Chlorophyllin Supplementation of Medicated or Unmedicated Swine Diets Impact on Fecal Escherichia coli and Enterococci
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Animals
2.2. Phase 1, Effects of Non- and ASP 250-Medicated (Chlortetracycline, Penicillin and Sulfamethazine) Diets without or with Chlorophyllin Supplementation on Fecal E. coli and Enterococcal Populations
2.3. Phase 2, Effects of Non- and Tylosin-Medicated Diets without or with Chlorophyllin Supplementation on Fecal E. coli and Enterococcal Populations
2.4. Diet Administration, Feed Refusals and Pig Weighing
2.5. Bacteriological Evaluations
2.6. Statistics
3. Results and Discussion
3.1. Study 1, Medicated Diet Effects on Feed Intake and Performance
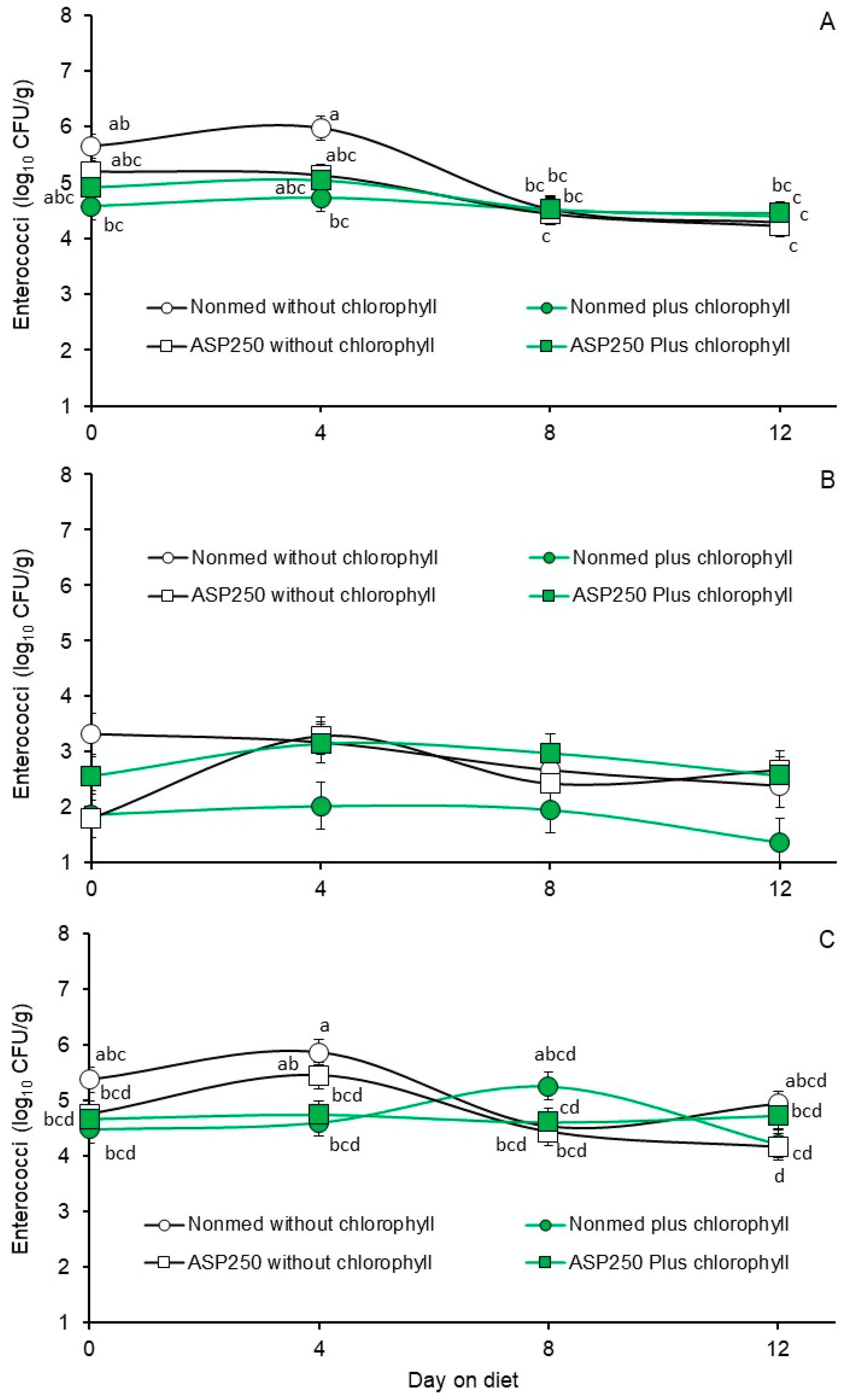
3.2. Phase 1, Effects of Non- and ASP 250-Medicated (Chlortetracycline, Penicillin and Sulfamethazine) Diets without or with Chlorophyllin Supplementation on Fecal E. coli and Enterococcal Populations
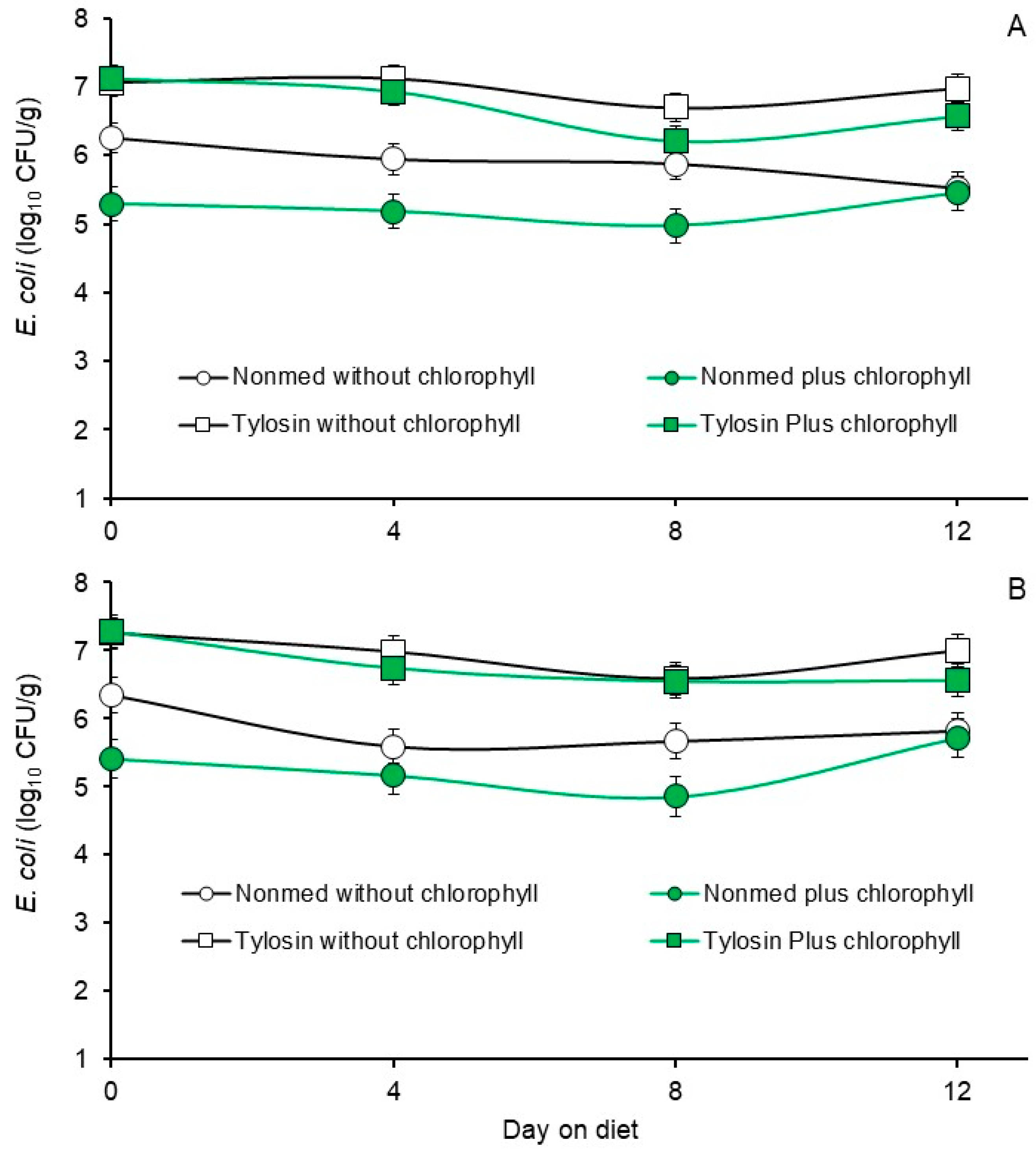
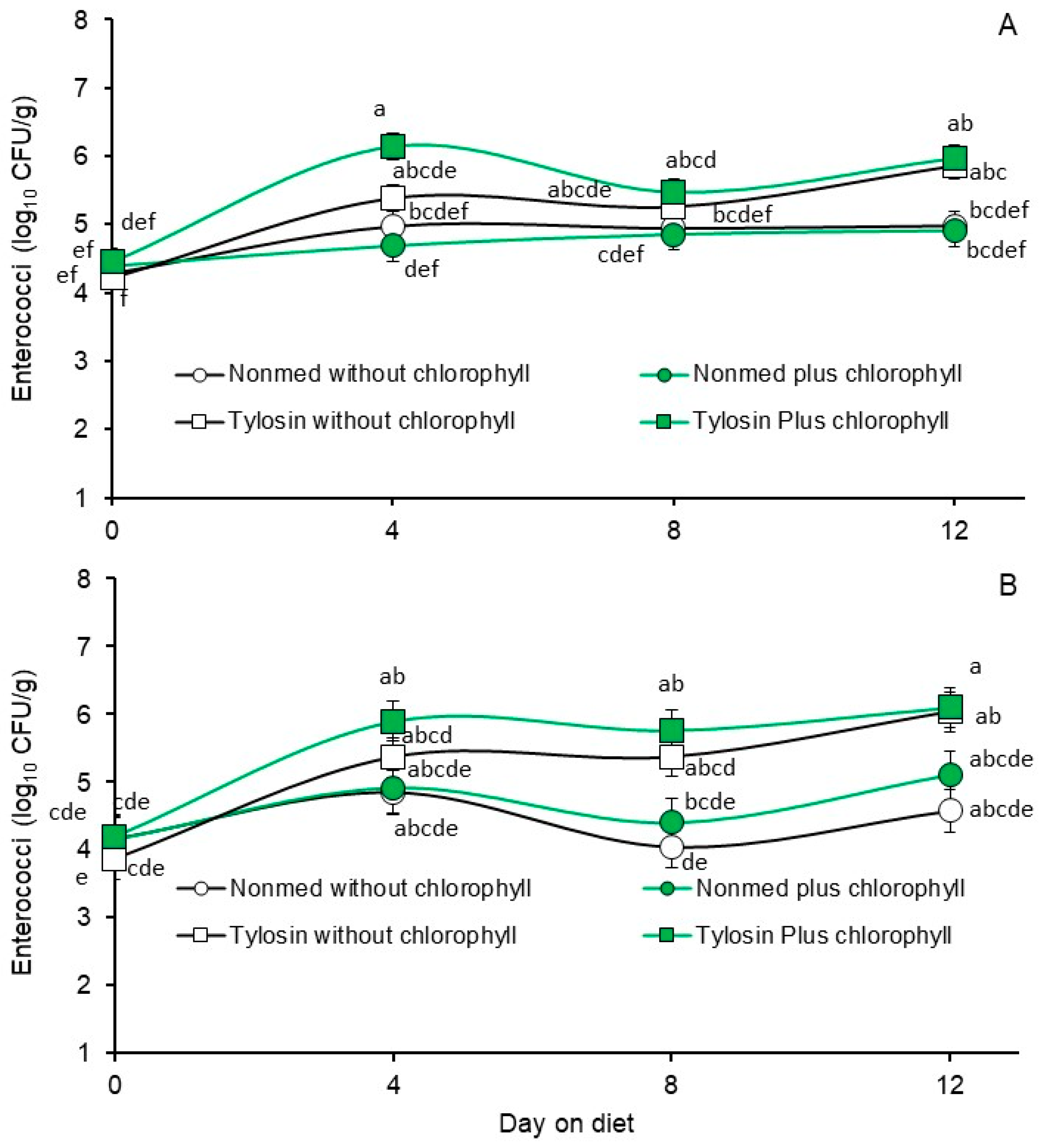
3.3. Phase 2, Effects Non- and Tylosin-Medicated Diets without or with Chlorophyllin Supplementation on Fecal E. coli and Enterococcal Populations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angulo, F.J.; Baker, N.L.; Olsen, S.J.; Anderson, A.; Barrett, T.J. Antimicrobial use in agriculture: Controlling the transfer of antimicrobial resistance to humans. Sem. Pediatric Infect. Dis. 2004, 15, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Gorbach, S.L. Antimicrobial use in animal feed—Time to stop. N. Engl. J. Med. 2001, 345, 1202–1203. [Google Scholar] [CrossRef] [PubMed]
- McEwen, S.A.; Fedorka-Cray, P.J. Antimicrobial use and resistance in animals. Clin. Infect. Dis. 2002, 34 (Suppl. S3), S93–S106. [Google Scholar] [CrossRef] [PubMed]
- Looft, T.; Johnson, T.A.; Allen, H.K.; Bayles, D.O.; Alt, D.P.; Stedtfeld, R.D.; Sul, W.J.; Stedtfeld, T.M.; Chai, B.; Cole, J.R.; et al. In-feed antibiotic effects on the swine intestinal microbiome. Proc. Nat. Acad. Sci. USA 2012, 109, 1691–1696. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Tokach, M.D.; DeRouchey, J.M.; Dritz, S.S.; Woodworth, J.C.; Goodband, R.D.; Chitakasempornkul, K.; Bello, N.M.; Capps, K.; Remfry, S.; et al. Effects of tylosin administration routes on the prevalence of antimicrobial resistance among fecal enterococci of finishing swine. Foodborne Pathog. Dis. 2019, 16, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Pagès, J.; Masi, M.; Barbe, J. Inhibitors of efflux pumps in Gram-negative bacteria. Trends Mol. Med. 2005, 11, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Starvi, M.; Piddock, L.J.V.; Gibbons, S. Bacterial efflux pump inhibitors from natural sources. J. Antimicrob. Chemother. 2007, 59, 1247–1260. [Google Scholar]
- Nikaido, H. The role of outer membrane and efflux pumps in the resistance of gram-negative bacteria. Can we improve drug access? Drug Resist. Updat. 1998, 1, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Poole, K. Efflux pumps as antimicrobial resistance mechanisms. Ann. Med. 2007, 39, 162–176. [Google Scholar] [CrossRef]
- Sáenz, Y.; Ruiz, J.; Zarazaga, M.; Teixido, M.; Torres, C.; Vila, J. Effect of the efflux pump inhibitor phe-arg-β-napththylamide on the MIC values of the quinolones, tetracyclines and chloramphenicol, in Escherichia coli isolates of different origin. J. Antimicrob. Chemother. 2004, 53, 544–545. [Google Scholar] [CrossRef]
- Stermitz, F.R.; Tawara-Matsuda, J.; Lorenz, P.; Mueller, P.; Zenewicz, L.; Lewis, K. 5′-Methoxyhydnocarpin-D and pheophorbide A: Berberis species components that potentiate berberine growth inhibition of resistant Staphylococcus aureus. J. Nat Prod. 2005, 63, 1146–1149. [Google Scholar] [CrossRef]
- Tegos, G.; Stermitz, F.R.; Lomovskaya, O.; Lewis, K. Multidrug pump inhibitors uncover remarkable activity of plant antimicrobials. Antimicrob. Agents Chemother. 2002, 46, 3133–3141. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.B.; Rasmussen, S.L.; Petrich, J.W.; Rasmussen, M.A. Determination of the concentration of potential efflux pump inhibitors, pheophorbide a and pyropheophorbide a, in the feces of animals by fluorescence spectroscopy. J. Agric. Food Chem. 2012, 60, 10456–10460. [Google Scholar] [CrossRef] [PubMed]
- Ferruzzi, M.G.; Blakeslee, J. Digestion, absorption, and cancer preventative activity of dietary chlorophyll derivatives. Nutr. Res. 2007, 27, 1–12. [Google Scholar] [CrossRef]
- Ferruzzi, M.G.; Failla, M.L.; Schwartz, S.J. Sodium copper chlorophyllin: In vitro digestive stability and accumulation by Caco-2 Human intestinal cells. J. Agric. Food Chem. 2002, 50, 2173–2179. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Bird, A.; Kopec, R.E. The metabolism and potential bioactivity of chlorophyll and metallo-chlorophyll derivatives in the gastrointestinal tract. Mol. Nutr. Food Res. 2021, 65, 2000761. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gálvez, A.; Viera, I.; Benito, I.; Roca, M. HPLC-hrTOF-MS study of copper chlorophylls: Composition of food colorants and biochemistry after ingestion. Food Chem. 2020, 321, 126721. [Google Scholar] [CrossRef] [PubMed]
- Tumulo, T.; Lanfer-Marquez, U.M. Copper chlorophyllin: A food colorant with bioactive properties? Food Res. Int. 2012, 46, 451–459. [Google Scholar] [CrossRef]
- Martins, C.F.; Lopes, P.A.; Palma, M.; Pinto, R.M.A.; Costa, M.; Alfaia, C.M.; Pestana, J.M.; Coelho, D.; Ribeiro, D.M.; Viegas, I.; et al. Impact of dietary Chlorella vulgaris and feed enzymes on health status, immune response and liver metabolites in weaned piglets. Sci. Rep. 2022, 12, 16816. [Google Scholar] [CrossRef]
- Martins, C.F.; Pestana Assunção, J.; Ribeiro Santos, D.M.; Madeira, M.S.M.D.S.; Alfaia, C.M.R.P.M.; Lopes, P.A.A.B.; Coelho, D.F.M.; Lemos, J.P.C.; de Almeida, A.M.; Prates, J.A.M.; et al. Effect of dietary inclusion of Spirulina on production performance, nutrient digestibility and meat quality traits in post-weaning piglets. J. Anim. Physiol. Anim. Nutr. 2021, 105, 247–259. [Google Scholar]
- Krüger, M.; Richter, P.; Strauch, S.M.; Nasir, A.; Burkovski, A.; Antunes, C.A.; Meißgeier, T.; Schlücker, E.; Schwab, S.; Lebert, M. What an Escherichia coli mutant can teach us about the antibacterial effect of chlorophyllin. Microorganisms 2019, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Kraatz, M.; Whitehead, T.R.; Cotta, M.A.; Berhow, M.A.; Rasmussen, M.A. Effect of chlorophyll-derived efflux pump inhibitor pheophorbide a and pyropheophorbide a on growth and macrolide antibiotic resistance of indicator and anaerobic swine manure bacteria. Int. J. Antibiotics. 2014, 2014, 185068. [Google Scholar] [CrossRef]
- Jørgensen, E.G. Antibiotic substances from cells and culture solutions of unicellular algae with special reference to some chlorophyll derivatives. Physiol. Plant. 1962, 15, 530–545. [Google Scholar] [CrossRef]
- Lee, A.; Mao, W.; Warren, M.; Mistry, A.; Hoshino, K.; Okumura, R.; Ishida, H.; Lomovskaya, O. Interplay between efflux pumps may provide either additive or multiplicative effects on drug resistance. J. Bact. 2000, 182, 3142–3150. [Google Scholar] [CrossRef] [PubMed]
- de Vogel, J.; Jonker-Termont, D.S.; Katan, M.B.; van der Meer, R. Natural chlorophyll but not chlorophyllin prevents heme-induced cytotoxic and hyperproliferative effects in rat colon. J. Nutr. 2005, 135, 1995–2000. [Google Scholar] [CrossRef] [PubMed]
- Zepka, L.Q.; Jacob-Lopes, E.; Roca, M. Catabolism and bioactive properties of chlorophylls. Curr. Opin. Food Sci. 2019, 26, 94–100. [Google Scholar] [CrossRef]
- Zheng, H.; You, Y.; Hua, M.; Liu, Y.; Chen, Z.; Zhang, L.; Wei, H.; Li, Y.; Luo, M.; Zeng, Y.; et al. Chlorophyllin modulates gut microbiota and inhibits intestinal inflammation to ameliorate hepatic fibrosis in mice. Front. Physiol. 2018, 9, 1671. [Google Scholar] [CrossRef]

| Study One (Diets) 1 | ||
|---|---|---|
| Phase 1 (Days 0 to 12) 512 Power Pig Starter 30–50 | Phase 2 (Days 13 to 24) 510 Power Pig Grower | |
| Crude protein | 21% | 20% |
| Lysine | 1.35% | 1.26% |
| Crude fat | 5.55% | 3.80% |
| Crude fiber | 6.00% | 6.00% |
| Calcium | 0.70 to 1.20% | 0.70 to 1.20% |
| Phosphorus | 0.65% | 0.68% |
| Salt | 0.35 to 0.85% | 0.35 to 0.85% |
| Selenium | 0.30 ppm | 0.30 ppm |
| Zinc | 250 ppm | 150 ppm |
| Phase 1 (ASP 250) Feeding Trial | Phase 2 (Tylosin) Feeding Trial | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diet | Initial Body Weight (kg) | Ending Body Weight (kg) 1 | Average Daily Feed Intake (kg/day as Fed) | Average Daily Gain (kg/day as Fed) | Feed Conversion Ratio | Initial Body Weight (kg) 1 | Final Body Weight (kg) | Average Daily Feed Intake (kg/day as Fed) | Average Daily Gain (kg/day as Fed) | Feed Conversion Ratio |
| Non-medicated starter without chlorophyllin | 76.7 | 92.9 | 4.25 | 1.35 | 3.32 | 92.9 | 106.3 | 4.36 | 1.12 | 4.04 |
| Non-medicated starter with chlorophyllin | 72.3 | 88.9 | 4.39 | 1.38 | 3.20 | 88.9 | 100.5 | 4.40 | 0.97 | 6.35 |
| Medicated starter without chlorophyllin | 71.0 | 91.4 | 4.15 | 1.71 | 2.47 | 91.4 | 100.7 | 4.28 | 0.98 | 5.86 |
| Medicated starter with chlorophyllin | 67.0 | 84.0 | 4.21 | 1.41 | 3.14 | 84.0 | 95.8 | 4.18 | 0.77 | 4.32 |
| p-value | 0.3953 | 0.4280 | 0.4981 | 0.1926 | 0.1625 | 0.4280 | 0.4281 | 0.0528 | 0.2184 | 0.3499 |
| SEM | 4.56 | 4.75 | 0.120 | 0.150 | 0.328 | 4.75 | 5.11 | 0.062 | 0.135 | 1.162 |
| Escherichia coli (log10 CFU/g Feces) | Enterococci (log10 CFU/g Feces) | |||||
|---|---|---|---|---|---|---|
| Diet | Population Recovered without Antibiotic Selection | Population Recovered with Penicillin Selection | Population Recovered with Chlortetracycline Selection | Population Recovered without Antibiotic Selection | Population Recovered with Penicillin Selection | Population Recovered with Chlortetracycline Selection |
| Non-medicated starter without chlorophyllin | 5.92 b | 5.89 b | 5.92 b | 5.22 a | 2.88 a | 5.18 a |
| Non-medicated starter with chlorophyllin | 5.38 c | 5.35 c | 5.38 c | 4.55 b | 1.80 b | 4.64 b |
| ASP 250-medicated starter without chlorophyllin | 6.84 a | 6.87 a | 6.82 a | 4.75 b | 2.54 ab | 4.70 b |
| ASP 250-medicated starter with chlorophyllin | 6.73 a | 6.77 a | 6.68 a | 4.73 b | 2.81 a | 4.69 b |
| p-value | 0.0001 | 0.0001 | 0.0001 | 0.0040 | 0.0325 | 0.0126 |
| SEM | 0.1264 | 0.1293 | 0.1453 | 0.1386 | 0.2903 | 0.1422 |
| Escherichia coli (log10 CFU/g Feces) | Enterococci (log10 CFU/g Feces) | |||||
|---|---|---|---|---|---|---|
| Days on Diet | Population Recovered without Antibiotic Selection | Population Recovered with Penicillin Selection | Population Recovered with Chlortetracycline Selection | Population Recovered without Antibiotic Selection | Population Recovered with Penicillin Selection | Population Recovered with Chlortetracycline Selection |
| 0 | 5.60 b | 5.51 b | 5.36 c | 5.08 a | 2.39 b | 4.82 b |
| 4 | 6.35 a | 6.32 a | 6.16 b | 5.21 a | 2.90 a | 5.17 a |
| 8 | 6.50 a | 6.64 a | 6.77 a | 4.50 b | 2.50 ab | 4.71 bc |
| 12 | 6.43 a | 6.41 a | 6.51 a | 4.45 b | 2.25 b | 4.51 c |
| p-value | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0227 | 0.0007 |
| SEM | 0.1326 | 0.1273 | 0.1294 | 0.1107 | 0.1851 | 0.1108 |
| Escherichia coli (log10 CFU/g Feces) | Enterococci (log10 CFU/g Feces) | |||
|---|---|---|---|---|
| Diet | Population Recovered without Antibiotic Selection | Population Recovered with Tylosin Selection | Population Recovered without Antibiotic Selection | Population Recovered with Tylosin Selection |
| Non-medicated starter without chlorophyllin | 5.90 b | 5.86 b | 4.91 bc | 4.40 c |
| Non-medicated starter with chlorophyllin | 5.22 c | 5.28 c | 4.71 c | 4.64 bc |
| Tylosin-medicated starter without chlorophyllin | 6.96 a | 6.95 a | 5.19 ab | 5.15 ab |
| Tylosin-medicated starter with chlorophyllin | 6.70 a | 6.78 a | 5.51 a | 5.49 a |
| p-value | 0.0001 | 0.0001 | 0.0011 | 0.0021 |
| SEM | 0.1633 | 0.1848 | 0.1572 | 0.2399 |
| Escherichia coli (log10 CFU/g Feces) | Enterococci (log10 CFU/g Feces) | |||
|---|---|---|---|---|
| Days on Diet | Population Recovered without Antibiotic Selection | Population Recovered with Tylosin Selection | Population Recovered without Antibiotic Selection | Population Recovered with Tylosin Selection |
| 0 | 6.43 a | 6.57 a | 4.45 c | 4.09 c |
| 4 | 6.29 a | 6.12 bc | 5.30 ab | 5.25 ab |
| 8 | 5.94 b | 5.91 c | 5.13 b | 4.89 b |
| 12 | 6.13 ab | 6.27 ab | 5.43 a | 5.45 a |
| p-value | 0.0088 | 0.0014 | 0.0001 | 0.0001 |
| SEM | 0.1097 | 0.1268 | 0.1021 | 0.1592 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feye, K.M.; Rasmussen, M.A.; Yeater, K.M.; Anderson, R.C.; Crippen, T.L.; Harvey, R.B.; Poole, T.L.; Ricke, S.C. Chlorophyllin Supplementation of Medicated or Unmedicated Swine Diets Impact on Fecal Escherichia coli and Enterococci. Animals 2024, 14, 1955. https://doi.org/10.3390/ani14131955
Feye KM, Rasmussen MA, Yeater KM, Anderson RC, Crippen TL, Harvey RB, Poole TL, Ricke SC. Chlorophyllin Supplementation of Medicated or Unmedicated Swine Diets Impact on Fecal Escherichia coli and Enterococci. Animals. 2024; 14(13):1955. https://doi.org/10.3390/ani14131955
Chicago/Turabian StyleFeye, Kristina M., Mark A. Rasmussen, Kathleen M. Yeater, Robin C. Anderson, Tawni L. Crippen, Roger B. Harvey, Toni L. Poole, and Steven C. Ricke. 2024. "Chlorophyllin Supplementation of Medicated or Unmedicated Swine Diets Impact on Fecal Escherichia coli and Enterococci" Animals 14, no. 13: 1955. https://doi.org/10.3390/ani14131955
APA StyleFeye, K. M., Rasmussen, M. A., Yeater, K. M., Anderson, R. C., Crippen, T. L., Harvey, R. B., Poole, T. L., & Ricke, S. C. (2024). Chlorophyllin Supplementation of Medicated or Unmedicated Swine Diets Impact on Fecal Escherichia coli and Enterococci. Animals, 14(13), 1955. https://doi.org/10.3390/ani14131955






