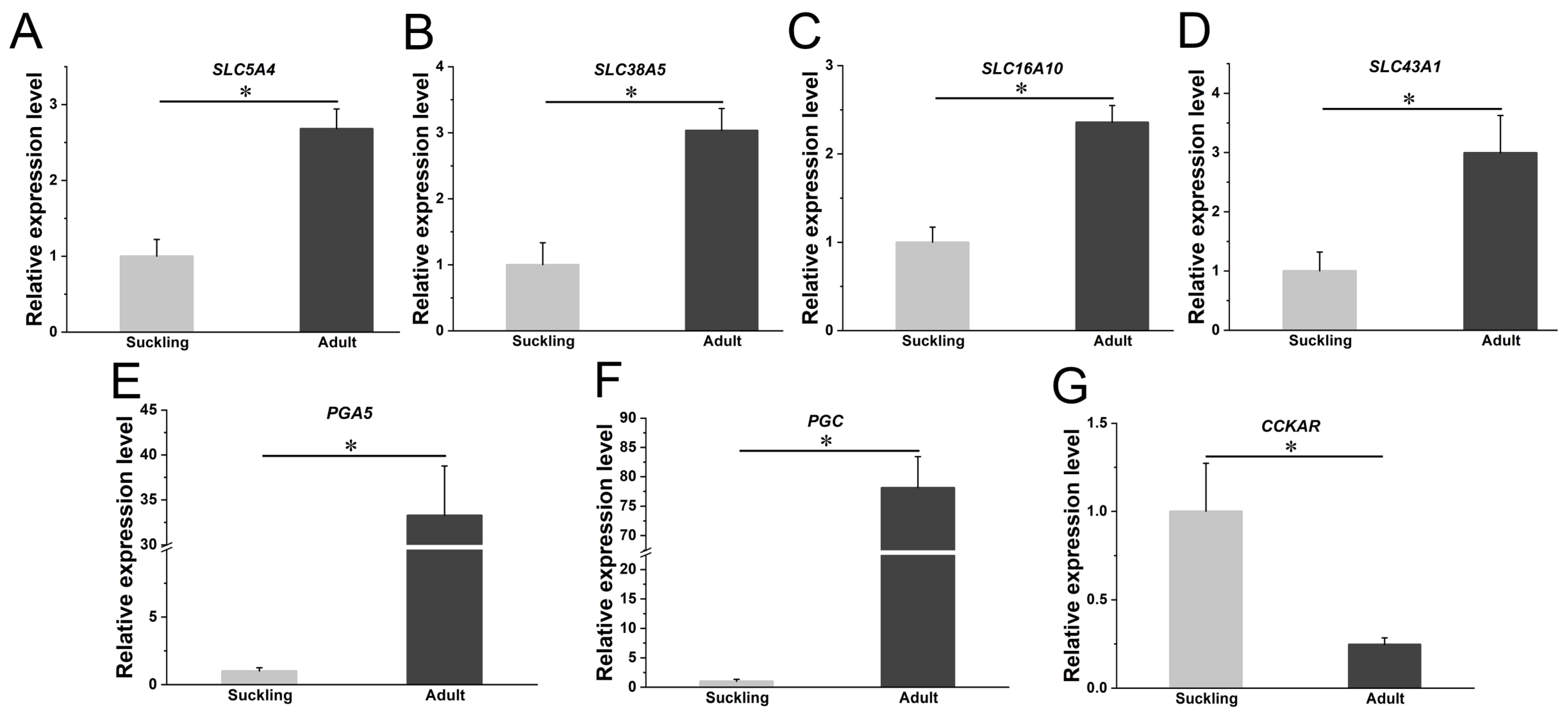
2.5. Real-Time Quantitative PCR
3. Results
3.1. RNA Sequencing
3.2. Identifications and General Characteristics of lncRNAs and miRNAs
3.3. Sample Cluster Analyses
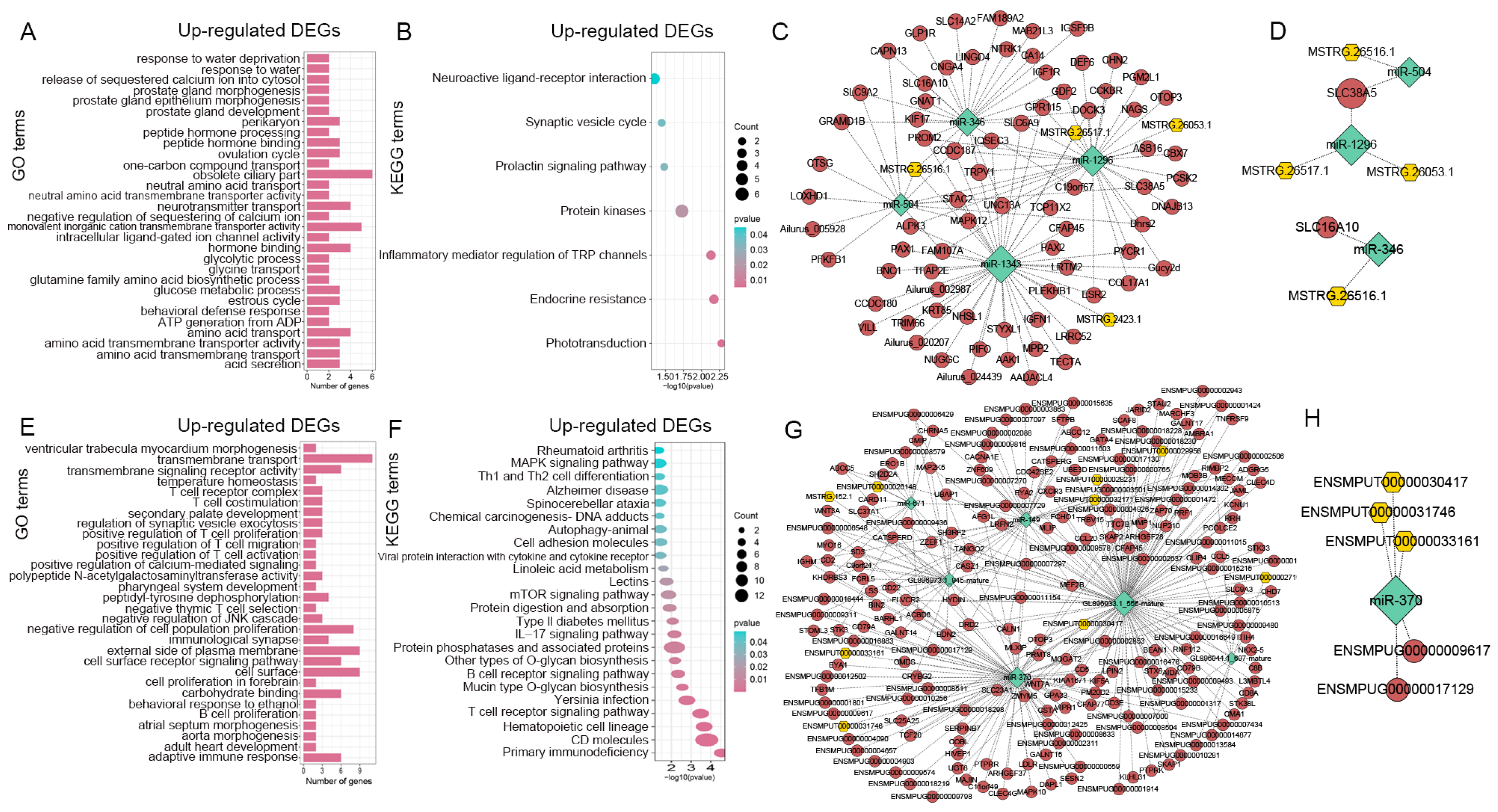
3.4. Enrichment Analyses of DE-mRNAs and Identifications of Digestion- and Metabolism-Related DE-mRNAs
3.5. Constructions of lncRNA-mRNA Networks
3.6. Constructions of miRNA-mRNA Networks
3.7. Constructions of lncRNA-miRNA-mRNA Networks
4. Discussion
4.1. Digestion and Metabolism of Carbohydrates
4.2. Digestion and Metabolism of Amino Acids and Proteins
4.3. Digestion and Metabolism of Lipids
5. Conclusions and Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mathieson, I. Human adaptation over the past 40,000 years. Curr. Opin. Genet. Dev. 2020, 62, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.N.; Ruczinski, I.; Yanek, L.R.; Becker, L.C.; Becker, D.M.; Guio, H.; Cui, T.; Chilton, F.H.; Mathias, R.A.; O’Connor, T.D. Evolution of Hominin Polyunsaturated Fatty Acid Metabolism: From Africa to the New World. Genome Biol. Evol. 2019, 11, 1417–1430. [Google Scholar] [CrossRef] [PubMed]
- Lillycrop, K.A.; Slater-Jefferies, J.L.; Hanson, M.A.; Godfrey, K.M.; Jackson, A.A.; Burdge, G.C. Induction of altered epigenetic regulation of the hepatic glucocorticoid receptor in the offspring of rats fed a protein-restricted diet during pregnancy suggests that reduced DNA methyltransferase-1 expression is involved in impaired DNA methylation and changes in histone modifications. Br. J. Nutr. 2007, 97, 1064–1073. [Google Scholar] [PubMed]
- Ehara, T.; Kamei, Y.; Yuan, X.; Takahashi, M.; Kanai, S.; Tamura, E.; Tsujimoto, K.; Tamiya, T.; Nakagawa, Y.; Shimano, H.; et al. Ligand-activated PPARalpha-dependent DNA demethylation regulates the fatty acid beta-oxidation genes in the postnatal liver. Diabetes 2015, 64, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Girard, J.; Ferre, P.; Pegorier, J.P.; Duee, P.H. Adaptations of glucose and fatty acid metabolism during perinatal period and suckling-weaning transition. Physiol. Rev. 1992, 72, 507–562. [Google Scholar] [CrossRef]
- Le Huerou, I.; Wicker, C.; Guilloteau, P.; Toullec, R.; Puigserver, A. Specific regulation of the gene expression of some pancreatic enzymes during postnatal development and weaning in the calf. Biochim. Biophys. Acta 1990, 1048, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Pierzynowski, S.G.; Westrom, B.R.; Erlanson-Albertsson, C.; Ahre’n, B.; Svendsen, J.; Karlsson, B.W. Induction of exocrine pancreas maturation at weaning in young developing pigs. J. Pediatr. Gastroenterol. Nutr. 1993, 16, 287–293. [Google Scholar] [PubMed]
- Ma, J.; Zhang, L.; Huang, Y.; Shen, F.; Wu, H.; Yang, Z.; Hou, R.; Song, Z.; Yue, B.; Zhang, X. Epigenomic profiling indicates a role for DNA methylation in the postnatal liver and pancreas development of giant pandas. Genomics 2022, 114, 110342. [Google Scholar] [CrossRef]
- Ma, J.; Shen, F.; Chen, L.; Wu, H.; Huang, Y.; Fan, Z.; Hou, R.; Yue, B.; Zhang, X. Gene expression profiles during postnatal development of the liver and pancreas in giant pandas. Aging 2020, 12, 15705–15729. [Google Scholar] [CrossRef]
- Roberts, M.S.; Gittleman, J.L. Ailurus fulgens. Mamm. Species 1984, 222, 1–8. [Google Scholar] [CrossRef]
- Glatston, A.R. Red Panda: Biology and Conservation of the First Panda; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Roberts, M.S.; Kessler, D.S. Reproduction in Red pandas, Ailurus fulgens (Carnivora: Ailuropodidae). J. Zool. 1979, 188, 235–249. [Google Scholar] [CrossRef]
- Anton, M.; Salesa, M.J.; Pastor, J.F.; Peigne, S.; Morales, J. Implications of the functional anatomy of the hand and forearm of Ailurus fulgens (Carnivora, Ailuridae) for the evolution of the ’false-thumb’ in pandas. J. Anat. 2006, 209, 757–764. [Google Scholar] [CrossRef]
- Salesa, M.J.; Anton, M.; Peigne, S.; Morales, J. Evidence of a false thumb in a fossil carnivore clarifies the evolution of pandas. Proc. Natl. Acad. Sci. USA 2006, 103, 379–382. [Google Scholar] [CrossRef]
- Wang, X.; Su, D.F.; Jablonski, N.G.; Ji, X.; Kelley, J.; Flynn, L.J.; Deng, T. Earliest giant panda false thumb suggests conflicting demands for locomotion and feeding. Sci. Rep. 2022, 12, 10538. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, Q.; Ma, S.; Ma, T.; Shan, L.; Wang, X.; Nie, Y.; Ning, Z.; Yan, L.; Xiu, Y.; et al. Comparative genomics reveals convergent evolution between the bamboo-eating giant and red pandas. Proc. Natl. Acad. Sci. USA 2017, 114, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Wang, X.; Hu, Y.; Wu, Q.; Nie, Y.; Dong, J.; Ding, Y.; Yan, L.; Wei, F. Diet drives convergent evolution of gut microbiomes in bamboo-eating species. Sci. China Life Sci. 2021, 64, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhang, L.; Shen, F.; Geng, Y.; Huang, Y.; Wu, H.; Fan, Z.; Hou, R.; Song, Z.; Yue, B.; et al. Gene expressions between obligate bamboo-eating pandas and non-herbivorous mammals reveal converged specialized bamboo diet adaptation. BMC Genom. 2023, 24, 23. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shen, F.; Jie, X.; Zhang, L.; Yan, G.; Wu, H.; Huang, Y.; Hou, R.; Yue, B.; Zhang, X. Comparative Transcriptomics and Methylomics Reveal Adaptive Responses of Digestive and Metabolic Genes to Dietary Shift in Giant and Red Pandas. Genes 2022, 13, 1446. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, G.; Heard, E. Advances in epigenetics link genetics to the environment and disease. Nature 2019, 571, 489–499. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, R. Regulatory non-coding RNAs: Revolutionizing the RNA world. Mol. Biol. Rep. 2014, 41, 3915–3923. [Google Scholar] [CrossRef]
- Nojima, T.; Proudfoot, N.J. Mechanisms of lncRNA biogenesis as revealed by nascent transcriptomics. Nat. Rev. Mol. Cell Biol. 2022, 23, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Z.; Wei, K. miRNA in cardiac development and regeneration. Cell Regen. 2021, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Guttman, M.; Amit, I.; Garber, M.; French, C.; Lin, M.F.; Feldser, D.; Huarte, M.; Zuk, O.; Carey, B.W.; Cassady, J.P.; et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature 2009, 458, 223–227. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Sim, G.; Kehling, A.C.; Park, M.S.; Secor, J.; Divoky, C.; Zhang, H.; Malhotra, N.; Bhagdikar, D.; Abd El-Wahab, E.W.; Nakanishi, K. Manganese-dependent microRNA trimming by 3’-->5’ exonucleases generates 14-nucleotide or shorter tiny RNAs. Proc. Natl. Acad. Sci. USA 2022, 119, e2214335119. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Zhao, J.; Yeung, P.Y.; Zhang, Q.C.; Kwok, C.K. Revealing lncRNA Structures and Interactions by Sequencing-Based Approaches. Trends Biochem. Sci. 2019, 44, 33–52. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.B.; Ulitsky, I. The functions of long noncoding RNAs in development and stem cells. Development 2016, 143, 3882–3894. [Google Scholar] [CrossRef]
- Chen, Y.; He, Y.; Zhou, H. The potential role of lncRNAs in diabetes and diabetic microvascular complications. Endocr. J. 2020, 67, 659–668. [Google Scholar] [CrossRef]
- Wang, J.; Yang, W.; Chen, Z.; Chen, J.; Meng, Y.; Feng, B.; Sun, L.; Dou, L.; Li, J.; Cui, Q.; et al. Long Noncoding RNA lncSHGL Recruits hnRNPA1 to Suppress Hepatic Gluconeogenesis and Lipogenesis. Diabetes 2018, 67, 581–593. [Google Scholar] [CrossRef]
- Kim, B.M.; Woo, J.; Kanellopoulou, C.; Shivdasani, R.A. Regulation of mouse stomach development and Barx1 expression by specific microRNAs. Development 2011, 138, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Kefas, B.; Comeau, L.; Erdle, N.; Montgomery, E.; Amos, S.; Purow, B. Pyruvate kinase M2 is a target of the tumor-suppressive microRNA-326 and regulates the survival of glioma cells. Neuro Oncol. 2010, 12, 1102–1112. [Google Scholar] [CrossRef] [PubMed]
- Davalos, A.; Goedeke, L.; Smibert, P.; Ramirez, C.M.; Warrier, N.P.; Andreo, U.; Cirera-Salinas, D.; Rayner, K.; Suresh, U.; Pastor-Pareja, J.C.; et al. miR-33a/b contribute to the regulation of fatty acid metabolism and insulin signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 9232–9237. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.V. MYC, microRNAs and glutamine addiction in cancers. Cell Cycle 2009, 8, 3243–3245. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Wang, J.; O’Brien, P.C.; Fu, B.; Ying, T.; Ferguson-Smith, M.A.; Yang, F. The genome phylogeny of domestic cat, red panda and five mustelid species revealed by comparative chromosome painting and G-banding. Chromosome Res. 2002, 10, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Harrer, S.; Schmidt, W.J. Annual rhythm and hormonal control of predatory behavior in female ferrets. Physiol. Behav. 1986, 37, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Ragg, J.R.; Mackintosh, C.G.; Moller, H. The scavenging behaviour of ferrets (Mustela furo), feral cats (Felis domesticus), possums (Trichosurus vulpecula), hedgehogs (Erinaceus europaeus) and harrier hawks (Circus approximans) on pastoral farmland in New Zealand: Implications for bovine tuberculosis transmission. N. Z. Vet. J. 2000, 48, 166–175. [Google Scholar] [PubMed]
- Davis, J.S.; Williams, S.H. The influence of diet on masticatory motor patterns in musteloid carnivorans: An analysis of jaw adductor activity in ferrets (Mustela putorius furo) and kinkajous (Potos. flavus). J. Exp. Zool. A Ecol. Integr. Physiol. 2017, 327, 551–561. [Google Scholar] [CrossRef]
- Schoknecht, P.A.; Cranford, J.A.; Akers, R.M. Variability in milk composition of the domestic ferret (Mustela putorius). Comp. Biochem. Physiol. A Comp. Physiol. 1985, 81, 589–591. [Google Scholar]
- Jenness, R. Proceedings: Biosynthesis and composition of milk. J. Investig. Dermatol. 1974, 63, 109–118. [Google Scholar] [CrossRef]
- Ahamad, M.N.; Ali, M.E.; Hossain, M.M.; Asing, A.; Sultana, S.; Jahurul, M.H. Multiplex PCR assay discriminates rabbit, rat and squirrel meat in food chain. Food Addit. Contam. A 2017, 34, 2043–2057. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Wang, Y.; Li, J.; Lu, D. Comparison of the fatty acid composition of milk, meat and oil of different livestock. Anim. Husb. 2019, 34, 18–23. [Google Scholar]
- Patel, R.K.; Jain, M. NGS QC Toolkit: A toolkit for quality control of next generation sequencing data. PLoS ONE 2012, 7, e30619. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef] [PubMed]
- Pertea, G.; Pertea, M. GFF Utilities: GffRead and GffCompare. F1000Res 2020, 9, 304. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, Y.; Ye, Z.Q.; Liu, X.Q.; Zhao, S.Q.; Wei, L.; Gao, G. CPC: Assess the protein-coding potential of transcripts using sequence features and support vector machine. Nucleic Acids Res. 2007, 35, W345–W349. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Luo, H.; Bu, D.; Zhao, G.; Yu, K.; Zhang, C.; Liu, Y.; Chen, R.; Zhao, Y. Utilizing sequence intrinsic composition to classify protein-coding and long non-coding transcripts. Nucleic Acids Res. 2013, 41, e166. [Google Scholar] [CrossRef]
- Mistry, J.; Finn, R. Pfam: A domain-centric method for analyzing proteins and proteomes. Methods Mol. Biol. 2007, 396, 43–58. [Google Scholar]
- Chen, Y.; Ye, W.; Zhang, Y.; Xu, Y. High speed BLASTN: An accelerated MegaBLAST search tool. Nucleic Acids Res. 2015, 43, 7762–7768. [Google Scholar] [CrossRef] [PubMed]
- Kalvari, I.; Nawrocki, E.P.; Argasinska, J.; Quinones-Olvera, N.; Finn, R.D.; Bateman, A.; Petrov, A.I. Non-Coding RNA Analysis Using the Rfam Database. Curr. Protoc. Bioinform. 2018, 62, e51. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, M.R.; Mackowiak, S.D.; Li, N.; Chen, W.; Rajewsky, N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 2012, 40, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010, 11, R25. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Zhang, Y.; Fan, G.; Xin, Y.; Yao, Y. Transcriptome analysis identified a novel 3-LncRNA regulatory network of transthyretin attenuating glucose induced hRECs dysfunction in diabetic retinopathy. BMC Med. Genom. 2019, 12, 134. [Google Scholar] [CrossRef]
- Betel, D.; Wilson, M.; Gabow, A.; Marks, D.S.; Sander, C. The microRNA.org resource: Targets and expression. Nucleic Acids Res. 2008, 36, D149–D153. [Google Scholar] [CrossRef]
- Rehmsmeier, M.; Steffen, P.; Hochsmann, M.; Giegerich, R. Fast and effective prediction of microRNA/target duplexes. RNA 2004, 10, 1507–1517. [Google Scholar] [CrossRef]
- Tay, Y.; Rinn, J.; Pandolfi, P.P. The multilayered complexity of ceRNA crosstalk and competition. Nature 2014, 505, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhuang, S.; Chen, X.; Du, J.; Zhong, L.; Ding, J.; Wang, L.; Yi, J.; Hu, G.; Tang, G.; et al. lncRNA ITGB8-AS1 functions as a ceRNA to promote colorectal cancer growth and migration through integrin-mediated focal adhesion signaling. Mol. Ther. 2022, 30, 688–702. [Google Scholar] [CrossRef] [PubMed]
- Dierenfeld, E.S.; Hintz, H.F.; Robertson, J.B.; Van Soest, P.J.; Oftedal, O.T. Utilization of bamboo by the giant panda. J. Nutr. 1982, 112, 636–641. [Google Scholar] [CrossRef]
- Fei, Y.; Hou, R.; Spotila, J.R.; Paladino, F.V.; Qi, D.; Zhang, Z. Metabolic rate of the red panda, Ailurus fulgens, a dietary bamboo specialist. PLoS ONE 2017, 12, e0173274. [Google Scholar] [CrossRef]
- Knott, K.K.; Christian, A.L.; Falcone, J.F.; Vance, C.K.; Bauer, L.L.; Fahey, G.C., Jr.; Kouba, A.J. Phenological changes in bamboo carbohydrates explain the preference for culm over leaves by giant pandas (Ailuropoda melanoleuca) during spring. PLoS ONE 2017, 12, e0177582. [Google Scholar] [CrossRef]
- Subramaniam, M.; Enns, C.B.; Loewen, M.E. Sigmoidal kinetics define porcine intestinal segregation of electrogenic monosaccharide transport systems as having multiple transporter population involvement. Physiol. Rep. 2019, 7, e14090. [Google Scholar] [CrossRef] [PubMed]
- Diez-Sampedro, A.; Eskandari, S.; Wright, E.M.; Hirayama, B.A. Na+-to-sugar stoichiometry of SGLT3. Am. J. Physiol. Ren. Physiol. 2001, 280, F278–F282. [Google Scholar] [CrossRef]
- Nakanishi, T.; Kekuda, R.; Fei, Y.J.; Hatanaka, T.; Sugawara, M.; Martindale, R.G.; Leibach, F.H.; Prasad, P.D.; Ganapathy, V. Cloning and functional characterization of a new subtype of the amino acid transport system N. Am. J. Physiol. Cell Physiol. 2001, 281, C1757–C1768. [Google Scholar] [CrossRef] [PubMed]
- Bodoy, S.; Fotiadis, D.; Stoeger, C.; Kanai, Y.; Palacin, M. The small SLC43 family: Facilitator system l amino acid transporters and the orphan EEG1. Mol. Asp. Med. 2013, 34, 638–645. [Google Scholar] [CrossRef]
- Uemura, S.; Mochizuki, T.; Kurosaka, G.; Hashimoto, T.; Masukawa, Y.; Abe, F. Functional analysis of human aromatic amino acid transporter MCT10/TAT1 using the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta Biomembr. 2017, 1859, 2076–2085. [Google Scholar] [CrossRef]
- Webb, L.A.; Sadri, H.; Schuh, K.; Egert, S.; Stehle, P.; Meyer, I.; Koch, C.; Dusel, G.; Sauerwein, H. Branched-chain amino acids: Abundance of their transporters and metabolizing enzymes in adipose tissue, skeletal muscle, and liver of dairy cows at high or normal body condition. J. Dairy. Sci. 2020, 103, 2847–2863. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, B.M.; Kiess, A.S.; Blemings, K.P. Alpha-aminoadipate delta-semialdehyde synthase mRNA knockdown reduces the lysine requirement of a mouse hepatic cell line. J. Nutr. 2008, 138, 2143–2147. [Google Scholar] [CrossRef] [PubMed]
- Boyce, S.W.; Zingg, B.M.; Lightfoot, T.L. Behavior of Mustela putorius furo (the domestic ferret). Vet. Clin. N. Am. Exot. Anim. Pract. 2001, 4, 697–712. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.R. Functional development of the stomach. Annu. Rev. Physiol. 1985, 47, 199–215. [Google Scholar] [CrossRef]
- Kumegawa, M.; Takuma, T.; Hosoda, S.; Kunii, S.; Kanda, Y. Precocious induction of pepsinogen in the stomach of suckling mice by hormones. Biochim. Biophys. Acta 1978, 543, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Seidel, E.R.; Johnson, L.R. Ontogeny of gastric mucosal muscarinic receptor and sensitivity to carbachol. Am. J. Physiol. 1984, 246, G550–G555. [Google Scholar] [CrossRef]
- Sun, L.P.; Gong, Y.H.; Dong, N.N.; Wang, L.; Yuan, Y. [Correlation of pepsinogen C (PGC) gene insertion/deletion polymorphism to PGC protein expression in gastric mucosa and serum]. Ai Zheng 2009, 28, 487–492. [Google Scholar]
- Athauda, S.B.; Tanji, M.; Kageyama, T.; Takahashi, K. A comparative study on the NH2-terminal amino acid sequences and some other properties of six isozymic forms of human pepsinogens and pepsins. J. Biochem. 1989, 106, 920–927. [Google Scholar] [CrossRef]
- Adaikan, P.G.; Karim, S.M. Effects of PGA and PGB compounds on gastrointestinal tract smooth muscle from man and laboratory animals. Prostaglandins 1976, 11, 15–22. [Google Scholar] [CrossRef]
- Schubert, M.L. Gastric secretion. Curr. Opin. Gastroenterol. 2002, 18, 639–649. [Google Scholar] [CrossRef]
- Huppi, K.; Siwarski, D.; Pisegna, J.R.; Wank, S. Chromosomal localization of the gastric and brain receptors for cholecystokinin (CCKAR and CCKBR) in human and mouse. Genomics 1995, 25, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Azemi, N.A.; Azemi, A.K.; Abu-Bakar, L.; Sevakumaran, V.; Muhammad, T.S.T.; Ismail, N. Xestospongia muta Fraction-7 and Linoleic Acid: Effects on SR-BI Gene Expression and HDL Cholesterol Uptake. Mar. Drugs 2022, 20, 762. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Zhang, L.; Luo, L.; Shen, F.; Zhao, Y.; Wu, H.; Huang, Y.; Hou, R.; Yue, B.; Zhang, X. Adaptive Expression and ncRNA Regulation of Genes Related to Digestion and Metabolism in Stomach of Red Pandas during Suckling and Adult Periods. Animals 2024, 14, 1795. https://doi.org/10.3390/ani14121795
Li L, Zhang L, Luo L, Shen F, Zhao Y, Wu H, Huang Y, Hou R, Yue B, Zhang X. Adaptive Expression and ncRNA Regulation of Genes Related to Digestion and Metabolism in Stomach of Red Pandas during Suckling and Adult Periods. Animals. 2024; 14(12):1795. https://doi.org/10.3390/ani14121795
Chicago/Turabian StyleLi, Lu, Liang Zhang, Lijun Luo, Fujun Shen, Yanni Zhao, Honglin Wu, Yan Huang, Rong Hou, Bisong Yue, and Xiuyue Zhang. 2024. "Adaptive Expression and ncRNA Regulation of Genes Related to Digestion and Metabolism in Stomach of Red Pandas during Suckling and Adult Periods" Animals 14, no. 12: 1795. https://doi.org/10.3390/ani14121795
APA StyleLi, L., Zhang, L., Luo, L., Shen, F., Zhao, Y., Wu, H., Huang, Y., Hou, R., Yue, B., & Zhang, X. (2024). Adaptive Expression and ncRNA Regulation of Genes Related to Digestion and Metabolism in Stomach of Red Pandas during Suckling and Adult Periods. Animals, 14(12), 1795. https://doi.org/10.3390/ani14121795





