The Omentum—A Forgotten Structure in Veterinary Surgery in Small Animals’ Surgery
Abstract
:Simple Summary
Abstract
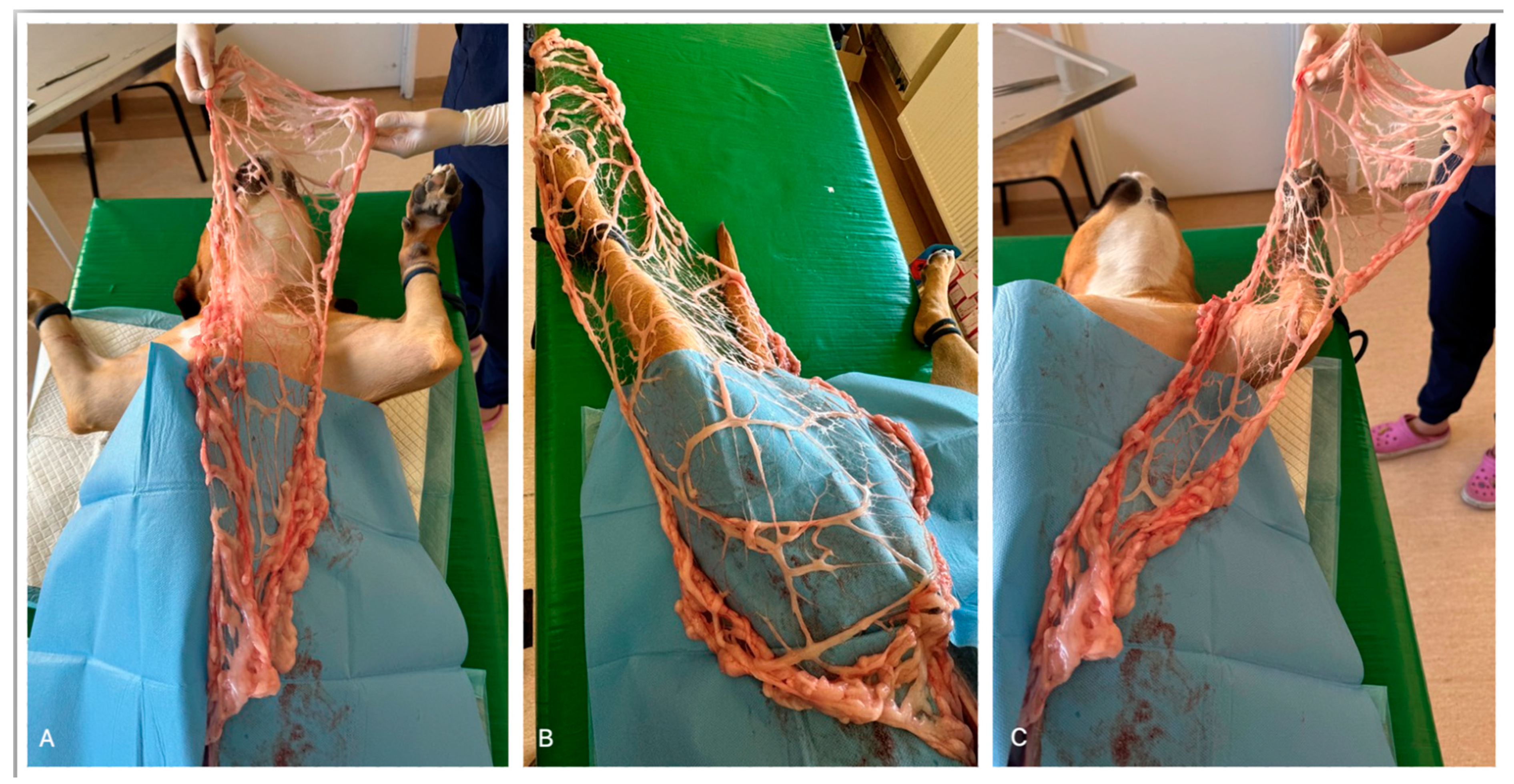
1. Introduction
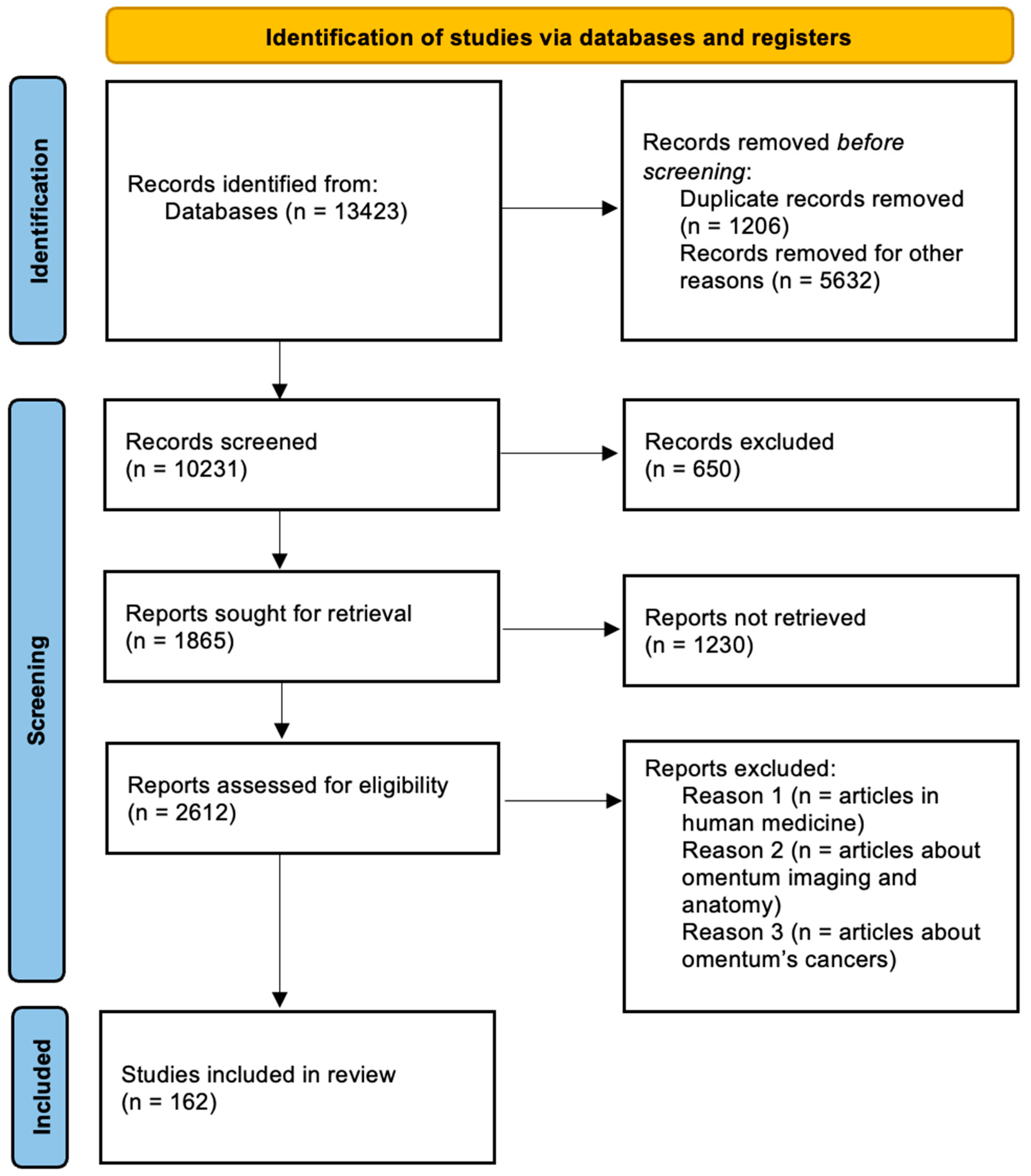
2. Materials and Methods
3. Omentum in the Treatment of Pancreatic Diseases
4. Omentum in Abscess Healing
5. Omentum in the Treatment of Cysts
6. Omentum in the Treatment of Prostatic Diseases
7. Omentum in Bone Healing
8. Omentum in Wound Healing
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barone, R. Appareil Digestif et Appareil Respiratoire. Anatomie Comparee des Mammiferes Domestiques. Tome 3, Splanchnologie 1; Editions Vigot: Paris, France, 2009; Volume 951. [Google Scholar]
- Doom, M.; de Rooster, H.; van Bergen, T.; Gielen, I.; Kromhout, K.; Simoens, P.; Cornillie, P. Morphology of the Canine Omentum Part 1: Arterial Landmarks that Define the Omentum. Anat. Histol. Embryol. 2016, 45, 37–43. [Google Scholar] [CrossRef] [PubMed]
- McGeady, T.A.; Quinn, P.J.; FitzPatrick, E.S.; Ryan, M.T. Coelomic Cavities. In Veterinary Embryology; Blackwell Publishing: Oxford, UK, 2006; pp. 59–65. [Google Scholar]
- Doom, M.; de Rooster, H.; van Bergen, T.; Gielen, I.; Kromhout, K.; Simoens, P.; Cornillie, P. Morphology of the Canine Omentum Part 2: The Omental Bursa and its Compartments Materialized and Explored by a Novel Technique. Anat. Histol. Embryol. 2016, 45, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Silva-Sanchez, A.; Randall, T.D.; Meza-Perez, S. Specialized immune responses in the peritoneal cavity and omentum. J. Leukoc. Biol. 2021, 109, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Budras, K.D. Abdominal Cavity. In Anatomy of the Dog, 5th ed.; Budras, K.-D., Ed.; Sclutersche: Hannover, Germany, 2007; pp. 50–61. [Google Scholar]
- Zietzschmann, O. Das Mesogastrium dorsale des Hundes mit einer schematischen Darstellung seiner Blatter. Morphol. Jahrb. 1939, 83, 327–358. [Google Scholar]
- Evans, H.; De Lahunta, A. The digestive apparatus and abdomen. In Miller’s Anatomy of the Dog; Evans, H.E., Ed.; Saunders/Elsevier: St. Louis, MI, USA, 2013; pp. 281–338. [Google Scholar]
- Hosgood, G. The omentum—The forgotten organ—Physiology and potential surgical applications in dogs and cats. Compend. Contin. Educ. Vet. 1990, 12, 45–51. [Google Scholar]
- Michailova, K.N.; Usunoff, K.G. The milky spots of the peritoneum and pleura: Structure, development and pathology. Biomed. Rev. 2004, 15, 47–66. [Google Scholar] [CrossRef]
- Huyghe, S.; de Rooster, H.; Doom, M.; Van den Broeck, W. The Microscopic Structure of the Omentum in Healthy Dogs: The Mystery Unravelle. Anat. Histol. Embryol. 2016, 45, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Ryan, G.B.; Grobety, J.; Majno, G. Postoperative peritoneal adhesions. A study of the mechanisms. Am. J. Pathol. 1971, 65, 117–148. [Google Scholar]
- Shimotsuma, M.J.; Shields, J.; Simpson-Morgan, M.W.; Sakuyama, A.; Shirasu, M.; Hagiwara, A.; Takahashi, T. Morpho-physiological function and role of omental milky spots as omentum-associated lymphoid tissue (OALT) in the peritoneal cavity. Lymphology 1993, 26, 90–101. [Google Scholar]
- Wilkosz, S.G.; Ireland, N.; Khwaja, N.; Walker, M.; Butt, R.; de Giorgio-Miller, A.; Herrick, S.E. A comparative study of the structure of human and murine greater omentum. Anat. Embryol. 2005, 209, 251–261. [Google Scholar] [CrossRef]
- Krist, L.F.; Eestermans, I.L.; Steenbergen, J.J.; Hoefsmit, E.C.; Cuesta, M.A.; Meyer, S.; Beelen, R.H. Cellular composition of milky spots in the human greater omentum: An immunochemical and ultrastructural study. Anat. Rec. 1995, 241, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Shimotsuma, M.; Kawata, M.; Hagiwara, A.; Takahashi, T. Milky spots in the human greater omentum. Macroscopic and histological identification. Acta Anat. 1989, 136, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Baptista, C.; Ferreira, R.; Fernandes, M.H.; Gomes, P.S.; Colaco, B. Influence of the Anatomical Site on Adipose Tissue-Derived Stromal Cells’ Biological Profile and Osteogenic Potential in Companion Animals. Vet. Sci. 2023, 24, 673. [Google Scholar] [CrossRef] [PubMed]
- Merlo, B.; Iacono, E. Beyond Canine Adipose Tissue-Derived Mesenchymal Stem/Stromal Cells Transplantation: An Update on Their Secretome Characterization and Applications. Animals 2023, 13, 3571. [Google Scholar] [CrossRef] [PubMed]
- Bach, F.S.; Rebelatto, C.L.K.; Fracaro, L.; Senegaglia, A.C.; Fragoso, F.Y.I.; Daga, D.R.; Brofman, P.R.S.; Pimpão, C.T.; Engracia Filho, J.R.; Montiani-Ferreira, F.; et al. Comparison of the Efficacy of Surgical Decompression Alone and Combined with Canine Adipose Tissue-Derived Stem Cell Transplantation in Dogs with Acute Thoracolumbar Disk Disease and Spinal Cord Injury. Front. Vet. Sci. 2019, 6, 383. [Google Scholar] [CrossRef] [PubMed]
- Bahamondes, F.; Flores, E.; Cattaneo, G.; Bruna, F.; Conget, P. Omental adipose tissue is a more suitable source of canine Mesenchymal stem cells. BMC Vet. Res. 2017, 13, 166. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.-K.; Song, W.-J.; Ahn, J.-O.; Li, Q.; Lee, B.-Y.; Kweon, K.; Park, S.-C.; Youn, H.-Y. Immunomodulatory effects of soluble factors secreted by feline adipose tissue-derived mesenchymal stem cells. Vet. Immunol. Immunopathol. 2017, 191, 22–29. [Google Scholar] [CrossRef] [PubMed]
- De Cesaris, V.; Grolli, S.; Bresciani, C.; Conti, V.; Basini, G.; Parmigiani, E.; Bigliardi, E. Isolation, proliferation and characterization of endometrial canine stem cells. Reprod. Domest. Anim. 2017, 52, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Enciso, N.; Ostronoff, L.L.K.; Mejías, G.; León, L.G.; Fermín, M.L.; Merino, E.; Fragio, C.; Avedillo, L.; Tejero, C. Stem cell factor supports migration in canine mesenchymal stem cells. Vet. Res. Commun. 2018, 42, 29–38. [Google Scholar] [CrossRef]
- Krueger, E.; Magri, L.M.S.; Botelho, A.S.; Bach, F.S.; Rebellato, C.L.K.; Fracaro, L.; Fragoso, F.Y.I.; Villanova, J.A.; Brofman, P.R.S.; Popović-Maneski, L. Effects of low-intensity electrical stimulation and adipose derived stem cells transplantation on the time-domain analysis-based electromyographic signals in dogs with SCI. Neurosci. Lett. 2019, 696, 38–45. [Google Scholar] [CrossRef]
- Rashid, U.; Yousaf, A.; Yaqoob, M.; Saba, E.; Moaeen-ud-Din, M.; Waseem, S.; Becker, S.K.; Sponder, G.; Aschenbach, J.R.; Sandhu, M.A. Characterization and differentiation potential of mesenchymal stem cells isolated from multiple canine adipose tissue sources. BMC Vet. Res. 2021, 17, 388. [Google Scholar] [CrossRef] [PubMed]
- Requicha, J.F.; Viegas, C.A.; Albuquerque, C.M.; Azevedo, J.M.; Reis, R.L.; Gomes, M.E. Effect of Anatomical Origin and Cell Passage Number on the Stemness and Osteogenic Differentiation Potential of Canine Adipose-Derived Stem Cells. Stem Cell Rev. Rep. 2012, 8, 1211–1222. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Baptista, C.; Queirós, A.; Ferreira, R.; Fernandes, M.H.; Gomes, P.S.; Colaço, B. Retinoic acid induces the osteogenic differentiation of cat adipose tissue-derived stromal cells from distinct anatomical sites. J. Anat. 2023, 242, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Linkova, D.D.; Rubtsova, Y.P.; Egorikhina, M.N. Cryostorage of Mesenchymal Stem Cells and Biomedical Cell-Based Products. Cells 2022, 11, 2691. [Google Scholar] [CrossRef] [PubMed]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef] [PubMed]
- Mocchi, M.; Dotti, S.; Del Bue, M.; Villa, R.; Bari, E.; Perteghella, S.; Torre, M.L.; Grolli, S. Veterinary Regenerative Medicine for Musculoskeletal Disorders: Can Mesenchymal Stem/Stromal Cells and Their Secretome Be the New Frontier? Cells 2020, 9, 1453. [Google Scholar] [CrossRef] [PubMed]
- Yaneselli, K.M.; Kuhl, C.P.; Terraciano, P.B.; de Oliveira, F.S.; Pizzato, S.B.; Pazza, K.; Magrisso, A.B.; Torman, V.; Rial, A.; Moreno, M.; et al. Comparison of the characteristics of canine adipose tissue-derived mesenchymal stem cells extracted from different sites and at different passage numbers. J. Vet. Sci. 2018, 19, 13–20. [Google Scholar] [CrossRef]
- Buote, N.J. Laparoscopic adipose-derived stem cell harvest technique with bipolar sealing device: Outcome in 12 dogs. Vet. Med. Sci. 2022, 8, 1421–1428. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Yokozeki, T.; Yokoyama, A.; Tabata, Y. Basic fibroblast growth factor enhances proliferation and hepatocyte growth factor expression of feline mesenchymal stem cells. Regen. Ther. 2020, 15, 10–17. [Google Scholar] [CrossRef]
- Kono, S.; Kazama, T.; Kano, K.; Harada, K.; Uechi, M.; Matsumoto, T. Phenotypic and functional properties of feline dedifferentiated fat cells and adipose-derived stem cells. Vet. J. 2014, 199, 88–96. [Google Scholar] [CrossRef]
- Li, D.; Luo, H.; Ruan, H.; Chen, Z.; Chen, S.; Wang, B.; Xie, Y. Isolation and identification of exosomes from feline plasma, urine and adipose-derived mesenchymal stem cells. BMC Vet. Res. 2021, 17, 272. [Google Scholar] [CrossRef] [PubMed]
- Parys, M.; Nelson, N.; Koehl, K.; Miller, R.; Kaneene, J.B.; Kruger, J.M.; Yuzbasiyan-Gurkan, V. Safety of Intraperitoneal Injection of Adipose Tissue-Derived Autologous Mesenchymal Stem Cells in Cats. J. Vet. Intern. Med. 2016, 30, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Quimby, J.M.; Webb, T.L.; Habenicht, L.M.; Dow, S.W. Safety and efficacy of intravenous infusion of allogeneic cryopreserved mesenchymal stem cells for treatment of chronic kidney disease in cats: Results of three sequential pilot studies. Stem Cell Res. Ther. 2013, 4, 48. [Google Scholar] [CrossRef] [PubMed]
- Villatoro, A.J.; Claros, S.; Fernández, V.; Alcoholado, C.; Fariñas, F.; Moreno, A.; Becerra, J.; Andrades, J.A. Safety and efficacy of the mesenchymal stem cell in feline eosinophilic keratitis treatment. BMC Vet. Res. 2018, 14, 116. [Google Scholar] [CrossRef]
- Villatoro, A.J.; Martín-Astorga, M.D.C.; Alcoholado, C.; Cárdenas, C.; Fariñas, F.; Becerra, J.; Visser, R. Altered Proteomic Profile of Adipose Tissue-Derived Mesenchymal Stem Cell Exosomes from Cats with Severe Chronic Gingivostomatitis. Animals 2021, 11, 2466. [Google Scholar] [CrossRef]
- Etzerodt, A.; Moulin, M.; Doktor, T.K.; Delfini, M.; Mossadegh-Keller, N.; Bajenoff, M.; Sieweke, M.K.; Moestrup, S.K.; Auphan-Anezin, N.; Lawrence, T. Tissue-resident macrophages in omentum promote metastatic spread of ovarian cancer. J. Exp. Med. 2020, 217, e20191869. [Google Scholar] [CrossRef]
- Schreiber, S.; Gehrckens, A.; Raedler, A. Activation of the major omentum-associated lymphoid tissue in Crohn disease. Zentralbl. Chir. 1995, 120, 135–144. [Google Scholar]
- Jani, K.; Saxena, A.K.; Vaghasia, R. Omental plugging for large-sized duodenal peptic perforations: A prospective Gene detection in peritonitis by DD RT-PCR 173 randomized study of 100 patients. South. Med. J. 2006, 99, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Azouz, A.; Razzaque, M.S.; El-Hallak, M.; Taguchi, T. Immunoinflammatory responses and fibrogenesis. Med. Electron. Microsc. 2004, 37, 141–148. [Google Scholar] [CrossRef]
- Hultman, C.S.; Carlson, G.W.; Losken, A.; Jones, G.; Culbertson, J.; Mackay, G.; Bostwick, J.; Jurkiewicz, M.J. Utility of the omentum in the reconstruction of complex extraperitoneal wounds and defects. Ann. Surg. 2002, 235, 782–795. [Google Scholar] [CrossRef]
- Ito, K.C.; Ferrigno, C.R.A.; Alves, F.R. Maximum length of greater omentum pedicle flap through subcutaneous tunnel for long bones in dogs. Cienc. Rural. 2010, 40, 594–599. [Google Scholar] [CrossRef]
- Lascelles, B.D.; Davison, L.; Dunning, M.; Bray, J.P.; White, R.A. Use of omental pedicle grafts in the management of non-healing axillary wounds in 10 cats. J. Small Anim. Pract. 1998, 39, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Black, M.G.; Ling, G.V.; Nyland, T.G.; Baker, T. Prevalence of Prostatic Cysts in Adult, Large-Breed Dogs. J. Am. Anim. Hosp. Assoc. 1998, 34, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Del Magno, S.; Pisani, G.; Dondi, F.; Cinti, F.; Morello, E.; Martano, M.; Foglia, A.; Giacobino, D.; Buracco, P. Surgical treatment and outcome of sterile prostatic cysts in dogs. Vet. Surg. 2021, 50, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Jerram, R.M.; Warman, C.G.; Davies, E.S.S.; Robson, M.C.; Walker, A.M. Successful treatment of a pancreatic pseudocyst by omentalisation in a dog. N. Z. Vet. J. 2004, 52, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.D.; Mann, F.A. Treatment for pancreatic abscesses via omentalization with abdominal closure versus open peritoneal drainage in dogs: 15 Cases (1994–2004). J. Am. Vet. Med. Assoc. 2006, 228, 397–402. [Google Scholar] [CrossRef] [PubMed]
- McAlinden, A.; Glyde, M.; McAllister, H.; Kirby, B. Omentalisation as adjunctive treatment of an infected femoral nonunion fracture: A case report. Ir. Vet. J. 2009, 62, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Pavletic, M.M. Surgery of the skin and management of wounds. In The Cat: Diseases and Clinical Management, 2nd ed.; Sherding, R.G., Ed.; Churchill Livingstone: Edinburgh, UK, 1994; pp. 1969–1997. [Google Scholar]
- Steiner, E.; Steinbach, L.S.; Schnarkowski, P.; Tirman, P.F.; Genant, H.K. Ganglia and cysts around joints. Radiol. Clin. N. Am. 1996, 34, 395–425. [Google Scholar] [CrossRef] [PubMed]
- Thrall, D.E. The mediastinum. In Textbook of Veterinary Diagnostic Radiology, 4th ed.; Thrall, D.E., Ed.; WB Saunders: Philadelphia, PA, USA, 2002; pp. 376–389. [Google Scholar]
- Woodbridge, N.; Martinoli, S.; Cherubini, G.B.; Caine, A.; Nelissen, P.; White, R. Omentalisation in the treatment of sublumbar abscessation: Long-term outcome in 10 dogs. Vet. Rec. 2014, 175, 625. [Google Scholar] [CrossRef]
- Branter, E.M.; Viviano, K.R. Multiple recurrent pancreatic cysts with associated pancreatic inflammation and atrophy in a cat. J. Feline Med. Surg. 2010, 12, 822–827. [Google Scholar] [CrossRef]
- Brückner, M. Laparoscopic omentalization of a pancreatic cyst in a cat. J. Am. Vet. Med. Assoc. 2019, 255, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Coleman, M.G.; Robson, M.C.; Harvey, C. Pancreatic cyst in a cat. N. Z. Vet. J. 2005, 53, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Hines, B.L.; Salisbury, S.K.; Jakovljevic, S.; DeNicola, D.B. Pancreatic pseudocyst associated with chronic-active necrotizing pancreatitis in a cat. J. Am. Anim. Hosp. Assoc. 1996, 32, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kang, J.H.; Chang, D.; Na, K.J.; Yang, M.P. Pancreatic abscess in a cat with diabetes mellitus. J. Am. Anim. Hosp. Assoc. 2015, 51, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Marchevsky, A.M.; Yovich, J.C.; Wyatt, K.M. Pancreatic pseudocyst causing extrahepatic biliary obstruction in a dog. Aust. Vet. J. 2000, 78, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Sadler, R.A.; Fields, E.L.; Whittemore, J.C. Attempted ultrasound-guided ethanol ablation of a suspected pancreatic pseudocyst in a dog. Can. Vet. J. 2016, 57, 1169–1174. [Google Scholar] [PubMed]
- Smith, S.A.; Biller, D.S. Resolution of a Pancreatic Pseudocyst in a Dog Following Percutaneous Ultrasonographic-Guided Drainage. J. Am. Anim. Hosp. Assoc. 1998, 34, 515–522. [Google Scholar] [CrossRef] [PubMed]
- VanEnkevort, B.A.; O’Brien, R.T.; Young, K.M. Pancreatic pseudocysts in 4 dogs and 2 cats: Ultrasonographic and clinicopathologic findings. J. Vet. Intern. Med. 1999, 13, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.J.; Seshadri, R.; Raffe, M.R. Characteristics and outcomes in surgical management of severe acute pancreatitis: 37 dogs (2001–2007). J. Vet. Emerg. Crit. Care 2009, 19, 165–173. [Google Scholar] [CrossRef]
- Tobias, K.M.; Johnston, S.A. Peritoneum and retroperitoneum. In Veterinary Surgery Small Animal; Kirby, B.M., Cornell, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 1391–1485. [Google Scholar]
- Lamb, C.R.; White, R.N.; Mcevoy, F.J. Sinography in the investigation of draining tracts in small animals: Retrospective review of 25 cases. Vet. Surg. 1994, 23, 129–134. [Google Scholar] [CrossRef]
- Slatter, D. Peritoneum and peritoneal cavity. In The Textbook of Small Animal Surgery; Kirby, B.M., Ed.; Saunders: Philadelphia, PA, USA, 2003; pp. 405–446. [Google Scholar]
- Frendin, J.; Funkquist, B.; Hansson, K.; Lonnemark, M.; Carlsten, J. Diagnostic imaging of foreign body reactions in dogs with diffuse back pain. J. Small Anim. Pract. 1999, 40, 278–285. [Google Scholar] [CrossRef]
- Frendin, J. Pyogranulomatous pleuritis with empyema in hunting dogs. Zentralbl Vet. A 1997, 44, 167–178. [Google Scholar] [CrossRef]
- Lotti, U.; Niebauer, G.W. Tracheobronchial foreign bodies of plant origin in 153 hunting dogs. Comp. Cont. Educ. Pract. Vet. 1992, 14, 900–903. [Google Scholar]
- Schultz, R.M.; Zwingenberger, A. Radiographic, computed tomographic, and ultrasonographic findings with migrating intrathoracic grass awns in dogs and cats. Vet. Radiol. Ultrasound 2008, 49, 249–255. [Google Scholar] [CrossRef]
- Johnson, M.S.; Martin, M.W.S. Successful medical treatment of 15 dogs with pyothorax. J. Small Anim. Pract. 2007, 48, 12–16. [Google Scholar] [CrossRef]
- Piek, C.J.; Robben, J.H. Pyothorax in nine dogs. Vet. Q. 2000, 22, 107–111. [Google Scholar] [CrossRef]
- Rooney, M.B.; Monnet, E. Medical and surgical treatment of pyothorax in dogs: 26 cases (1991–2001). J. Am. Vet. Med. Assoc. 2002, 221, 86–92. [Google Scholar] [CrossRef]
- Scott, J.A.; Macintire, D.K. Canine pyothorax: Clinical presentation, diagnosis, and treatment. Comp. Cont. Educ. Pract. Vet. 2003, 25, 180–194. [Google Scholar]
- Knight, H.D.; Hietala, S.K.; Jang, S. Antibacterial treatment of abscesses. J. Am. Vet. Med. Assoc. 1980, 15, 1095–1098. [Google Scholar]
- Nylander, G.; Tjernberg, B. The lymphatics of the greater omentum: An experimental study in the dog. Lymphology 1969, 2, 3–7. [Google Scholar]
- Ross, W.E.; Pardo, A.D. Evaluation of an omental pedicle extension technique in the dog. Vet. Surg. 1993, 22, 37–43. [Google Scholar] [CrossRef]
- Brown, J.R. Human actinomycosis: A study of 181 subjects. Hum. Pathol. 1973, 4, 319–330. [Google Scholar] [CrossRef]
- Smego, R.A.; Foglia, G. Actinomycosis. Clin. Infect. Dis. 1998, 26, 1255–1261. [Google Scholar] [CrossRef]
- Franklin, A.D.; Fearnside, S.M.; Brain, P.H. Omentalisation of a caudal mediastinal abscess in a dog. Aust. Vet. J. 2011, 89, 217–220. [Google Scholar] [CrossRef]
- Best, E.J.; Bush, D.J.; Dye, C. Suspected choledochal cyst in a domestic shorthair cat. J. Feline Med. Surg. 2010, 12, 814–817. [Google Scholar] [CrossRef]
- Domanjko-Petric, A.; Cernec, D.; Cotman, M. Polycystic kidney disease: A review and occurrence in Slovenia with comparison between ultrasound and genetic testing. J. Feline Med. Surg. 2008, 10, 115.e9. [Google Scholar] [CrossRef]
- Adler, R.; Wilson, D.W. Biliary cystadenoma of cats: Review. Vet. Pathol. 1995, 32, 415–418. [Google Scholar] [CrossRef]
- Van den Ingh, T.S.G.A.M.; Cullen, J.M.; Twedt, D.C.; Winkle, T.V.; Desmet, V.J.; Rothuizen, J. Morphological classification of biliary disorders of the canine and feline liver. In WSAVA Standards for Clinical and Histological Diagnosis of Canine and Feline Liver Diseases; Saunders: Oxford, UK, 2006; pp. 61–76. [Google Scholar]
- Besner, E.; Paddock, H.N.; Nguyen, L.T.; Kay, S.M. Choledochal Cyst: A Surgical Perspective. e-Med. Rev. 2008, 53, 51–56. [Google Scholar]
- Kligman, K.C.; Kim, S.E.; Winter, M.D.; Bacon, N.J.; Krellner, H.L.; Levy, J.K. What is your diagnosis? Synovial cysts. J. Am. Vet. Med. Assoc. 2009, 235, 945–946. [Google Scholar] [CrossRef]
- Prymak, C.; Goldschmidt, M.H. Synovial cysts in five dogs and one cat. J. Am. Anim. Hosp. Assoc. 1991, 27, 151–154. [Google Scholar]
- Stead, A.C.; Else, R.W.; Stead, M.C. Synovial cysts in cats. J. Small Anim. Pract. 1995, 36, 450–454. [Google Scholar] [CrossRef]
- White, J.D.; Martin, P.; Hudson, D.; Clark, A.; Malik, R. What is your diagnosis? J. Feline Med. Surg. 2004, 6, 339–344. [Google Scholar] [CrossRef]
- Hittmair, K.M.; Maedl, I.; Reifinger, M.; Mayrhofer, E. Synovial cyst of the fifth digit in a cat. J. Feline Med. Surg. 2010, 12, 175–178. [Google Scholar] [CrossRef]
- Bray, J.P.; White, R.A.S.; Williams, J.M. Partial resection and omentalization: A new technique for management of prostatic retention cysts in dogs. Vet. Surg. 1997, 26, 202–209. [Google Scholar] [CrossRef]
- White, R.A.; Williams, J.M. Intracapsular prostatic omentalization: A new technique for management of prostatic abscesses in dogs. Vet. Surg. 1995, 24, 390–395. [Google Scholar] [CrossRef]
- Stegan, L.; Van Goethem, B.; Beerden, C.; Grussendorf, C.; de Rooster, H. Use of greater omentum in the surgical treatment of a synovial cyst in a cat. Tierarztl. Prax. Ausg. K Kleintiere Heimtiere 2012, 43, 115–119. [Google Scholar]
- Arnold, E.P. Pararenal pseudocyst. Br. J. Urol. 1972, 44, 40–46. [Google Scholar] [CrossRef]
- Abdinoor, D.J. Perinephric pseudocysts in a cat. J. Am. Anim. Hosp. Assoc. 1980, 16, 763–767. [Google Scholar]
- Carlson, R.A.; Badertscher, R.N. Feline renal pseudocyst with metastatic carcinoma of the contralateral kidney. Feline Pract. 1993, 21, 23–27. [Google Scholar]
- Chastain, C.B.; Grier, R.L. Bilateral retroperitoneal cysts in a cat. Feline Pract. 1975, 5, 51–53. [Google Scholar]
- Geel, J.K. Perinephric extravasation of urine with pseudocyst formation in a cat. J. S. Afr. Vet. Assoc. 1986, 57, 33–34. [Google Scholar]
- Inns, J.H. Treatment of perinephric pseudocysts by ornental drainage. Aust. Vet. Pract. 1997, 27, 174–176. [Google Scholar]
- Kirberger, R.M.; Jacobsen, L.S. Perinephric pseudocysts in a cat. Aust. Vet. Pract. 1992, 22, 160–163. [Google Scholar]
- Kraft, A.M.; Kraft, C.G. Renal capsular cyst in a domestic cat. Vet. Med. Small Anim. Clin. 1970, 65, 692. [Google Scholar]
- Lemire, T.D.; Reao, W.K. Macroscopic and microscopic characterization of a urineferous perirenal pseudocyst in a domestic short hair cat. Vet. Pathol. 1998, 35, 68–70. [Google Scholar] [CrossRef]
- Miles, K.G.; Jergens, A.E. Unilateral perinephric pseudocyst of undetermined origin in a dog. Vet. Radiol. Ultrasound 1992, 33, 277–281. [Google Scholar] [CrossRef]
- Mitten, R.A. Pararenal pseudocysts in a cat. Iowa State Univ. Vet. 1978, 40, 65–67. [Google Scholar]
- Moon, M.L.; Dallman, M.A. Calcium oxalate ureterolith in a cat. Vet. Radiol. 1991, 32, 261. [Google Scholar] [CrossRef]
- Rishniw, M.; Weioman, J.; Hornof, W. Hydrothorax secondary to a perinephric pseudocyst in a cat. Vet. Radiol. Ultrasound 1998, 39, 193–196. [Google Scholar] [CrossRef]
- Robotham, G.R. What is your diagnosis? J. Am. Vet. Med. 1999, 182, 44–45. [Google Scholar]
- Ticer, J.W. Capsulogenic renal cyst in a cat. J. Am. Vet. Med. 1963, 143, 613–614. [Google Scholar]
- Tidwell, A.S.; Ullman, S.L.; Schelling, S.H. Urinorna (para-ureteral pseudocyst) in a dog. Vet. Radiol. 1990, 31, 203206. [Google Scholar] [CrossRef]
- Ochoa, V.B.; Dibartow, S.P.; Chew, D.J.; Westropp, J.; Carothers, M.; Biller, D. Perinephric pseudocysts in the cat: A retrospective study and review of the literature. J. Vet. Intern. Med. 1999, 13, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Hill, T.P.; Odesnik, B.J. Omentalisation of perinephric pseudocysts in a cat. J. Small Anim. Pract. 2000, 41, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Borisov, A.V. Lymphatic capillaries and blood vessels of milky spots in the human greater omentum. Fed. Proc. Transl. Suppl. 1964, 23, 150–154. [Google Scholar]
- González Domínguez, M.; Hernandez, C.; Maldonado-Estrada, J. Protective compromise of great omentum in an asymptomatic uterine rupture in a bitch: A case report. Rev. Colomb. Cienc. Pecuarias 2010, 23, 369. [Google Scholar]
- Platell, C.; Cooper, D.; Papadimitriou, J.M.; Hall, J.C. The omentum. World J. Gastroenterol. 2000, 6, 169–176. [Google Scholar]
- Berry, S.J.; Strandberg, J.D.; Coffey, D.S.; Saunders, W.J. Development of canine benign prostatic hyperplasia with age. Prostate 1986, 9, 363–373. [Google Scholar] [CrossRef]
- Polisca, A.; Troisi, A.; Fontaine, E.; Menchetti, L.; Fontbonne, A. A retrospective study of canine prostatic diseases from 2002 to 2009 at the Alfort Veterinary College in France. Theriogenology 2016, 85, 835–840. [Google Scholar] [CrossRef]
- Smith, J. Canine prostatic disease: A review of anatomy, pathology, diagnosis, and treatment. Theriogenology 2008, 70, 375–383. [Google Scholar] [CrossRef]
- Freitag, T.; Jerram, R.M.; Walker, A.M.; Warman, C.G.A. Surgical management of common canine prostatic conditions. Compend. Contin. Educ. Vet. 2007, 29, 656–658. [Google Scholar] [PubMed]
- Huggins, C.; Sommer, J.L. Quantitative studies of prostatic secretion. J. Exp. Med. 1940, 30, 747–762. [Google Scholar] [CrossRef] [PubMed]
- Bokemeyer, J.; Peppler, C.; Thiel, C.; Failing, K.; Kramer, M.; Gerwing, M. Prostatic cavitary lesions containing urine in dogs. J. Small Anim. Pract. 2011, 52, 132–138. [Google Scholar] [CrossRef] [PubMed]
- McGill, J.; Thieman Mankin, K.M.; Parambeth, J.C.; Edwards, J.; Cook, A. Urine-Filled Large Prostatic Cystic Structure in Two Unrelated Male Miniature Dachshunds. J. Am. Anim. Hosp. Assoc. 2018, 54, e54606. [Google Scholar] [CrossRef] [PubMed]
- Hardie, E.M.; Barsanti, J.A.; Rawlings, C.A. Complications of prostatic surgery. J. Am. Anim. Hosp. Assoc. 1984, 20, 50–56. [Google Scholar]
- Mullen, H.; Matthirsen, D.; Sacvelli, D. Results of Surgery and Postoperative Complications in 92 Dogs Treated for Prostatic Abscessation by a Multiple Penrose Drain Technique. J. Am. Anim. Hosp. Assoc. 1990, 26, 369–379. [Google Scholar]
- Sisson, D.D.; Hoffer, R.E. Osteocollagenous prostatic retention cyst: Report of a canine case. J. Am. Anim. Hosp. Assoc. 1997, 13, 61–64. [Google Scholar]
- White, R.; Herrtage, M.E.; Dennis, R. The diagnosis and management of paraprostatic and prostatic retention cysts in the dog. J. Small Anim. Pract. 1987, 28, 551–574. [Google Scholar] [CrossRef]
- Zolton, G.M. Surgical techniques for the prostate. Vet. Clin. N. Am. Small Anim. Pract. 1979, 9, 349–355. [Google Scholar] [CrossRef]
- Hoffer, R.E.; Dykes, N.L.; Greiner, T.P. Marsupialization as a treatment for prostatic disease. J. Am. Anim. Hosp. Assoc. 1977, 13, 98–104. [Google Scholar]
- Lee, J.; Lee, K.S.; Kim, C.L.; Byeon, J.S.; Gu, N.Y.; Cho, I.S.; Cha, S.H. Effect of donor age on the proliferation and multipotency of canine adipose-derived mesenchymal stem cells. J. Vet. Sci. 2017, 18, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Breitbach, M.; Schildberg, F.A.; Hesse, M.; Fleischmann, B.K. Bone marrow CD73+ mesenchymal stem cells display increased stemness in vitro and promote fracture healing in vivo. Bone Rep. 2021, 15, 101133. [Google Scholar] [CrossRef] [PubMed]
- Ode, A.; Schoon, J.; Kurtz, A.; Gaetjen, M.; Ode, J.E.; Geissler, S.; Duda, G.N. CD73/5′-ecto-nucleotidase acts as a regulatory factor in osteo-/chondrogenic differentiation of mechanically stimulated mesenchymal stromal cells. Eur. Cells Mater. 2013, 25, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.T.; Liu, C.; Hyun, J.S.; Lo, D.D.; Montoro, D.T.; Hasegawa, M.; Li, S.; Sorkin, M.; Rennert, R.; Keeney, M.; et al. CD90 (Thy-1)-positive selection enhances osteogenic capacity of human adipose-derived stromal cells. Tissue Eng. Part A 2013, 19, 989–997. [Google Scholar] [CrossRef]
- Nakamura, H.; Yukita, A.; Ninomiya, T.; Hosoya, A.; Hiraga, T.; Ozawa, H. Localization of Thy-1-positive cells in the perichondrium during endochondral ossification. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2010, 58, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Vimalraj, S.; Arumugam, B.; Miranda, P.J.; Selvamurugan, N. Runx2: Structure, function, and phosphorylation in osteoblast differentiation. Int. J. Biol. Macromol. 2015, 78, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.X.; Magovern, C.J.; Mack, C.A.; Budenbender, K.T.; Ko, W.; Rosengart, T.K. Vascular endothelial growth factor is the major angiogenic factor in omentum: Mechanism of the omentum mediated angiogenesis. J. Surg. Res. 1997, 67, 147–154. [Google Scholar] [CrossRef]
- Saifzadeh, S.; Pourreza, B.; Hobbenaghi, R.; Naghadeh, B.D.; Kazemi, S. Autogenous greater omentum, as a free nonvascularized graft, enhances bone healing: An experimental nonunion model. J. Investig. Surg. 2009, 22, 129–137. [Google Scholar] [CrossRef]
- Piermattei, D.; Johnson, K.A. The Hindlimb. In An Atlas of Surgical Approaches to the Bones and Joints of the Dog and Cat; Piermattei, D.L., Johnson, K.A., Eds.; Saunders: Philadelphia, PA, USA, 2004. [Google Scholar]
- Baltzer, W.I.; Cooley, S.; Warnock, J.J.; Nemanic, S.; Stieger-Vanagas, S.M. Augmentation of radius and ulna diaphyseal fracture repair in toy breed dogs using free autogenous omental graft. Vet. Comp. Orthop. Traumatol. 2015, 28, 131–135. [Google Scholar]
- Ree, J.J.; Baltzer, W.I.; Nemanic, S. Randomized, controlled, prospective clinical trial of autologous greater omentum free graft versus autogenous cancellous bone graft in radial and ulnar fractures in miniature breed dogs. Vet. Surg. 2018, 47, 392–405. [Google Scholar] [CrossRef]
- Levy, Y.; Miko, I.; Hauck, M.; Mathesz, K.; Furka, I.; Orda, R. Effect of omental angiogenic lipid factor on revascularization of autotransplanted spleen in dogs. Eur. Surg. Res. 1998, 30, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Silverman, K.J.; Lund, D.P.; Zetter, B.R.; Lainey, L.L.; Shahood, J.A.; Freiman, D.G.; Folkman, J.; Barger, A.C. Angiogenic activity of adipose tissue. Biochem. Biophys. Res. Commun. 1988, 153, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Konturek, S.J.; Brzozowski, T.; Majka, I.; Pawlik, W.; Stachura, J. Omentum and basic fibroblast growth factor in healing of chronic gastric ulcerations in rats. Dig. Dis. Sci. 1994, 39, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.K.; Carlson, G.W.; Austin, G.E.; Austin, E.D.; Rand, R.P.; Jurkiewicz, M.J. Short gut syndrome: Treatment by neovascularization of the small intestine. Ann. Plast. Surg. 1996, 37, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Song, D. The greater omentum: Its use in the surgical management of severely infected leg soft tissue and bone injuries. Report of two cases. Cent. Afr. J. Med. 1989, 35, 423–425. [Google Scholar] [PubMed]
- Kos, J.; Nadinic, V.; Huljev, D.; Nadinić, I.; Turić, J.; Košuta, D.; Anić, T.; Babić, T.; Vnuk, D.; Kreszinger, M.; et al. Healing of bone defect by application of free transplant of greater omentum. Vet. Arh. 2006, 76, 367–379. [Google Scholar]
- Hausman, M.R.; Schaffler, M.B.; Majeska, R.J. Prevention of fracture healing in rats by an inhibitor of angiogenesis. Bone 2001, 29, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Rhinelander, F.W. The normal microcirculation of diaphyseal cortex and its response to fracture. J. Bone Jt. Surg. Am. 1968, 50, 784–800. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Patel, J.; Litbarg, N.O.; Gudehithlu, K.P.; Sethupathi, P.; Arruda, J.A.L.; Dunea, G. Stromal cells cultured from omentum express pluripotent markers, produce high amounts of VEGF, and engraft to injured sites. Cell Tissue Res. 2008, 332, 81–88. [Google Scholar] [CrossRef]
- Oloumi, M.M.; Derakhshanfar, A.; Molaei, M.M.; Tayyebi, M. The angiogenic potential of autogenous free omental graft in experimental tibial defects in rabbit: Short-term preliminary histopathological study. J. Exp. Anim. Sci. 2006, 43, 179–187. [Google Scholar] [CrossRef]
- Street, J.; Bao, M.; deGuzman, L.; Bunting, S.; Peale, F.V.; Ferrara, N.; Steinmetz, H.; Hoeffel, J.; Cleland, J.L.; Daugherty, A.; et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc. Natl. Acad. Sci. USA 2002, 99, 9656–9661. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Pun, S.; Wronski, T.J. Bone anabolic effects of basic fibroblast growth factor in ovariectomized rats. Endocrinology 1999, 140, 5780–5788. [Google Scholar] [CrossRef] [PubMed]
- Matoba, Y.; Katayama, H.; Ohami, H. Evaluation of omental implantation for perforated gastric ulcer therapy: Findings in a rat model. J. Gastroenterol. 1996, 31, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Rosier, R.N.; O’Keefe, R.J.; Hicks, D.G. The potential role of transforming growth factor beta in fracture healing. Clin. Orthop. Relat. Res. 1998, 355, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Brockman, D.J.; Pardo, A.D.; Conzemius, M.G.; Cabell, L.M.; Trout, N.J. Omentum enhanced reconstruction of chronic non-healing wounds in cats: Techniques and clinical use. Vet. Surg. 1996, 25, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.J. Chronic axillary wound repair in a cat with omentalisation and omocervical skin flap. J. Small Anim. Pract. 2005, 46, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Deboer, D.J. Nonhealing cutaneous wounds. In Consultations in Feline Internal Medicine; August, J.R., Ed.; W. B. Saunders: Philadelphia, PA, USA, 1991; pp. 101–106. [Google Scholar]
- Swain, S.F.; Henderson, R.A. Small Animal Wound Management; Lea & Febiger: Philadelphia, PA, USA, 1990. [Google Scholar]
- Dupont, C.; Menaro, Y. Transposition of the greater omentum for reconstruction of the chest wall. Plast. Reconstr. Surg. 1972, 49, 263–267. [Google Scholar] [CrossRef] [PubMed]
- McLean, D.H.; Bijncke, H.J. Autotransplant of omentum to a large scalp defect with microsurgical revascularisation. Plast. Reconstr. Surg. 1972, 49, 260–273. [Google Scholar] [CrossRef]
- Quimby, G.F.; Diamono, D.A.; Mor, Y.; Zaidi, Z.; Ransley, P.G. Bladder neck reconstruction: Long-term follow-up of reconstruction with omentum and silicone sheath. J. Urol. 1996, 156, 629–632. [Google Scholar] [CrossRef]
- Lascelles, B.D.; White, R.A.S. Combined omental pedicle grafts and thoracodorsal axial pattern flaps for the reconstruction of chronic, nonhealing axillary wounds in cats. Vet. Surg. 2001, 30, 380–385. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morawska-Kozłowska, M.; Wilkosz, A.; Zhalniarovich, Y. The Omentum—A Forgotten Structure in Veterinary Surgery in Small Animals’ Surgery. Animals 2024, 14, 1848. https://doi.org/10.3390/ani14131848
Morawska-Kozłowska M, Wilkosz A, Zhalniarovich Y. The Omentum—A Forgotten Structure in Veterinary Surgery in Small Animals’ Surgery. Animals. 2024; 14(13):1848. https://doi.org/10.3390/ani14131848
Chicago/Turabian StyleMorawska-Kozłowska, Magdalena, Aleksandra Wilkosz, and Yauheni Zhalniarovich. 2024. "The Omentum—A Forgotten Structure in Veterinary Surgery in Small Animals’ Surgery" Animals 14, no. 13: 1848. https://doi.org/10.3390/ani14131848




