Coloration in Equine: Overview of Candidate Genes Associated with Coat Color Phenotypes
Abstract
Simple Summary
Abstract
1. Introduction
2. Coat Color Classification in Equines
2.1. Coat Color Classification in Horses
- (1)
- Black: The entire coat is primarily black.
- (2)
- Bay: The coat is brown with black points and shades.
- (3)
- Chestnut: The coat color is varying shades of red and have non-black points.
- (4)
- Diluted colors: The coat color becomes lighter and sometimes nearly white.
- (5)
- White spotting: The patterns of white spotting are added independently to any colored coat.
2.2. Coat Color Classification in Donkey
- (1)
- Black: The entire coat is primarily black.
- (2)
- Sanfen: The entire coat is black, with white fur around the mouth, eyes, and underbelly.
- (3)
- Gray: The coat is predominantly greenish-gray, greenish-brown, or greenish-white, with lighter coloring on the belly and nose. The long hairs are black or nearly black.
- (4)
- Cyan: Mixed black and white hairs cover the body, and the number of white hairs increases with age.
- (5)
- Chestnut: The coat is mostly red all over the body, with lighter, almost white coloring around the mouth, nose, eyes, underbelly, and inside the limbs.
- (6)
- White: The entire coat is white or light gray.
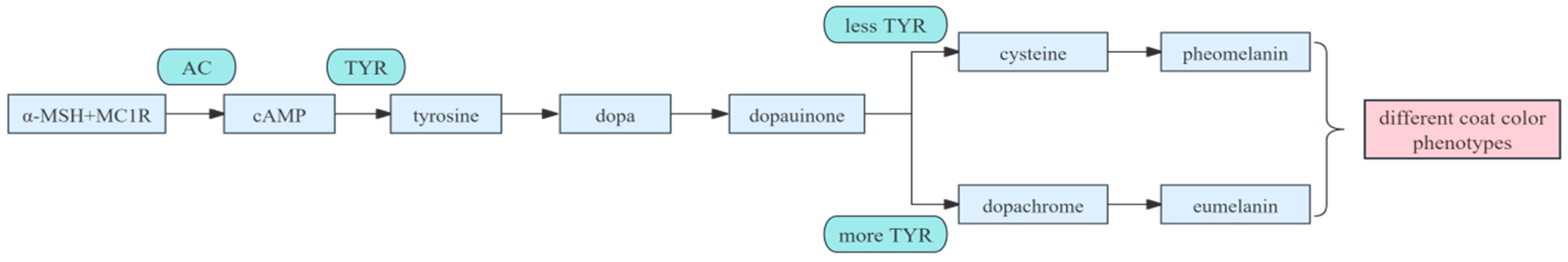
3. Mechanisms of Pigmentation
4. Candidate Genes Associated with Coat Color Phenotypes
4.1. Candidate Genes Associated with Coat Color Phenotypes in Horses
4.1.1. MC1R and ASIP
4.1.2. KIT
4.1.3. Diluted Genes and Their Association with Coat Colors
4.1.4. Other Genes
4.2. Candidate Genes Associated with Coat Color Phenotypes in Donkey
5. Coat Color Genetic Applications
5.1. Coat Color Genetic Applications in Breeding
5.2. Coat Color Genetic Applications in Diseases
5.2.1. Vitiligo and Melanoma
5.2.2. Health Risks
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodrigues, J.B.; Raw, Z.; Santurtun, E.; Cooke, F.; Clancy, C. Donkeys in transition: Changing use in a changing world. Braz. J. Vet. Res. Anim. Sci. 2021, 58, e174325. [Google Scholar] [CrossRef]
- Raudsepp, T.; Finno, C.J.; Bellone, R.R.; Petersen, J.L. Ten years of the horse reference genome: Insights into equine biology, domestication and population dynamics in the post-genome era. Anim. Genet. 2019, 50, 569–597. [Google Scholar] [CrossRef] [PubMed]
- Orlando, L.; Librado, P. Origin and Evolution of Deleterious Mutations in Horses. Genes 2019, 10, 649. [Google Scholar] [CrossRef] [PubMed]
- Togtokh, M.; Haige, H.; Ruoyang, Z.; Tugeqin, B.; Manglai, D.; Bai, D. The origins and genetic characteristics of domestic horses. Biodivers. Sci. 2020, 28, 734–748. [Google Scholar]
- Camillo, F.; Rota, A.; Biagini, L.; Tesi, M.; Fanelli, D.; Panzani, D. The Current Situation and Trend of Donkey Industry in Europe. J. Equine Vet. Sci. 2018, 65, 44–49. [Google Scholar] [CrossRef]
- Wang, C.; Li, H.; Guo, Y.; Huang, J.; Sun, Y.; Min, J.; Wang, J.; Fang, X.; Zhao, Z.; Wang, S.; et al. Donkey genomes provide new insights into domestication and selection for coat color. Nat. Commun. 2020, 11, 6014. [Google Scholar] [CrossRef]
- Ahmad, H.I.; Ahmad, M.J.; Jabbir, F.; Ahmar, S.; Ahmad, N.; Elokil, A.A.; Chen, J. The Domestication Makeup: Evolution, Survival, and Challenges. Front. Ecol. Evol. 2020, 8, 103. [Google Scholar] [CrossRef]
- Hansen Wheat, C.; van der Bijl, W.; Wheat, C.W. Morphology does not covary with predicted behavioral correlations of the domestication syndrome in dogs. Evol. Lett. 2020, 4, 189–199. [Google Scholar] [CrossRef]
- Jensen, P. Domestication—From behaviour to genes and back again. Appl. Anim. Behav. Sci. 2006, 97, 3–15. [Google Scholar] [CrossRef]
- Oli, M.K.; Kenney, A.J.; Boonstra, R.; Boutin, S.; Murray, D.L.; Peers, M.J.L.; Gilbert, B.S.; Jung, T.S.; Chaudhary, V.; Hines, J.E.; et al. Does coat colour influence survival? A test in a cyclic population of snowshoe hares. Proc. R. Soc. B Biol. Sci. 2023, 290, 20221421. [Google Scholar] [CrossRef]
- Kennah, J.L.; Peers, M.J.L.; Vander Wal, E.; Majchrzak, Y.N.; Menzies, A.K.; Studd, E.K.; Boonstra, R.; Humphries, M.M.; Jung, T.S.; Kenney, A.J.; et al. Coat color mismatch improves survival of a keystone boreal herbivore: Energetic advantages exceed lost camouflage. Ecology 2023, 104, e3882. [Google Scholar] [CrossRef] [PubMed]
- Thapa, P.C.; Do, D.N.; Manafiazar, G.; Miar, Y. Coat color inheritance in American mink. BMC Genom. 2023, 24, 234. [Google Scholar] [CrossRef] [PubMed]
- Brancalion, L.; Haase, B.; Wade, C.M. Canine coat pigmentation genetics: A review. Anim. Genet. 2022, 53, 3–34. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; He, X.; Zhao, Y.; Unierhu; Bai, D.; Manglai, D. Tyrosinase-related protein 1 (TYRP1) gene polymorphism and skin differential expression related to coat color in Mongolian horse. Livest. Sci. 2014, 167, 58–64. [Google Scholar] [CrossRef]
- Dugatkin, L.A. The silver fox domestication experiment. Evol. Educ. Outreach 2018, 11, 16. [Google Scholar] [CrossRef][Green Version]
- Terfa, Z.G.; Haile, A.; Baker, D.; Kassie, G.T. Valuation of traits of indigenous sheep using hedonic pricing in Central Ethiopia. Agric. Food Econ. 2013, 1, 6. [Google Scholar] [CrossRef]
- Kassie, G.T.; Abdulai, A.; Wollny, C. Heteroscedastic hedonic price model for cattle in the rural markets of central Ethiopia. Appl. Econ. 2011, 43, 3459–3464. [Google Scholar] [CrossRef]
- Sánchez-Guerrero, M.J.; Negro-Rama, S.; Demyda-Peyras, S.; Solé-Berga, M.; Azor-Ortiz, P.J.; Valera-Córdoba, M. Morphological and genetic diversity of Pura Raza Español horse with regard to the coat colour. Anim. Sci. J. 2019, 90, 14–22. [Google Scholar] [CrossRef]
- Zhong, H.; Zhang, J.; Tan, C.; Shi, J.; Yang, J.; Cai, G.; Wu, Z.; Yang, H. Pig Coat Color Manipulation by MC1R Gene Editing. Int. J. Mol. Sci. 2022, 23, 10356. [Google Scholar] [CrossRef]
- Jia, X.; Ding, P.; Chen, S.; Zhao, S.; Wang, J.; Lai, S. Analysis of MC1R, MITF, TYR, TYRP1, and MLPH Genes Polymorphism in Four Rabbit Breeds with Different Coat Colors. Animals 2021, 11, 81. [Google Scholar] [CrossRef]
- Yao, L.; Bao, A.; Hong, W.; Hou, C.; Zhang, Z.; Liang, X.; Aniwashi, J. Transcriptome profiling analysis reveals key genes of different coat color in sheep skin. PeerJ 2019, 7, e8077. [Google Scholar] [CrossRef]
- Fernández, A.; Hayashi, M.; Garrido, G.; Montero, A.; Guardia, A.; Suzuki, T.; Montoliu, L. Genetics of non-syndromic and syndromic oculocutaneous albinism in human and mouse. Pigment. Cell Melanoma Res. 2021, 34, 786–799. [Google Scholar] [CrossRef]
- Philipp, U.; Lupp, B.; Mömke, S.; Stein, V.; Tipold, A.; Eule, J.C.; Rehage, J.; Distl, O. A MITF Mutation Associated with a Dominant White Phenotype and Bilateral Deafness in German Fleckvieh Cattle. PLoS ONE 2011, 6, e28857. [Google Scholar] [CrossRef]
- Dessinioti, C.; Stratigos, A.J.; Rigopoulos, D.; Katsambas, A.D. A review of genetic disorders of hypopigmentation: Lessons learned from the biology of melanocytes. Exp. Dermatol. 2009, 18, 741–749. [Google Scholar] [CrossRef]
- Platt, S.; Freeman, J.; di Stefani, A.; Wieczorek, L.; Henley, W. Prevalence of Unilateral and Bilateral Deafness in Border Collies and Association with Phenotype. J. Vet. Intern. Med. 2006, 20, 1355–1362. [Google Scholar] [CrossRef]
- Gauly, M.; Vaughan, J.; Hogreve, S.K.; Erhardt, G. Brainstem Auditory-Evoked Potential Assessment of Auditory Function and Congenital Deafness in Llamas (Lama glama) and Alpacas (L. pacos). J. Vet. Intern. Med. 2005, 19, 756–760. [Google Scholar]
- Jannot, A.; Meziani, R.; Bertrand, G.; Gérard, B.; Descamps, V.; Archimbaud, A.; Picard, C.; Ollivaud, L.; Basset-Seguin, N.; Kerob, D.; et al. Allele variations in the OCA2 gene (pink-eyed-dilution locus) are associated with genetic susceptibility to melanoma. Eur. J. Hum. Genet. 2005, 13, 913–920. [Google Scholar] [CrossRef]
- Marklund, L.; Moller, M.J.; Sandberg, K.; Andersson, L. A missense mutation in the gene for melanocyte-stimulating hormone receptor (MCIR) is associated with the chestnut coat color in horses. Mamm. Genome 1996, 7, 895–899. [Google Scholar] [CrossRef]
- Abitbol, M.; Legrand, R.; Tiret, L. A missense mutation in melanocortin 1 receptor is associated with the red coat colour in donkeys. Anim. Genet. 2014, 45, 878–880. [Google Scholar] [CrossRef]
- Rieder, S.; Taourit, S.; Mariat, D.; Langlois, B.; Guérn, G. Mutations in the agouti (ASIP), the extension (MC1R), and the brown (TYRP1) loci and their association to coat color phenotypes in horses (Equus caballus). Mamm. Genome 2001, 12, 450–455. [Google Scholar] [CrossRef]
- Abitbol, M.; Legrand, R.; Tiret, L. A missense mutation in the agouti signaling protein gene (ASIP) is associated with the no light points coat phenotype in donkeys. Genet. Sel. Evol. 2015, 47, 28. [Google Scholar] [CrossRef]
- Sun, T.; Li, S.; Xia, X.; Ji, C.; Zhang, G.; Yu, J.; Jiang, G.; Dang, R.; Lei, C. ASIP gene variation in Chinese donkeys. Anim. Genet. 2017, 48, 372–373. [Google Scholar] [CrossRef]
- Utzeri, V.J.; Bertolini, F.; Ribani, A.; Schiavo, G.; Dall’Olio, S.; Fontanesi, L. The albinism of the feral Asinara white donkeys (Equus asinus) is determined by a missense mutation in a highly conserved position of the tyrosinase (TYR) gene deduced protein. Anim. Genet. 2016, 47, 120–124. [Google Scholar] [CrossRef]
- Reissmann, M.; Bierwolf, J.; Brockmann, G.A. Two SNPs in the SILV gene are associated with silver coat colour in ponies. Anim. Genet. 2007, 38, 1–6. [Google Scholar] [CrossRef]
- Hauswirth, R.; Haase, B.; Blatter, M.; Brooks, S.A.; Burger, D.; Drögemüller, C.; Gerber, V.; Henke, D.; Janda, J.; Jude, R.; et al. Mutations in MITF and PAX3 Cause “Splashed White” and Other White Spotting Phenotypes in Horses. PLoS Genet. 2012, 8, e1002653. [Google Scholar] [CrossRef]
- McFadden, A.; Vierra, M.; Martin, K.; Brooks, S.A.; Everts, R.E.; Lafayette, C. Spotting the Pattern: A Review on White Coat Color in the Domestic Horse. Animals 2024, 14, 451. [Google Scholar] [CrossRef]
- Pasternak, M.; Krupiński, J.; Gurgul, A.; Bugno-Poniewierska, M. Genetic, historical and breeding aspects of the occurrence of the tobiano pattern and white markings in the Polish population of Hucul horses—A review. J. Appl. Anim. Res. 2020, 48, 21–27. [Google Scholar] [CrossRef]
- Oyebanjo, M.O.; Obi, E.A.; Salako, A.E. Genes affecting coat colour and the resulting variation in horses (Equus caballus)—A Review. J. Anim. Sci. Vet. Med. 2022, 7, 127–149. [Google Scholar] [CrossRef]
- Grilz-Seger, G.; Reiter, S.; Neuditschko, M.; Wallner, B.; Rieder, S.; Leeb, T.; Jagannathan, V.; Mesarič, M.; Cotman, M.; Pausch, H.; et al. A Genome-Wide Association Analysis in Noriker Horses Identifies a SNP Associated with Roan Coat Color. J. Equine Vet. Sci. 2020, 88, 102950. [Google Scholar] [CrossRef]
- Haase, B.; Rieder, S.; Leeb, T. Two variants in the KIT gene as candidate causative mutations for a dominant white and a white spotting phenotype in the donkey. Anim. Genet. 2015, 46, 321–324. [Google Scholar] [CrossRef]
- Pielberg, G.R.; Golovko, A.; Sundström, E.; Curik, I.; Lennartsson, J.; Seltenhammer, M.H.; Druml, T.; Binns, M.; Fitzsimmons, C.; Lindgren, G.; et al. A cis-acting regulatory mutation causes premature hair graying and susceptibility to melanoma in the horse. Nat. Genet. 2008, 40, 1004–1009. [Google Scholar] [CrossRef]
- Bisbee, D.; Carpenter, M.L.; Hofes-Martin, K.; Brooks, S.A.; Lafayette, C. Identification of a novel missense variant in SLC45A2 associated with dilute snowdrop phenotype in Gypsy horses. Anim. Genet. 2020, 51, 342–343. [Google Scholar] [CrossRef]
- Holl, H.M.; Pflug, K.M.; Yates, K.M.; Hoefs-Martin, K.; Shepard, C.; Cook, D.G.; Lafayette, C.; Brooks, S.A. A candidate gene approach identifies variants in SLC45A2 that explain dilute phenotypes, pearl and sunshine, in compound heterozygote horses. Anim. Genet. 2019, 50, 271–274. [Google Scholar] [CrossRef]
- Cook, D.; Brooks, S.; Bellone, R.; Bailey, E. Missense Mutation in Exon 2 of SLC36A1 Responsible for Champagne Dilution in Horses. PLoS Genet. 2008, 4, e1000195. [Google Scholar] [CrossRef]
- Imsland, F.; McGowan, K.; Rubin, C.; Henegar, C.; Sundström, E.; Berglund, J.; Schwochow, D.; Gustafson, U.; Imsland, P.; Lindblad-Toh, K. Regulatory mutations in TBX3 disrupt asymmetric hair pigmentation that underlies Dun camouflage color in horses. Nat. Genet. 2016, 48, 152–158. [Google Scholar] [CrossRef]
- Brunberg, E.; Andersson, L.; Gothran, G.; Sandberg, K.; Mikko, S.; Lindgren, G. A missense mutation in PMEL17 is associated with the Silver coat color in horses. BMC Genet. 2006, 7, 46. [Google Scholar] [CrossRef]
- Lauvergne, J.J.; Silvestrelli, M.; Langlois, B.; Renieri, C.; Poirel, D.; Antaldi, G.G.V. A new scheme for describing horse coat colour. Livest. Prod. Sci. 1991, 27, 219–229. [Google Scholar] [CrossRef]
- Sponenberg, D.P.; Bellone, R. Equine Color Genetics, 4th ed.; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Thiruvenkadan, A.K.; Kandasamy, N.; Panneerselvam, S. Coat colour inheritance in horses. Livest. Sci. 2008, 117, 109–129. [Google Scholar] [CrossRef]
- Lin, X.; Zhu, L.; He, J. Morphogenesis, Growth Cycle and Molecular Regulation of Hair Follicles. Front. Cell Dev. Biol. 2022, 10, 899095. [Google Scholar] [CrossRef]
- Ji, S.; Zhu, Z.; Sun, X.; Fu, X. Functional hair follicle regeneration: An updated review. Signal Transduct. Target Ther. 2021, 6, 66. [Google Scholar] [CrossRef]
- Wu, W.; Yang, J.; Tao, H.; Lei, M. Environmental Regulation of Skin Pigmentation and Hair Regeneration. Stem Cells Dev. 2022, 31, 91–96. [Google Scholar] [CrossRef]
- Carrasco, E.; Soto-Heredero, G.; Mittelbrunn, M. The Role of Extracellular Vesicles in Cutaneous Remodeling and Hair Follicle Dynamics. Int. J. Mol. Sci. 2019, 20, 2758. [Google Scholar] [CrossRef]
- Arenas-Báez, P.; Torres-Hernández, G.; Castillo-Hernández, G.; Hernández-Rodríguez, M.; Sánchez-Gutiérrez, R.A.; Vargas-López, S.; González-Maldonado, J.; Domínguez-Martínez, P.A.; Granados-Rivera, L.A.; Maldonado-Jáquez, L.A. Coat Color in Local Goats: Influence on Environmental Adaptation and Productivity, and Use as a Selection Criterion. Biology 2023, 12, 929. [Google Scholar] [CrossRef]
- Hu, S.; Chen, Y.; Zhao, B.; Yang, N.; Chen, S.; Shen, J.; Bao, G.; Wu, X. KIT is involved in melanocyte proliferation, apoptosis and melanogenesis in the Rex Rabbit. PeerJ 2020, 8, e9402. [Google Scholar] [CrossRef]
- Rachmin, I.; Lee, J.H.; Zhang, B.; Sefton, J.; Jung, I.; Lee, Y.I.; Hsu, Y.; Fisher, D.E. Stress-associated ectopic differentiation of melanocyte stem cells and ORS amelanotic melanocytes in an ex vivo human hair follicle model. Exp. Dermatol. 2021, 30, 578–587. [Google Scholar] [CrossRef]
- Zhang, B.; Ma, S.; Rachmin, I.; He, M.; Baral, P.; Choi, S.; Goncalves, W.A.; Shwartz, Y.; Fast, E.M.; Su, Y.; et al. Hyperactivation of sympathetic nerves drives depletion of melanocyte stem cells. Nature 2020, 577, 676–681. [Google Scholar] [CrossRef]
- Qiu, W.; Chuong, C.; Lei, M. Regulation of melanocyte stem cells in the pigmentation of skin and its appendages: Biological patterning and therapeutic potentials. Exp. Dermatol. 2019, 28, 395–405. [Google Scholar] [CrossRef]
- Lee, S.; An, L.; Soloway, P.D.; White, A.C. Dynamic regulation of chromatin accessibility during melanocyte stem cell activation. Pigment Cell Melanoma Res. 2023, 36, 531–541. [Google Scholar] [CrossRef]
- Henkel, J.; Saif, R.; Jagannathan, V.; Schmocker, C.; Zeindler, F.; Bangerter, E.; Herren, U.; Posantzis, D.; Bulut, Z.; Ammann, P.; et al. Selection signatures in goats reveal copy number variants underlying breed-defining coat color phenotypes. PLoS Genet. 2019, 15, e1008536. [Google Scholar] [CrossRef]
- Van Deventer, R.; Rhode, C.; Marx, M.; Roodt-Wilding, R. Elucidation of coat colour genetics in blue wildebeest. Mamm. Biol. 2021, 101, 439–449. [Google Scholar] [CrossRef]
- Grabolus, D.; Wacławik, P.; Zatoń-Dobrowolska, M. Differences in melanin type and content among color variations in American mink (Neovison vison). Can. J. Anim. Sci. 2020, 100, 418–425. [Google Scholar] [CrossRef]
- Tsatmali, M.; Ancans, J.; Thody, A.J. Melanocyte Function and Its Control by Melanocortin Peptides. J. Histochem. Cytochem. 2002, 50, 125–133. [Google Scholar] [CrossRef]
- Sugumaran, M. Reactivities of Quinone Methides versus o-Quinones in Catecholamine Metabolism and Eumelanin Biosynthesis. Int. J. Mol. Sci. 2016, 17, 1576. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K. Chemistry of Mixed Melanogenesis—Pivotal Roles of Dopaquinone. Photochem. Photobiol. 2008, 84, 582–592. [Google Scholar] [CrossRef]
- Ito, S. A Chemist’s View of Melanogenesis. Pigment Cell Res. 2003, 16, 230–236. [Google Scholar] [CrossRef]
- Solano, F. On the Metal Cofactor in the Tyrosinase Family. Int. J. Mol. Sci. 2018, 19, 633. [Google Scholar] [CrossRef]
- Esposito, R.; D’Aniello, S.; Squarzoni, P.; Pezzotti, M.R.; Ristoratore, F.; Spagnuolo, A. New Insights into the Evolution of Metazoan Tyrosinase Gene Family. PLoS ONE 2012, 7, e35731. [Google Scholar] [CrossRef]
- Cosso, G.; Carcangiu, V.; Luridiana, S.; Fiori, S.; Columbano, N.; Masala, G.; Careddu, G.M.; Passino, E.S.; Mura, M.C. Characterization of the Sarcidano Horse Coat Color Genes. Animals 2022, 12, 2677. [Google Scholar] [CrossRef]
- Shang, S.; Yu, Y.; Zhao, Y.; Dang, W.; Zhang, J.; Qin, X.; Irwin, D.M.; Wang, Q.; Liu, F.; Wang, Z.; et al. Synergy between MC1R and ASIP for coat color in horses (Equus caballus). J. Anim. Sci. 2019, 97, 1578–1585. [Google Scholar] [CrossRef]
- Reissmann, M.; Musa, L.; Zakizadeh, S.; Ludwig, A. Distribution of coat-color-associated alleles in the domestic horse population and Przewalski’s horse. J. Appl. Genet. 2016, 57, 519–525. [Google Scholar] [CrossRef]
- Mura, M.C.; Carcangiu, V.; Cosso, G.; Columbano, N.; Passino, E.S.; Luridiana, S. Discrepancies between Genetic and Visual Coat Color Assignment in Sarcidano Horse. Animals 2024, 14, 543. [Google Scholar] [CrossRef]
- Ji, R.; Tao, Y. Melanocortin-1 receptor mutations and pigmentation: Insights from large animals. Prog. Mol. Biol. Transl. Sci. 2022, 189, 179–213. [Google Scholar]
- Hida, T.; Wakamatsu, K.; Sviderskaya, E.V.; Donkin, A.J.; Mobtoliu, L.; Lamoreux, M.L.; Yu, B.; Millhauser, G.L.; Ito, S.; Barsh, G.S.; et al. Agouti protein, mahogunin, and attractin in pheomelanogenesis and melanoblast-like alteration of melanocytes: A cAMP-independent pathway. Pigment Cell Melanoma Res. 2009, 22, 623–634. [Google Scholar] [CrossRef]
- García-Borrón, J.C.; Sánchez-Laorden, B.L.; Jiménez-Cervantes, C. Melanocortin-1 receptor structure and functional regulation. Pigment Cell Res. 2005, 18, 393–410. [Google Scholar] [CrossRef]
- Wagner, H.; Reissmann, M. New polymorphism detected in the horse MC1R gene. Anim. Genet. 2000, 31, 289–290. [Google Scholar] [CrossRef]
- Corbin, L.J.; Pope, J.; Sanson, J.; Antczak, D.F.; Miller, D.; Sadeghi, R.; Brooks, S.A. An Independent Locus Upstream of ASIP Controls Variation in the Shade of the Bay Coat Colour in Horses. Genes 2020, 11, 606. [Google Scholar] [CrossRef]
- Brooks, S.A.; Bailey, E. Exon skipping in the KIT gene causes a Sabino spotting pattern in horses. Mamm. Genome 2005, 16, 893–902. [Google Scholar]
- Mau, C.; Poncet, P.A.; Bucher, B.; Stranzinger, G.; Rider, S. Genetic mapping of dominant white (W), a homozygous lethal condition in the horse (Equus caballus). J. Anim. Breed. Genet. 2004, 121, 374–383. [Google Scholar] [CrossRef]
- McFadden, A.; Vierra, M.; Robilliard, H.; Martin, K.; Brooks, S.A.; Everts, R.E.; Lafayette, C. Population Analysis Identifies 15 Multi-Variant Dominant White Haplotypes in Horses. Animals 2024, 14, 517. [Google Scholar] [CrossRef]
- Patterson Rosa, L.; Martin, K.; Vierra, M.; Lundquist, E.; Foster, G.; Brooks, S.A.; Lafayette, C. A KIT Variant Associated with Increased White Spotting Epistatic to MC1R Genotype in Horses (Equus caballus). Animals 2022, 12, 1958. [Google Scholar] [CrossRef]
- McFadden, A.; Martin, K.; Foster, G.; Vierra, M.; Lundquist, E.W.; Everts, R.E.; Martin, E.; Mcloone, K.; Brooks, S.A.; Lafayette, C. 5′UTR Variant in KIT Associated with White Spotting in Horses. J. Equine Vet. Sci. 2023, 127, 104563. [Google Scholar] [CrossRef]
- Hug, P.; Jude, R.; Henkel, J.; Jagannathan, V.; Leeb, T. A novel KIT deletion variant in a German Riding Pony with white-spotting coat colour phenotype. Anim. Genet. 2019, 50, 761–763. [Google Scholar] [CrossRef]
- Patterson Rosa, L.; Martin, K.; Vierra, M.; Foster, G.; Lundquist, E.; Brooks, S.A.; Lafayette, C. Two Variants of KIT Causing White Patterning in Stock-Type Horses. J. Hered. 2021, 112, 447–451. [Google Scholar] [CrossRef]
- Brooks, S.A.; Terry, R.B.; Bailey, E. A PCR-RFLP for KIT associated with tobiano spotting pattern in horses. Anim. Genet. 2002, 33, 301–303. [Google Scholar] [CrossRef]
- Brooks, S.A.; Lear, T.L.; Adelson, D.L.; Bailey, E. A chromosome inversion near the KIT gene and the Tobiano spotting pattern in horses. Cytogenet. Genome Res. 2008, 119, 225–230. [Google Scholar] [CrossRef]
- Voß, K.; Tetens, J.; Thaller, G.; Becker, D. Coat Color Roan Shows Association with KIT Variants and No Evidence of Lethality in Icelandic Horses. Genes 2020, 11, 680. [Google Scholar] [CrossRef]
- Mariat, D.; Taourit, S.; Guérin, G. A mutation in the MATP gene causes the cream coat colour in the horse. Genet. Sel. Evol. 2003, 35, 119–133. [Google Scholar] [CrossRef][Green Version]
- Sevane, N.; Sanz, C.R.; Dunner, S. Explicit evidence for a missense mutation in exon 4 of SLC45A2 gene causing the pearl coat dilution in horses. Anim. Genet. 2019, 50, 275–278. [Google Scholar] [CrossRef]
- Cieslak, J.; Brooks, S.A.; Wodas, L.; Mantaj, W.; Borowska, A.; Sliwowska, J.H.; Ziarniak, K.; Mackowski, M. Genetic Background of the Polish Primitive Horse (Konik) Coat Color Variation—New Insight into Dun Dilution Phenotypic Effect. J. Hered. 2021, 112, 436–442. [Google Scholar] [CrossRef]
- Rubin, C.; Hodge, M.; Naboulsi, R.; Beckman, M.; Bellone, R.R.; Kallenberg, A.; J’Usrey, R.; Ohmura, H.; Seki, K.; Ohnuma, A.; et al. An intronic copy number variation in Syntaxin 17 determines speed of greying and melanoma incidence in Grey horses. bioRxiv 2023, 2011–2023. [Google Scholar]
- Nowacka-Woszuk, J.; Mackowski, M.; Stefaniuk-Szmukier, M.; Cieslak, J. The equine graying with age mutation of the STX17 gene: A copy number study using droplet digital PCR reveals a new pattern. Anim. Genet. 2021, 52, 223–227. [Google Scholar] [CrossRef]
- Flesher, J.L.; Paterson-Coleman, E.K.; Vasudeva, P.; Ruiz-Vega, R.; Marshall, M.; Pearlman, E.; MacGregor, G.R.; Neumann, J.; Ganesan, A.K. Delineating the role of MITF isoforms in pigmentation and tissue homeostasis. Pigment Cell Melanoma Res. 2020, 33, 279–292. [Google Scholar] [CrossRef]
- Henkel, J.; Lafayette, C.; Brooks, S.A.; Martin, K.; Patterson-Rosa, L.; Cook, D.; Jagannathan, V.; Leeb, T. Whole-genome sequencing reveals a large deletion in the MITF gene in horses with white spotted coat colour and increased risk of deafness. Anim. Genet. 2019, 50, 172–174. [Google Scholar] [CrossRef]
- Magdesian, K.G.; Tanaka, J.; Bellone, R.R. A De Novo MITF Deletion Explains a Novel Splashed White Phenotype in an American Paint Horse. J. Hered. 2020, 111, 287–293. [Google Scholar] [CrossRef]
- Patterson Rosa, L.; Martin, K.; Vierra, M.; Foster, G.; Brooks, S.A.; Lafayette, C. Non-frameshift deletion on MITF is associated with a novel splashed white spotting pattern in horses (Equus caballus). Anim. Genet. 2022, 53, 538–540. [Google Scholar] [CrossRef]
- Bellone, R.R.; Tanaka, J.; Esdaile, E.; Sutton, R.B.; Payette, F.; Leduc, L.; Till, B.J.; Abdel-Ghaffar, A.K.; Hammond, M.; Magdesian, K.G. A de novo 2.3 kb structural variant in MITF explains a novel splashed white phenotype in a Thoroughbred family. Anim. Genet. 2023, 54, 752–762. [Google Scholar] [CrossRef]
- McFadden, A.; Martin, K.; Foster, G.; Vierra, M.; Lundquist, E.W.; Everts, R.E.; Martin, E.; McLoone, K.; Brooks, S.A.; Lafayette, C. Two Novel Variants in MITF and PAX3 Associated with Splashed White Phenotypes in Horses. J. Equine Vet. Sci. 2023, 128, 104875. [Google Scholar] [CrossRef]
- Kim, N.; Chae, H.; Baek, K.; Cho, I.; Jung, Y.; Woo, J.; Park, S.; Kim, J.; Lee, S.; Yang, Y. A Study on the Changes of Coat Color-Related Genes according to Generational Changes in Jeju Horses. JET 2015, 30, 183–188. [Google Scholar] [CrossRef]
- Wang, D.; Ru, W.; Xu, Y.; Zhang, J.; He, X.; Fan, G.; Mao, B.; Zhuou, X.; Qin, Y. Chemical constituents and bioactivities of Colla corii asini. Drug Discov. Ther. 2014, 8, 201–207. [Google Scholar] [CrossRef]
- Liu, S.; Su, J.; Yang, Q.; Sun, M.; Wang, Z.; Yu, J.; Jafari, H.; Lei, C.; Sun, Y.; Dang, R. Genome-wide analyses based on a novel donkey 40K liquid chip reveal the gene responsible for coat color diversity in Chinese Dezhou donkey. Anim. Genet. 2024, 55, 140–146. [Google Scholar] [CrossRef]
- Sánchez-Guerrero, M.J.; Solé, M.; Azor, P.J.; Sölkner, J.; Valera, M. Genetic and environmental risk factors for vitiligo and melanoma in Pura Raza Español horses. Equine Vet. J. 2019, 51, 606–611. [Google Scholar] [CrossRef]
- Stiegler, J.; Brickley, S. Vitiligo: A Comprehensive Overview. J. Dermatol. Nurses Assoc. 2021, 13, 18–27. [Google Scholar]
- Druml, T.; Brem, G.; Horna, M.; Ricard, A.; Grilz-Seger, G. DPF3, A Putative Candidate Gene for Melanoma Etiopathogenesis in Gray Horses. J. Equine Vet. Sci. 2022, 108, 103797. [Google Scholar] [CrossRef]
- Pulos, W.L.; Hutt, F.B. Lethal dominant white in horses. Heredity 1969, 60, 59–63. [Google Scholar] [CrossRef]
- Esdaile, E.; Kallenberg, A.; Avila, F.; Bellone, R.R. Identification of W13 in the American Miniature Horse and Shetland Pony Populations. Genes 2021, 12, 1985. [Google Scholar] [CrossRef]
- Magdesian, K.G.; Williams, D.C.; Aleman, M.; LeCouteur, R.A.; Madigan, J.E. Evaluation of deafness in American Paint Horses by phenotype, brainstem auditory-evoked responses, and endothelin receptor B genotype. J. Am. Vet. Med. Assoc. 2009, 235, 1204–1211. [Google Scholar] [CrossRef]
- Sandmeyer, L.S.; Kingsley, N.B.; Walder, C.; Archer, S.; Leis, M.L.; Bellone, R.R.; Bauer, B.S. Risk factors for equine recurrent uveitis in a population of Appaloosa horses in western Canada. Vet. Ophthalmol. 2020, 23, 515–525. [Google Scholar] [CrossRef]
- Rockwell, H.; Mack, M.; Famula, T.; Sandmeyer, L.; Bauer, B.; Dwyer, A.; Lassaline, M.; Besson, S.; Archer, S.; McCue, M.; et al. Genetic investigation of equine recurrent uveitis in Appaloosa horses. Anim. Genet. 2020, 51, 111–116. [Google Scholar] [CrossRef]
- Gilger, B.C. Equine recurrent uveitis: The viewpoint from the USA. Equine Vet. J. 2010, 42, 57–61. [Google Scholar] [CrossRef]
- Cappai, M.G.; Picciau, M.; Nieddu, G.; Sogos, I.; Cherchi, R.; Pinna, W. Cutaneous metabolic pathway of tyrosine as a precursor to melanin in Asinara’s white donkey, Equus asinus L., 1758. Ital. J. Anim. Sci. 2015, 14, 502–507. [Google Scholar] [CrossRef]

| Gene | Species | Coat Color | References |
|---|---|---|---|
| MC1R | Horses | Chestnut | [28] |
| Donkeys | Red | [29] | |
| ASIP | Horses | Black | [30] |
| Donkeys | Black | [31,32] | |
| TYR | Donkeys | White | [33] |
| SILV | Horses | Sliver dapple | [34] |
| MITF | Horses | Splashed white | [35] |
| KIT | Horses | Dominant white | [36] |
| Horses | Tobiano | [37] | |
| Horses | Sabino | [38] | |
| Horses | Roan | [39] | |
| Donkeys | White | [40] | |
| STX17 | Horses | Gray | [41] |
| SLC45A2 | Horses | Snowdrop | [42] |
| Horses | Pearl/cream | [43] | |
| SLC36A1 | Horses | Champagne | [44] |
| TBX3 | Donkeys | Dun | [6] |
| Horses | Dun | [45] | |
| TRPM1 | Horses | Leopard complex spotting | [36] |
| PMEL17 | Horses | Sliver dapple | [46] |
| Coat Color | Extension Genotype | Agouti Genotype | Genotype |
|---|---|---|---|
| Black | E- | aa | EEaa/Eeaa |
| Bay | E- | A- | EEAA/EeAA/EEAa/EeAa |
| Chestnut | ee | A-/aa | eeAA/eeAa/eeaa |
| Trait | Allele Symbol | Gene | Phenotype | References |
|---|---|---|---|---|
| Dominant White | W1–W35 | White patterning or an entirely white coat with pink skin underneath | [36] | |
| Tobiano | TO | KIT | Large markings on legs and small head markings | [37] |
| Sabino | SB1 | A white spotting pattern with towering uneven stockings and a blaze on the face | [38] | |
| Roan | RN | Dispersed white hair and dark points | [39] |
| Gene | Allele | Mutation | Phenotype | Reference |
|---|---|---|---|---|
| MITF | SW1 | 10 bp insertion | Splashed white | [35] |
| MITF | SW3 | Small deletion | Splashed white | [35] |
| MITF | SW5 | 63 kb deletion | White spotting, blue eyes | [94] |
| MITF | SW6 | 8.7 kb deletion | Splashed white, blue eyes | [95] |
| MITF | SW7 | A novel three-base pair deletion | Splashed white | [96] |
| MITF | SW8 | 2.3 kb deletion | Splashed white, blue eyes | [97] |
| MITF | SW9 | Missense mutation | Splashed white, blue eyes | [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Peng, Y.; Zhang, X.; Wang, X.; Chen, W.; Kou, X.; Liang, H.; Ren, W.; Khan, M.Z.; Wang, C. Coloration in Equine: Overview of Candidate Genes Associated with Coat Color Phenotypes. Animals 2024, 14, 1802. https://doi.org/10.3390/ani14121802
Liu X, Peng Y, Zhang X, Wang X, Chen W, Kou X, Liang H, Ren W, Khan MZ, Wang C. Coloration in Equine: Overview of Candidate Genes Associated with Coat Color Phenotypes. Animals. 2024; 14(12):1802. https://doi.org/10.3390/ani14121802
Chicago/Turabian StyleLiu, Xiaotong, Yongdong Peng, Xinhao Zhang, Xinrui Wang, Wenting Chen, Xiyan Kou, Huili Liang, Wei Ren, Muhammad Zahoor Khan, and Changfa Wang. 2024. "Coloration in Equine: Overview of Candidate Genes Associated with Coat Color Phenotypes" Animals 14, no. 12: 1802. https://doi.org/10.3390/ani14121802
APA StyleLiu, X., Peng, Y., Zhang, X., Wang, X., Chen, W., Kou, X., Liang, H., Ren, W., Khan, M. Z., & Wang, C. (2024). Coloration in Equine: Overview of Candidate Genes Associated with Coat Color Phenotypes. Animals, 14(12), 1802. https://doi.org/10.3390/ani14121802







