Therapeutic Application of Extracellular Vesicles Derived from Mesenchymal Stem Cells in Domestic Animals
Abstract
Simple Summary
Abstract
1. Introduction
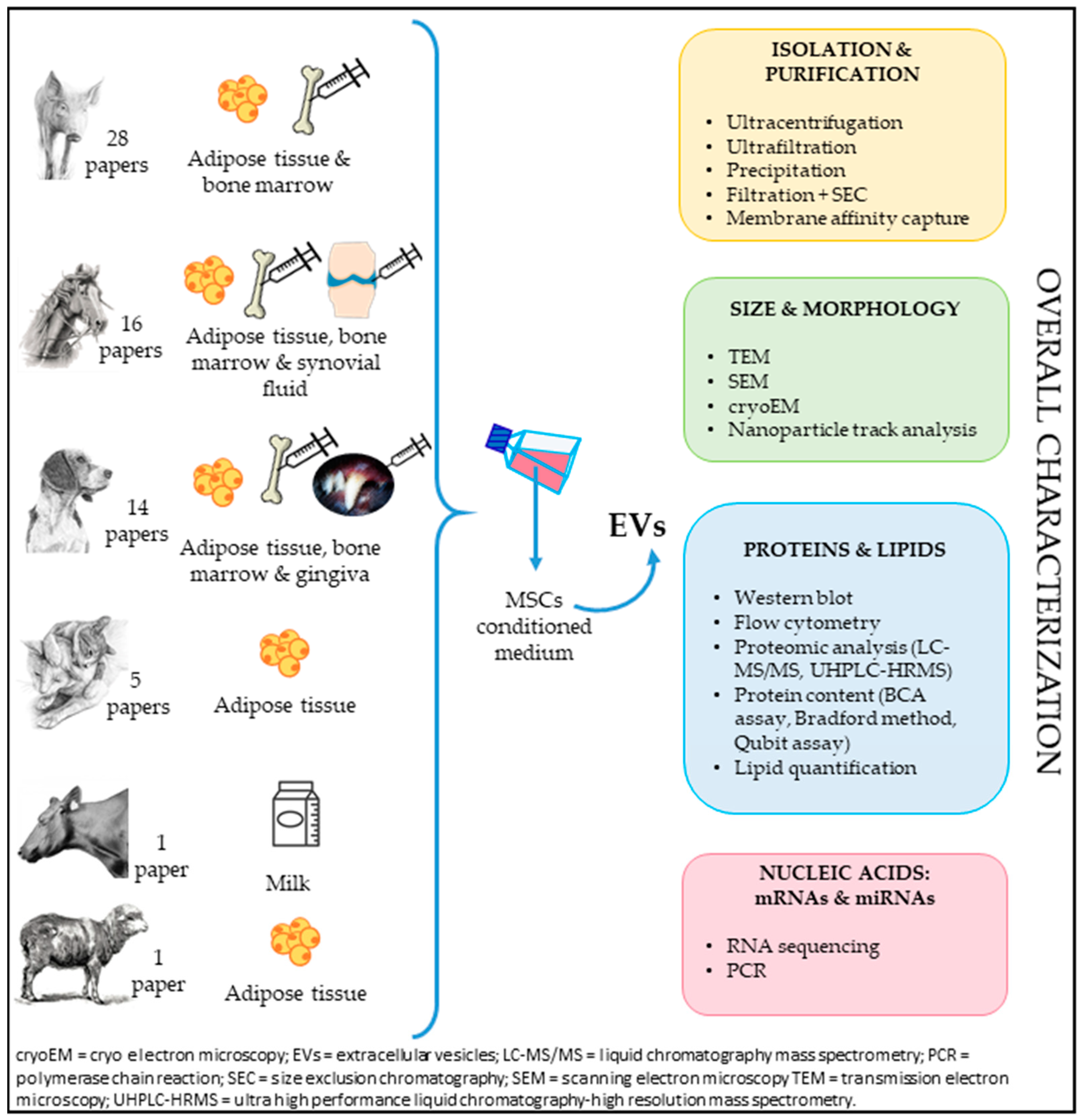
2. Characterization and Sources
2.1. Adult Tissues
2.2. Fetal Adnexa
3. Clinical Application of EVs
3.1. Preclinical Studies
3.2. Clinical Studies/Applications
3.2.1. Orthopedic Field
3.2.2. Reproductive Field
3.2.3. Skin-Wound Field
3.2.4. Urinary Tract
3.2.5. Mammary Gland
3.3. Future Perspectives and Challenges in Clinical Applications
3.4. Large-Scale Production, Isolation Methods and Factors That Affect EV Quality/Quantity
3.5. Safety Profile
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lalu, M.M.; McIntyre, L.; Pugliese, C.; Fergusson, D.; Winston, B.W.; Marshall, J.C.; Granton, J.; Stewart, D.J.; Canadian Critical Care Trials Group. Safety of Cell Therapy with Mesenchymal Stromal Cells (SafeCell): A Systematic Review and Meta-Analysis of Clinical Trials. PLoS ONE 2012, 7, e47559. [Google Scholar] [CrossRef] [PubMed]
- Brooke, G.; Cook, M.; Blair, C.; Han, R.; Heazlewood, C.; Jones, B.; Kambouris, M.; Kollar, K.; McTaggart, S.; Pelekanos, R.; et al. Therapeutic Applications of Mesenchymal Stromal Cells. Semin. Cell Dev. Biol. 2007, 18, 846–858. [Google Scholar] [CrossRef]
- Iacono, E.; Pascucci, L.; Rossi, B.; Bazzucchi, C.; Lanci, A.; Ceccoli, M.; Merlo, B. Ultrastructural characteristics and immune profile of equine MSCs from fetal adnexa. Reproduction 2017, 154, 509–519. [Google Scholar] [CrossRef][Green Version]
- Merlo, B.; Teti, G.; Lanci, A.; Burk, J.; Mazzotti, E.; Falconi, M.; Iacono, E. Comparison between Adult and Foetal Adnexa Derived Equine Post-Natal Mesenchymal Stem Cells. BMC Vet. Res. 2019, 15, 277. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, N.A.; Ortner, A.; Jacamo, R.O.; Reinisch, A.; Schallmoser, K.; Rohban, R.; Etchart, N.; Fruehwirth, M.; Beham-Schmid, C.; Andreeff, M.; et al. Oxygen Sensing Mesenchymal Progenitors Promote Neo-Vasculogenesis in a Humanized Mouse Model In Vivo. PLoS ONE 2012, 7, e44468. [Google Scholar] [CrossRef]
- Bell, G.I.; Meschino, M.T.; Hughes-Large, J.M.; Broughton, H.C.; Xenocostas, A.; Hess, D.A. Combinatorial Human Progenitor Cell Transplantation Optimizes Islet Regeneration Through Secretion of Paracrine Factors. Stem Cells Dev. 2012, 21, 1863–1876. [Google Scholar] [CrossRef]
- Abumaree, M.H.; Abomaray, F.M.; Alshabibi, M.A.; AlAskar, A.S.; Kalionis, B. Immunomodulatory Properties of Human Placental Mesenchymal Stem/Stromal Cells. Placenta 2017, 59, 87–95. [Google Scholar] [CrossRef]
- Carrade, D.D.; Borjesson, D.L. Immunomodulation by Mesenchymal Stem Cells in Veterinary Species. Comp. Med. 2013, 63, 207–217. [Google Scholar] [PubMed]
- Le Blanc, K.; Davies, L.C. Mesenchymal Stromal Cells and the Innate Immune Response. Immunol. Lett. 2015, 168, 140–146. [Google Scholar] [CrossRef]
- Zhao, S.; Wehner, R.; Bornhäuser, M.; Wassmuth, R.; Bachmann, M.; Schmitz, M. Immunomodulatory Properties of Mesenchymal Stromal Cells and Their Therapeutic Consequences for Immune-Mediated Disorders. Stem Cells Dev. 2010, 19, 607–614. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular Vesicles: Exosomes, Microvesicles, and Friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Siljander, P.R.M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological Properties of Extracellular Vesicles and Their Physiological Functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- George, J.N.; Thoi, L.L.; McManus, L.M.; Reimann, T.A. Isolation of Human Platelet Membrane Microparticles from Plasma and Serum. Blood 1982, 60, 834–840. [Google Scholar] [CrossRef]
- Ratajczak, J.; Wysoczynski, M.; Hayek, F.; Janowska-Wieczorek, A.; Ratajczak, M.Z. Membrane-Derived Microvesicles: Important and Underappreciated Mediators of Cell-to-Cell Communication. Leukemia 2006, 20, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Akyurekli, C.; Le, Y.; Richardson, R.B.; Fergusson, D.; Tay, J.; Allan, D.S. A Systematic Review of Preclinical Studies on the Therapeutic Potential of Mesenchymal Stromal Cell-Derived Microvesicles. Stem Cell Rev. 2015, 11, 150–160. [Google Scholar] [CrossRef]
- Rani, S.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Toward Cell-Free Therapeutic Applications. Mol. Ther. 2015, 23, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Park, S.R.; Jung, B.K.; Jeon, Y.K.; Lee, Y.S.; Kim, M.K.; Kim, Y.G.; Jang, J.Y.; Kim, C.W. Exosomes Derived from Mesenchymal Stem Cells Suppress Angio-genesis by Down-Regulating VEGF Expression in Breast Cancer Cells. PLoS ONE 2013, 8, e84256. [Google Scholar] [CrossRef] [PubMed]
- Roccaro, A.M.; Sacco, A.; Maiso, P.; Azab, A.K.; Tai, Y.T.; Reagan, M.; Azab, F.; Flores, L.M.; Campigotto, F.; Weller, E.; et al. BM mesenchymal stromal cell–derived exosomes facilitate multiple myeloma progression. J. Clin. Investig. 2012, 123, 1542–1555. [Google Scholar] [CrossRef] [PubMed]
- Im, H.; Shao, H.; Park, Y.I.; Peterson, V.M.; Castro, C.M.; Weissleder, R.; Lee, H. Label-Free Detection and Molecular Profiling of Exosomes with a Nano-Plasmonic Sensor. Nat. Biotechnol. 2014, 32, 490–495. [Google Scholar] [CrossRef]
- Caplan, A.I.; Dennis, J.E. Mesenchymal Stem Cells as Trophic Mediators. J. Cell Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.Z. The Emerging Role of Microvesicles in Cellular Therapies for Organ/Tissue Regeneration. Nephrol. Dial. Transpl. 2011, 26, 1453–1456. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.T.; Johnstone, R.M. Fate of the Transferrin Receptor during Maturation of Sheep Reticulocytes in Vitro: Selective Externalization of the Receptor. Cell 1983, 33, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wen, J.; Lu, T.; Han, W.; Jiao, K.; Li, H. Mesenchymal Stem Cell-Derived Extracellular Vesicles in Bone-Related Diseases: Intercellular Communication Messengers and Therapeutic Engineering Protagonists. Int. J. Nanomed. 2024, 19, 3233–3257. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to- cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; Carter, D.R.F.; Clayton, A.; Lambert, D.W.; Raposo, G.; Vader, P. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2022, 23, 369–382. [Google Scholar] [CrossRef]
- Varderidou-Minasian, S.; Lorenowicz, M.J. Mesenchymal stromal/stem cell-derived extracellular vesicles in tissue repair: Challenges and opportunities. Theranostics 2020, 10, 5979–5997. [Google Scholar] [CrossRef]
- Sluijter, J.P.; Verhage, V.; Deddens, J.C.; van den Akker, F.; Doevendans, P.A. Microvesicles and exosomes for intracardiac communication. Cardiovasc. Res. 2014, 102, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.; Zhang, L.; Duan, L.; Wang, X.; Min, Y.; Yu, H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J. Mol. Med. 2014, 92, 387–397. [Google Scholar] [CrossRef]
- Khoei, S.G.; Dermani, F.K.; Malih, S.; Fayazi, N.; Sheykhhasan, M. The use of mesenchymal stem cells and their derived extracellular vesicles in cardiovascular disease treatment. Curr. Stem Cell Res. Ther. 2020, 15, 623–638. [Google Scholar] [CrossRef]
- Bruno, S.; Grange, C.; Collino, F.; Deregibus, M.C.; Cantaluppi, V.; Biancone, L.; Tetta, C.; Camussi, G. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS ONE 2012, 7, e33115. [Google Scholar] [CrossRef] [PubMed]
- Kilpinen, L.; Impola, U.; Sankkila, L.; Ritamo, I.; Aatonen, M.; Kilpinen, S.; Tuimala, J.; Valmu, L.; Levijoki, J.; Finckenberg, P. Extracellular membrane vesicles from umbilical cord blood-derived MSC protect against ischemic acute kidney injury, a feature that is lost after inflammatory conditioning. J. Extracell. Vesicles 2013, 2, 21927. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Meng, H.; Wan, W.; Xie, M.; Wen, C. Application potential of stem/progenitor cell-derived extracellular vesicles in renal diseases. Curr. Stem Cell Res. Ther. 2019, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Mardpour, S.; Hassani, S.N.; Mardpour, S.; Sayahpour, F.; Vosough, M.; Ai, J.; Aghdami, N.; Hamidieh, A.A.; Baharvand, H. Extra-cellular vesicles derived from human embryonic stem cell-MSCs ameliorate cirrhosis in thioacetamide-induced chronic liver injury. J. Cell Physiol. 2018, 233, 9330–9344. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Fan, X.; Wang, Y.; Shen, M.; Zheng, Y.; Zhao, S.; Yang, L. Mesenchymal Stem Cell-Derived Extracellular Vesicles in Liver immunity and therapy. Front. Immunol. 2022, 13, 833878. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.M.; Zhuansun, Y.X.; Chen, R.; Lin, L.; Lin, Y.; Li, J.G. Mesenchymal stem cell exosomes promote immunosuppression of regulatory T cells in asthma. Exp. Cell Res. 2018, 363, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Nataliya, B.; Mikhail, A.; Vladimir, P.; Olga, G.; Maksim, V.; Ivan, Z.; Novoseletskaya, E.; Sagaradze, G.; Danilova, N.; Malkov, P.; et al. Mesenchymal stromal cells facilitate resolution of pulmonary fibrosis by miR-29c and miR-129 intercellular transfer. Exp. Mol. Med. 2023, 55, 1399–1412. [Google Scholar] [CrossRef] [PubMed]
- Casado-Díaz, A.; Quesada-Gómez, J.M.; Dorado, G. Extracellular vesicles derived from mesenchymal stem cells (MSC) in regenerative medicine: Applications in skin wound healing. Front. Bioeng. Biotechnol. 2020, 8, 146. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.H.; Kim, H.K.; Lee, J.; Kwon, H.H.; Park, G.H.; Yang, S.H.; Jung, J.Y.; Choi, H.; Lee, J.H.; Sung, S.; et al. Mesenchymal Stem/Stromal Cell-Derived Exosomes for Immunomodulatory Therapeutics and Skin Regeneration. Cells 2020, 9, 1157. [Google Scholar] [CrossRef]
- Kim, J.; Kim, E.H.; Lee, H.; Sung, J.H.; Bang, O.Y. Clinical-Scale Mesenchymal Stem Cell-Derived Extracellular Vesicle Therapy for Wound Healing. Int. J. Mol. Sci. 2023, 24, 4273. [Google Scholar] [CrossRef]
- Dong, L.; Pu, Y.; Zhang, L.; Qi, Q.; Xu, L.; Li, W.; Wei, C.; Wang, X.; Zhou, S.; Zhu, J.; et al. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles promote lung adenocarcinoma growth by transferring miR-410. Cell Death Dis. 2018, 9, 218. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Hai, B.; Kelly, J.; Wu, S.; Liu, F. Extracellular vesicle mimics made from iPS cell-derived mesenchymal stem cells improve the treatment of metastatic prostate cancer. Stem Cell Res. Ther. 2021, 12, 29. [Google Scholar] [CrossRef]
- Weng, Z.; Zhang, B.; Wu, C.; Yu, F.; Han, B.; Li, B.; Li, L. Therapeutic roles of mesenchymal stem cell-derived extracellular vesi-cles in cancer. J. Hematol. Oncol. 2021, 14, 136. [Google Scholar] [CrossRef] [PubMed]
- Mathew, B.; Torres, L.A.; Gamboa Acha, L.; Tran, S.; Liu, A.; Patel, R.; Chennakesavalu, M.; Aneesh, A.; Huang, C.C.; Feinstein, D.L.; et al. Uptake and distribution of administered bone marrow mesenchymal stem cell extracellular vesicles in retina. Cells 2021, 10, 730. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.K.; Ballal, A.R.; Shailaja, S.; Seetharam, R.N.; Raghu, C.H.; Sankhe, R.; Pai, K.; Tender, T.; Mathew, M.; Aroor, A.; et al. Small extracellular vesicle-loaded bevacizumab reduces the frequency of intravitreal injection required for diabetic retinopathy. Theranostics 2023, 13, 2241–2255. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yuan, Q.; Xie, L. Mesenchymal Stem Cell-Based Immunomodulation: Properties and Clinical Application. Stem Cells Int. 2018, 2018, 3057624. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, Q.; Hu, Y.; Shi, Y. Current Research and Use of Mesenchymal Stem Cells in the Therapy of Autoimmune Diseases. Curr. Stem Cell Res. Ther. 2019, 14, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ferrer, M.; Amaro-Prellezo, E.; Dorronsoro, A.; Sánchez-Sánchez, R.; Vicente, Á.; Cosín-Roger, J.; Barrachina, M.D.; Baquero, M.C.; Valencia, J.; Sepúlveda, P. HIF-Overexpression and Pro-Inflammatory Priming in Human Mesenchymal Stro-mal Cells Improves the Healing Properties of Extracellular Vesicles in Experimental Crohn’s Disease. Int. J. Mol. Sci. 2021, 22, 11269. [Google Scholar] [CrossRef]
- Madel, R.J.; Börger, V.; Dittrich, R.; Bremer, M.; Tertel, T.; Phuong, N.N.T.; Baba, H.A.; Kordelas, L.; Staubach, S.; Stein, F.; et al. Independent human mesenchymal stromal cell-derived extracellular vesicle preparations differentially attenuate symptoms in an advanced murine graft-versus-host disease model. Cytotherapy 2023, 25, 821–836. [Google Scholar] [CrossRef]
- Hackel, A.; Vollmer, S.; Bruderek, K.; Lang, S.; Brandau, S. Immunological priming of mesenchymal stromal/stem cells and their extracellular vesicles augments their therapeutic benefits in experimental graft-versus-host disease via engagement of PD-1 ligands. Front. Immunol. 2023, 14, 1078551. [Google Scholar] [CrossRef]
- van Velthoven, C.T.; Braccioli, L.; Willemen, H.L.; Kavelaars, A.; Heijnen, C.J. Therapeutic potential of genetically modified mesenchymal stem cells after neonatal hypoxic-ischemic brain damage. Mol. Ther. 2014, 22, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Nguyen, P.D.; Shanti, R.M.; Shi, S.; Shakoori, P.; Zhang, Q.; Le, A.D. Gingiva-derived mesenchymal stem cell-extracellular vesicles activate schwann cell repair phenotype and promote nerve regeneration. Tissue Eng. Part A 2019, 25, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Guy, R.; Herman, S.; Benyamini, H.; Ben-Zur, T.; Kobo, H.; Pasmanik-Chor, M.; Yaacobi, D.; Barel, E.; Yagil, C.; Yagil, Y.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles as Proposed Therapy in a Rat Model of Cerebral Small Vessel Disease. Int. J. Mol. Sci. 2022, 23, 11211. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yuan, L.; Cao, H.; Guo, J.; Zhou, X.; Zeng, Z. Application and Molecular Mechanisms of Extracellular Vesicles De-rived from Mesenchymal Stem Cells in Osteoporosis. Curr. Issues Mol. Biol. 2022, 44, 6346–6367. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.; Ni, J.; Witherel, C.E.; Yang, M.; Burdick, J.A.; Wen, C.; Wong, S.H.D. Harnessing Tissue-derived Extracellular Vesicles for Osteoarthritis theranostics. Theranostics 2022, 12, 207–231. [Google Scholar] [CrossRef]
- Zou, J.; Yang, W.; Cui, W.; Li, C.; Ma, C.; Ji, X.; Hong, J.; Qu, Z.; Chen, J.; Liu, A.; et al. Therapeutic potential and mechanisms of mesenchymal stem cell-derived exosomes as bioactive materials in tendon-bone healing. J. Nanobiotechnol. 2023, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- Lötvall, J.; Hill, A.F.; Hochberg, F.; Buzás, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions (MISEV2014): A position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef] [PubMed]
- Moccia, V.; Sammarco, A.; Cavicchioli, L.; Castagnaro, M.; Bongiovanni, L.; Zappulli, V. Extracellular Vesicles in Veterinary Medicine. Animals 2022, 12, 2716. [Google Scholar] [CrossRef] [PubMed]
- Arévalo-Turrubiarte, M.; Baratta, M.; Ponti, G.; Chiaradia, E.; Martignani, E. Extracellular vesicles from equine mesenchymal stem cells decrease inflammation markers in chondrocytes in vitro. Equine Vet. J. 2022, 54, 1133–1143. [Google Scholar] [CrossRef]
- Navarrete, F.; Wong, Y.S.; Cabezas, J.; Riadi, G.; Manríquez, J.; Rojas, D.; Furlanetto Mancanares, A.C.; Rodriguez-Alvarez, L.; Saravia, F.; Castro, F.O. Distinctive cellular transcriptomic signature and MicroRNA cargo of extracellular vesicles of horse adipose and endometrial mesenchymal stem cells from the same donors. Cell Reprogramming 2020, 22, 311–327. [Google Scholar] [CrossRef]
- Ji, Y.; Jiang, W.; Zeng, F.; Zou, D.; Li, S.; Zhang, X.; Zhu, Q.; Liang, Q.; Li, M.; Li, D. Characterization of Canine Gingival-Derived Mesenchymal Stem Cells and Their Exosomes. J. Vet. Dent. 2023. [Google Scholar] [CrossRef] [PubMed]
- Pipino, C.; Mandatori, D.; Buccella, F.; Lanuti, P.; Preziuso, A.; Castellani, F.; Grotta, L.; Di Tomo, P.; Marchetti, S.; Di Pietro, N.; et al. Identification and characterization of a stem cell-like population in bovine milk: A potential new source for regenerative medicine in veterinary. Stem Cells Dev. 2018, 27, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Eirin, A.; Riester, S.M.; Zhu, X.Y.; Tang, H.; Evans, J.M.; O’Brien, D.; van Wijnen, A.J.; Lerman, L.O. MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene 2014, 551, 55–64. [Google Scholar] [CrossRef]
- Chen, K.H.; Chen, C.H.; Wallace, C.G.; Yuen, C.M.; Kao, G.S.; Chen, Y.L.; Shao, P.L.; Chen, Y.L.; Chai, H.T.; Lin, K.C.; et al. Intravenous administration of xenogenic adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes markedly reduced brain infarct volume and preserved neurological function in rat after acute ischemic stroke. Oncotarget 2016, 7, 74537. [Google Scholar] [CrossRef] [PubMed]
- Eirin, A.; Zhu, X.Y.; Puranik, A.S.; Woollard, J.R.; Tang, H.; Dasari, S.; Lerman, A.; van Wijnen, A.J.; Lerman, L.O. Comparative proteomic analysis of extracellular vesicles isolated from porcine adipose tissue-derived mesenchymal stem/stromal cells. Sci. Rep. 2016, 6, 36120. [Google Scholar] [CrossRef]
- Eirin, A.; Zhu, X.Y.; Puranik, A.S.; Woollard, J.R.; Tang, H.; Dasari, S.; Lermna, A.; van Wijnen, A.J.; Lerman, L.O. Comparative Integrated transcriptomic and proteomic analysis of the molecular cargo of extracellular vesicles derived from porcine adipose tissue-derived mesenchymal stem cells. PLoS ONE 2017, 12, e0174303. [Google Scholar] [CrossRef]
- Eirin, A.; Zhu, X.Y.; Puranik, A.S.; Tang, H.; McGurren, K.A.; van Wijnen, A.J.; Lerman, A.; Lerman, L.O. Mesenchymal stem cell–derived extracellular vesicles attenuate kidney inflammation. Kidney Int. 2017, 92, 114–124. [Google Scholar] [CrossRef]
- Eirin, A.; Zhu, X.Y.; Jonnada, S.; Lerman, A.; van Wijnen, A.J.; Lerman, L.O. Mesenchymal stem cell-derived extracellular vesicles improve the renal microvasculature in metabolic renovascular disease in swine. Cell Transplant. 2018, 27, 1080–1095. [Google Scholar] [CrossRef]
- Meng, Y.; Eirin, A.; Zhu, X.Y.; O’Brien, D.R.; Lerman, A.; Van Wijnen, A.J.; Lerman, L.O. The metabolic syndrome modifies the mRNA expression profile of extracellular vesicles derived from porcine mesenchymal stem cells. Diabetol. Metab. Syndr. 2018, 10, 58. [Google Scholar] [CrossRef]
- Khatri, M.; Richardson, L.A.; Meulia, T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res. Ther. 2018, 9, 17. [Google Scholar] [CrossRef]
- Meng, Y.; Eirin, A.; Zhu, X.Y.; Tang, H.; Chanana, P.; Lerman, A.; Van Wijnen, A.J.; Lerman, L.O. The metabolic syndrome alters the miRNA signature of porcine adipose tissue-derived mesenchymal stem cells. Cytom. Part A 2018, 93, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Eirin, A.; Zhu, X.Y.; Woollard, J.R.; Tang, H.; Dasari, S.; Lerman, A.; Lerman, L.O. Metabolic syndrome interferes with packaging of proteins within porcine mesenchymal stem cell-derived extracellular vesicles. Stem Cells Transl. Med. 2019, 8, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Conley, S.M.; Shook, J.E.; Zhu, X.Y.; Eirin, A.; Jordan, K.L.; Woollard, J.R.; Isik, B.; Hickson, L.J.; Puranik, A.S.; Lerman, L.O. Metabolic syndrome induces release of smaller extracellular vesicles from porcine mesenchymal stem cells. Cell Transplant. 2019, 28, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Thankam, F.G.; Chandra, I.; Diaz, C.; Dilisio, M.F.; Fleegel, J.; Gross, R.M.; Agrawal, D.K. Matrix regeneration proteins in the hypoxia-triggered exosomes of shoulder tenocytes and adipose-derived mesenchymal stem cells. Mol. Cell Biochem. 2020, 465, 75–87. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, X.Y.; Zhao, Y.; Eirin, A.; Liu, L.; Ferguson, C.M.; Tang, H.; Lerman, A.; Lerman, L.O. Selective intrarenal delivery of mesenchymal stem cell-derived extracellular vesicles attenuates myocardial injury in experimental metabolic renovascular disease. Basic Res. Cardiol. 2020, 115, 16. [Google Scholar] [CrossRef]
- Pawar, A.S.; Eirin, A.; Tang, H.; Zhu, X.Y.; Lerman, A.; Lerman, L.O. Upregulated tumor necrosis factor-α transcriptome and proteome in adipose tissue-derived mesenchymal stem cells from pigs with metabolic syndrome. Cytokine 2020, 130, 155080. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, X.; Zhang, L.; Ferguson, C.M.; Song, T.; Jiang, K.; Conley, S.M.; Krier, J.D.; Tang, H.; Saadiq, I.; et al. Mesenchymal stem/stromal cells and their extracellular vesicle progeny decrease injury in poststenotic swine kidney through different mechanisms. Stem Cells Dev. 2020, 29, 1190–1200. [Google Scholar] [CrossRef]
- Song, T.; Eirin, A.; Zhu, X.; Zhao, Y.; Krier, J.D.; Tang, H.; Jordan, K.L.; Woollard, J.R.; Taner, T.; Lerman, A.; et al. Mesenchymal stem cell–derived extracellular vesicles induce regulatory t cells to ameliorate chronic kidney injury. Hypertension 2020, 75, 1223–1232. [Google Scholar] [CrossRef]
- Li, Y.; Meng, Y.; Zhu, X.; Saadiq, I.M.; Jordan, K.L.; Eirin, A.; Lerman, L.O. Metabolic syndrome increases senescence-associated micro-RNAs in extracellular vesicles derived from swine and human mesenchymal stem/stromal cells. Cell Commun. Signal. 2020, 18, 124. [Google Scholar] [CrossRef]
- Farahani, R.A.; Zhu, X.Y.; Tang, H.; Jordan, K.L.; Lerman, A.; Lerman, L.O.; Eirin, A. Metabolic syndrome alters the cargo of mitochondria-related microRNAs in swine mesenchymal stem cell-derived extracellular vesicles, impairing their capacity to repair the stenotic kidney. Stem Cells Int. 2020, 2020, 8845635. [Google Scholar] [CrossRef]
- Monguió-Tortajada, M.; Prat-Vidal, C.; Moron-Font, M.; Clos-Sansalvador, M.; Calle, A.; Gastelurrutia, P.; Cserkoova, A.; Morancho, A.; Ramírez, M.A.; Rosell, A.; et al. Local administration of porcine immunomodulatory, chemotactic and angiogenic extracellular vesicles using engineered cardiac scaffolds for myocardial infarction. Bioact. Mater. 2021, 6, 3314–3327. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, C.M.; Farahani, R.A.; Zhu, X.Y.; Tang, H.; Jordan, K.L.; Saadiq, I.M.; Lerman, A.; Lerman, L.O.; Eirin, A. Mesenchymal stem/stromal cell-derived extracellular vesicles elicit better preservation of the intra-renal microvasculature than renal revascularization in pigs with renovascular disease. Cells 2021, 10, 763. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Hong, S.; Zhu, X.; Zhang, L.; Tang, H.; Jordan, K.L.; Saadiq, I.M.; Huang, W.; Lerman, A.; Eirin, A.; et al. IL-10 partly mediates the ability of MSC-derived extracellular vesicles to attenuate myocardial damage in experimental metabolic renovascular hypertension. Front. Immunol. 2022, 13, 940093. [Google Scholar] [CrossRef] [PubMed]
- Monguió-Tortajada, M.; Prat-Vidal, C.; Martínez-Falguera, D.; Teis, A.; Soler-Botija, C.; Courageux, Y.; Munizaga-Larroudé, M.; Moron-Font, M.; Bayes-Genis, A.; Borràs, F.E.; et al. Acellular cardiac scaffolds enriched with MSC-derived extracellular vesicles limit ventricular remodelling and exert local and systemic immunomodulation in a myocardial infarction porcine model. Theranostics 2022, 12, 4656. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Cui, Y.; Li, X.; Xiong, Y.; Yue, G.; Yang, Y. Isolation and Identification of Porcine Bone Marrow Mesenchymal Stem Cells and their Derived Extracellular Vesicles. JoVE 2022, 182, e63785. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Zhu, X.Y.; Jiang, Y.; Zhang, L.; Tang, H.; Jordan, K.L.; Saadiq, I.M.; Huang, W.; Lerman, A.; Eirin, A.; et al. Autologous Extracellular Vesicles attenuate cardiac injury in experimental atherosclerotic renovascular disease more effectively than their parent Mesenchymal Stem/Stromal Cells. Stem Cell Rev. Rep. 2023, 19, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Piao, C.; Liu, T.; Lu, X.; Ma, Y.; Zhang, J.; Liu, G.; Wang, H. Effects of the exosomes of adipose-derived mesenchymal stem cells on apoptosis and pyroptosis of injured liver in miniature pigs. Biomed. Pharmacother. 2023, 169, 115873. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, T.; Jiao, G.; Lv, Y.; Piao, C.; Lu, X.; Ma, H.; Wang, H. Exosomes from adipose-derived mesenchymal stem cells can attenuate liver injury caused by minimally invasive hemihepatectomy combined with ischemia-reperfusion in minipigs by modulating the endoplasmic reticulum stress response. Life Sci. 2023, 321, 121618. [Google Scholar] [CrossRef] [PubMed]
- Shulman, I.; Ageeva, T.; Kostennikov, A.; Ogurcov, S.; Tazetdinova, L.; Kabdesh, I.; Rogozhin, A.; Ganiev, I.; Rizvanov, I.; Mukhamedshina, Y. Intrathecal Injection of Autologous Mesenchymal Stem-Cell-Derived Extracellular Vesicles in Spinal Cord Injury: A Feasibility Study in Pigs. Int. J. Mol. Sci. 2023, 24, 8240. [Google Scholar] [CrossRef]
- Aggarwal, R.; Shao, A.; Potel, K.N.; So, S.W.; Swingen, C.M.; Wright, C.A.; Hocum Stone, L.L.; McFalls, E.O.; Butterick, T.A.; Kelly, R.F. Stem cell-derived exosome patch with coronary artery bypass graft restores cardiac function in chronically ischemic porcine myocardium. J. Thorac. Cardiovasc. Surg. 2023, 166, e512–e530. [Google Scholar] [CrossRef]
- Pascucci, L.; Alessandri, G.; Dall’Aglio, C.; Mercati, F.; Coliolo, P.; Bazzucchi, C.; Dante, S.; Petrini, S.; Curina, G.; Ceccarelli, P. Membrane vesicles mediate pro-angiogenic activity of equine adipose-derived mesenchymal stromal cells. Vet. J. 2014, 202, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Pascucci, L.; Dall’Aglio, C.; Bazzucchi, C.; Mercati, F.; Mancini, M.G.; Pessina, A.; Alessandri, G.; Giammarioli, M.; Dante, S.; Brunati, G.; et al. Horse adipose-derived mesenchymal stromal cells constitutively produce membrane vesicles: A morphological study. Histol. Histopathol. 2015, 30, 549–557. [Google Scholar] [PubMed]
- Marycz, K.; Michalak, I.; Kocherova, I.; Marędziak, M.; Weiss, C. The Cladophora glomerata enriched by biosorption process in Cr (III) improves viability, and reduces oxidative stress and apoptosis in equine metabolic syndrome derived adipose mesenchymal stromal stem cells (ASCs) and their extracellular vesicles (MV’s). Mar. Drugs 2017, 15, 385. [Google Scholar] [CrossRef] [PubMed]
- Capomaccio, S.; Cappelli, K.; Bazzucchi, C.; Coletti, M.; Gialletti, R.; Moriconi, F.; Passamonti, F.; Pepe, M.; Petrini, S.; Mecocci, S.; et al. Equine adipose-derived mesenchymal stromal cells release extracellular vesicles enclosing different subsets of small RNAs. Stem Cells Int. 2019, 2019, 4957806. [Google Scholar] [CrossRef] [PubMed]
- Klymiuk, M.C.; Balz, N.; Elashry, M.I.; Heimann, M.; Wenisch, S.; Arnhold, S. Exosomes isolation and identification from equine mesenchymal stem cells. BMC Vet. Res. 2019, 15, 42. [Google Scholar] [CrossRef] [PubMed]
- Kornicka-Garbowska, K.; Pędziwiatr, R.; Woźniak, P.; Kucharczyk, K.; Marycz, K. Microvesicles isolated from 5-azacytidine-and-resveratrol-treated mesenchymal stem cells for the treatment of suspensory ligament injury in horse—A case report. Stem Cell Res. Ther. 2019, 10, 394. [Google Scholar] [CrossRef] [PubMed]
- Hotham, W.E.; Thompson, C.; Szu-Ting, L.; Henson, F.M.D. The anti-inflammatory effects of equine bone marrow stem cell-derived extracellular vesicles on autologous chondrocytes. Vet. Rec. 2021, 8, e22. [Google Scholar] [CrossRef] [PubMed]
- Mocchi, M.; Grolli, S.; Dotti, S.; Di Silvestre, D.; Villa, R.; Berni, P.; Conti, V.; Passignani, G.; Brambilla, F.; Del Bue, M.; et al. Equine mesenchymal stem/stromal cells freeze-dried secretome (Lyosecretome) for the treatment of musculoskeletal diseases: Production process validation and batch release test for clinical use. Pharmaceuticals 2021, 14, 553. [Google Scholar] [CrossRef]
- Contentin, R.; Jammes, M.; Bourdon, B.; Cassé, F.; Bianchi, A.; Audigié, F.; Branly, T.; Velot, E.; Galéra, P. Bone marrow MSC secretome increases equine articular chondrocyte collagen accumulation and their migratory capacities. Int. J. Mol. Sci. 2022, 23, 5795. [Google Scholar] [CrossRef]
- Soukup, R.; Gerner, I.; Gültekin, S.; Baik, H.; Oesterreicher, J.; Grillari, J.; Jenner, F. Characterisation of extracellular vesicles from equine mesenchymal stem cells. Int. J. Mol. Sci. 2022, 23, 5858. [Google Scholar] [CrossRef]
- Clarke, E.J.; Johnson, E.; Caamaño Gutierrez, E.; Andersen, C.; Berg, L.C.; Jenkins, R.E.; Lindegaard, C.; Uvebrant, K.; Lundgren-Åkerlund, E.; Turlo, A.; et al. Temporal extracellular vesicle protein changes following intraarticular treatment with integrin α10β1-selected mesenchymal stem cells in equine osteoarthritis. Front. Vet. Sci. 2022, 9, 1057667. [Google Scholar] [CrossRef] [PubMed]
- Soukup, R.; Gerner, I.; Mohr, T.; Gueltekin, S.; Grillari, J.; Jenner, F. Mesenchymal Stem Cell Conditioned Medium Modulates Inflammation in Tenocytes: Complete Conditioned Medium Has Superior Therapeutic Efficacy than Its Extracellular Vesicle Fraction. Int. J. Mol. Sci. 2023, 24, 10857. [Google Scholar] [CrossRef] [PubMed]
- Abdelnaby, E.A.; Abdallah, A.N.; Anwar, I.M.; El-Tookhy, O.S.; Shamaa, A.A. The therapeutic effect of stem cell-derived exosomes in the treatment of chronic endometritis as assessed by histopathological, Doppler and hormonal expression in Arabian mares. Equine Vet. Educ. 2023, 36, 347–356. [Google Scholar] [CrossRef]
- Cassé, F.; Velot, E.; Bianchi, A.; Audigié, F.; Contentin, R.; Galéra, P. Pro-Inflammatory Cytokine Priming and Purification Method Modulate the Impact of Exosomes Derived from Equine Bone Marrow Mesenchymal Stromal Cells on Equine Articular Chondrocytes. Int. J. Mol. Sci. 2023, 24, 14169. [Google Scholar] [CrossRef] [PubMed]
- El-Tookhy, O.S.; Shamaa, A.A.; Shehab, G.G.; Abdallah, A.N.; Azzam, O.M. Histological evaluation of experimentally induced critical size defect skin wounds using exosomal solution of mesenchymal stem cells derived microvesicles. Int. J. Stem Cells 2017, 10, 144–153. [Google Scholar] [CrossRef]
- Qamar, A.Y.; Fang, X.; Kim, M.J.; Cho, J. Improved post-thaw quality of canine semen after treatment with exosomes from conditioned medium of adipose-derived mesenchymal stem cells. Animals 2019, 9, 865. [Google Scholar] [CrossRef]
- Villatoro, A.J.; Alcoholado, C.; Martín-Astorga, M.C.; Fernández, V.; Cifuentes, M.; Becerra, J. Comparative analysis and characterization of soluble factors and exosomes from cultured adipose tissue and bone marrow mesenchymal stem cells in canine species. Vet. Immunol. Immunopathol. 2019, 208, 6–15. [Google Scholar] [CrossRef]
- An, J.H.; Li, Q.; Bhang, D.H.; Song, W.J.; Youn, H.Y. TNF-α and INF-γ primed canine stem cell-derived extracellular vesicles alleviate experimental murine colitis. Sci. Rep. 2020, 10, 2115. [Google Scholar] [CrossRef]
- An, J.H.; Li, Q.; Ryu, M.O.; Nam, A.R.; Bhang, D.H.; Jung, Y.C.; Song, W.J.; Youn, H.Y. TSG-6 in extracellular vesicles from canine mesenchymal stem/stromal is a major factor in relieving DSS-induced colitis. PLoS ONE 2020, 15, e0220756. [Google Scholar] [CrossRef]
- Park, S.M.; An, J.H.; Lee, J.H.; Kim, K.B.; Chae, H.K.; Oh, Y.I.; Song, W.J.; Youn, H.Y. Extracellular vesicles derived from DFO-preconditioned canine AT-MSCs reprogram macrophages into M2 phase. PLoS ONE 2021, 16, e0254657. [Google Scholar] [CrossRef]
- Teshima, T.; Yuchi, Y.; Suzuki, R.; Matsumoto, H.; Koyama, H. Immunomodulatory effects of canine adipose tissue mesenchymal stem cell-derived extracellular vesicles on stimulated CD4+ T cells isolated from peripheral blood mononuclear cells. J. Immunol. Res. 2021, 2021, 2993043. [Google Scholar] [CrossRef]
- Mocchi, M.; Bari, E.; Dotti, S.; Villa, R.; Berni, P.; Conti, V.; Del Bue, M.; Squassino, G.P.; Segale, L.; Ramoni, R.; et al. Canine mesenchymal cell lyosecretome production and safety evaluation after allogenic intraarticular injection in osteoarthritic dogs. Animals 2021, 11, 3271. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Yoshizaki, K.; Nishida, H.; Kamishina, H.; Maeda, S.; Takano, K.; Fujita, N.; Nishimura, R.; Jo, J.I.; Tabata, Y.; et al. Extracellular vesicles derived from canine mesenchymal stromal cells in serum free culture medium have anti-inflammatory effect on microglial cells. Front. Vet. Sci. 2021, 8, 633426. [Google Scholar] [CrossRef]
- Kim, S.Y.; Yoon, T.H.; Na, J.; Yi, S.J.; Jin, Y.; Kim, M.; Oh, T.H.; Chung, T.W. Mesenchymal stem cells and extracellular vesicles derived from canine adipose tissue ameliorates inflammation, skin barrier function and pruritus by reducing JAK/STAT signaling in atopic dermatitis. Int. J. Mol. Sci. 2022, 23, 4868. [Google Scholar] [CrossRef]
- Cho, B.S.; Kim, S.B.; Kim, S.; Rhee, B.; Yoon, J.; Lee, J.W. Canine Mesenchymal-Stem-Cell-Derived Extracellular Vesicles Attenuate Atopic Dermatitis. Animals 2023, 13, 2215. [Google Scholar] [CrossRef]
- Liu, H.; Huang, L.; Chen, F.; Zhong, Z.; Ma, X.; Zhou, Z.; Cao, S.; Shen, L.; Peng, G. Adipose-derived mesenchymal stem cells secrete extracellular vesicles: A potential cell-free therapy for canine renal ischaemia-reperfusion injury. Vet. Med. Sci. 2023, 9, 1134–1142. [Google Scholar] [CrossRef]
- Yoshizaki, K.; Nishida, H.; Tabata, Y.; Jo, J.I.; Nakase, I.; Akiyoshi, H. Controlled release of canine MSC-derived extracellular vesicles by cationized gelatin hydrogels. Regen. Ther. 2023, 22, 1–6. [Google Scholar] [CrossRef]
- Sung, S.E.; Seo, M.S.; Kang, K.K.; Choi, J.H.; Lee, S.; Sung, M.; Kim, K.; Lee, G.W.; Lim, J.H.; Yang, S.Y.; et al. Mesenchymal stem cell exosomes derived from feline adipose tissue enhance the effects of anti-inflammation compared to fibroblasts-derived exosomes. Vet. Sci. 2021, 8, 182. [Google Scholar] [CrossRef]
- Villatoro, A.J.; Martín-Astorga, M.D.C.; Alcoholado, C.; Cárdenas, C.; Fariñas, F.; Becerra, J.; Visser, R. Altered proteomic profile of adipose tissue-derived mesenchymal stem cell exosomes from cats with severe chronic gingivostomatitis. Animals 2021, 11, 2466. [Google Scholar] [CrossRef]
- Villatoro, A.J.; Martín-Astorga, M.D.C.; Alcoholado, C.; Sánchez-Martín, M.D.M.; Becerra, J. Proteomic analysis of the secretome and exosomes of feline adipose-derived mesenchymal stem cells. Animals 2021, 11, 295. [Google Scholar] [CrossRef]
- Li, D.; Luo, H.; Ruan, H.; Chen, Z.; Chen, S.; Wang, B.; Xie, Y. Isolation and identification of exosomes from feline plasma, urine and adipose-derived mesenchymal stem cells. BMC Vet. Res. 2021, 17, 272. [Google Scholar] [CrossRef]
- Li, W.; Wang, W.; He, X.; Liao, Z.; Aierken, A.; Hua, J.; Wang, Y.; Lu, D.; Zhang, S. Rapid recovery of male cats with postrenal acute kidney injury by treating with allogeneic adipose mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res. Ther. 2022, 13, 379. [Google Scholar] [CrossRef]
- von Stade, D.; Meyers, M.; Johnson, J.; Schlegel, T.T.; Romeo, A.; Regan, D.; McGilvray, K. Exosome Cell Origin Affects In Vitro Markers of Tendon Repair in Ovine Macrophages and Tenocytes. Tissue Eng. Part A 2023, 29, 282–291. [Google Scholar] [CrossRef]
- De Coppiz, P.; Bartsch, G.J.; Siddiqui, M.M.; Xu, T.; Santos, C.C.; Perin, L.; Mostoslavsky, G.; Serre, A.C.; Snyder, E.Y.; Yoo, J.J.; et al. Isolation of amniotic stem cell lines with potential for therapy. Nat. Biotechnol. 2007, 25, 100–106. [Google Scholar] [CrossRef]
- Delo, D.M.; De Coppi, P.; Bartsch, G.J.; Atala, A. Amniotic fluid e placental stem cells. Methods Enzymol. 2006, 419, 426–438. [Google Scholar]
- Gucciardo, L.; Lories, R.; Ochsenbein-Kolble, N.; Done, E.; Zwijsen, A.; Depresta, J. Fetal mesenchymal stem cells: Isolation, properties e potential use in perinatology e regenerative medicine. BJOG 2009, 116, 166–172. [Google Scholar] [CrossRef]
- In’t Anker, P.S.; Scherjon, S.A.; Kleijburg-van der Keur, C.; De Groot Swings, G.M.; Claas, F.H.; Fibbe, W.E.; Kanhai, H.H.H. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 2004, 22, 1338–1345. [Google Scholar] [CrossRef]
- Kern, S.; Eichler, H.; Stoeve, J.; Kluter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef]
- Kögler, G.; Sensken, S.; Airey, J.A.; Trapp, T.; Mschen, M.; Feldhahn, N.; Liedtke, S.; Sorg, R.V.; Fischer, J.; Rosenbaum, C.; et al. New human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J. Exp. Med. 2004, 200, 123–135. [Google Scholar] [CrossRef]
- Lange-Consiglio, A.; Rossi, D.; Tassan, S.; Perego, R.; Cremonesi, F.; Parolini, O. Conditioned medium from horse amniotic membrane-derived multipotent progenitor cells: Immunomodulatory activity in vitro and first clinical application in tendon and ligament injuries in vivo. Stem Cells Dev. 2013, 22, 3015–3024. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.L.; Persaud, T.V.N. The developing human: Clinically oriented embryology. 1998, Sanders.
- Evangelista, M.; Soncini, M.; Parolini, O. Placenta-derived stem cells: New hope for cell therapy? Cytotechnology 2008, 58, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Zucca, E.; Corsini, E.; Galbiati, V.; Lange-Consiglio, A.; Ferrucci, F. Evaluation of amniotic mesenchymal cell derivatives on cytokine production in equine alveolar macrophages: An in vitro approach to lung inflammation. Stem Cell Res. Ther. 2016, 7, 137. [Google Scholar] [CrossRef] [PubMed]
- Perrini, C.; Strillacci, M.G.; Bagnato, A.; Esposti, P.; Marini, M.G.; Corradetti, B.; Bizzaro, D.; Idda, A.; Ledda, S.; Capra, E.; et al. Microvesicles secreted from equine amniotic-derived cells and their potential role in reducing inflammation in endometrial cells in an in-vitro model. Stem Cell Res. Ther. 2016, 7, 169. [Google Scholar] [CrossRef]
- Lange-Consiglio, A.; Lazzari, B.; Perrini, C.; Pizzi, F.; Stella, A.; Cremonesi, F.; Capra, E. MicroRNAs of equine amniotic mesenchymal cell-derived microvesicles and their involvement in anti-inflammatory processes. Cell Transplant. 2018, 27, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Lange-Consiglio, A.; Perrini, C.; Tasquier, R.; Deregibus, M.C.; Camussi, G.; Pascucci, L.; Marini, M.G.; Corradetti, B.; Bizzaro, D.; De Vita, B.; et al. Equine amniotic microvesicles and their anti-inflammatory potential in a tenocyte model in vitro. Stem Cells Dev. 2016, 25, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Desantis, S.; Accogli, G.; Albrizio, M.; Rossi, R.; Cremonesi, F.; Lange Consiglio, A. Glycan profiling analysis of Equine amniotic progenitor mesenchymal cells and their derived extracellular microvesicles. Stem Cells Dev. 2019, 28, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Crain, S.K.; Robinson, S.R.; Thane, K.E.; Davis, A.M.; Meola, D.M.; Barton, B.A.; Yang, V.K.; Hoffman, A.M. Extracellular vesicles from Wharton’s Jelly mesenchymal stem cells suppress CD4 expressing T Cells through Transforming Growth Factor Beta and Adenosine Signaling in a canine model. Stem Cells Dev. 2019, 28, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.; Snyder, O.L.; He, H.; Christenson, L.K.; Fleming, S.; Weiss, M.L. Procoagulant Activity of Umbilical Cord-Derived Mesenchymal Stromal Cells’ Extracellular Vesicles (MSC-EVs). Int. J. Mol. Sci. 2023, 24, 9216. [Google Scholar] [CrossRef] [PubMed]
- Karam, R.G.; Motta, L.C.B.; de Almeida, M.F.; Bridi, A.; da Silveira, J.C.; Ambrósio, C.E. Secretion pattern of canine amniotic stem cells derived extracellular vesicles. Anim. Reprod. 2022, 19, e20220063. [Google Scholar] [CrossRef]
- Scassiotti, R.F.; de Paula Coutinho, M.; Pinto Santos, S.I.; Ferreira Pinto, P.A.; Ferreira de Almeida, M.; Karam, R.G.; da Silva Rosa, P.M.; Dos Santos Martins, D.; Coelho da Silveira, J.; Ambrósio, C.E. Adipose and amnion-derived mesenchymal stem cells: Extracellular vesicles characterization and implication for reproductive biotechnology. Theriogenology 2023, 198, 264–272. [Google Scholar] [CrossRef]
- Pastore, S.; Troisi, A.; Romani, R.; Bellezza, I.; Gargaro, M.; De Michele, A.; Orlandi, R.; Guerrera, G.; Bazzano, M.; Polisca, A. Isolation of extracellular vesicles from bitch’s amnion-derived cells culture and their CD59 expression: Preliminary results. Theriogenology 2023, 198, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Ophelders, D.R.; Wolfs, T.G.; Jellema, R.K.; Zwanenburg, A.; Andriessen, P.; Delhaas, T.; Ludwig, A.K.; Radtke, S.; Peters, V.; Janssen, L.; et al. Mesenchymal stromal cell-derived extracellular vesicles protect the fetal brain after hypoxia-ischemia. Stem Cells Transl. Med. 2016, 5, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Jenner, F.; Wagner, A.; Gerner, I.; Ludewig, E.; Trujanovic, R.; Rohde, E.; von Rechenberg, B.; Gimona, M.; Traweger, A. Evaluation of the potential of umbilical cord mesenchymal stromal cell–derived small extracellular vesicles to improve rotator cuff healing: A pilot ovine study. Am. J. Sports Med. 2023, 51, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Homma, K.; Bazhanov, N.; Hashimoto, K.; Shimizu, M.; Heathman, T.; Hao, Q.; Nawgiri, R.; Muthukumarana, V.; Lee, J.W.; Prough, D.S.; et al. Mesenchymal stem cell-derived exosomes for treatment of sepsis. Front. Immunol. 2023, 14, 1136964. [Google Scholar] [CrossRef] [PubMed]
- Lange-Consiglio, A.; Lazzari, B.; Pizzi, F.; Idda, A.; Cremonesi, F.; Capra, E. Amniotic microvesicles impact hatching and pregnancy percentages of in vitro bovine embryos and blastocyst microRNA expression versus in vivo controls. Sci. Rep. 2020, 10, 501. [Google Scholar] [CrossRef] [PubMed]
- Lange-Consiglio, A.; Gusmara, C.; Manfredi, E.; Idda, A.; Soggiu, A.; Greco, V.; Bonizzi, L.; Cremonesi, F.; Zecconi, A. Antimicrobial effects of conditioned medium from amniotic progenitor cells in vitro and in vivo: Toward tissue regenerative therapies for bovine mastitis. Front. Vet. Sci. 2019, 6, 443. [Google Scholar] [CrossRef] [PubMed]
- Lange-Consiglio, A.; Funghi, F.; Cantile, C.; Idda, A.; Cremonesi, F.; Riccaboni, P. Case report: Use of amniotic microvesicles for regenerative medicine treatment of a mare with chronic endometritis. Front. Vet. Sci. 2020, 7, 347. [Google Scholar] [CrossRef] [PubMed]
- Lange-Consiglio, A.; Gaspari, G.; Funghi, F.; Capra, E.; Cretich, M.; Frigerio, R.; Bosi, G.; Cremonesi, F. Amniotic Mesenchymal-Derived Extracellular Vesicles and Their Role in the Prevention of Persistent Post-Breeding Induced Endometritis. Int. J. Mol. Sci. 2023, 24, 5166. [Google Scholar] [CrossRef] [PubMed]
- Mocchi, M.; Bari, E.; Marrubini, G.; Bonda, A.F.; Perteghella, S.; Tartara, F.; Cofano, F.; di Perna, G.; Giovannelli, L.; Mandracchia, D.; et al. Freeze-Dried Mesenchymal Stem Cell-Secretome pharmaceuticalization: Optimization of formulation and manufacturing process robustness. Pharmaceutics 2021, 13, 1129. [Google Scholar] [CrossRef]
- Bemis, L.T.; McCue, P.M.; Hatzel, J.N.; Bemis, J.; Ferris, R.A. Evidence for production of early pregnancy factor (Hsp10), micro RNAs and exosomes by day 8 equine embryos. 8th ISEET Abstracts. J. Equine Vet. Sci. 2012, 32, 398. [Google Scholar] [CrossRef]
- Ng, Y.H.; Rome, S.; Jalabert, A.; Forterre, A.; Singh, H.; Hincks, C.L.; Salamonsen, L.A. Endometrial exosomes/microvesicles in the uterine microenvironment: A new paradigm for embryo-endometrial cross talk at implantation. PLoS ONE 2013, 8, e58502. [Google Scholar] [CrossRef]
- Rebordao, M.R.; Amaral, A.; Lukasik, K.; Szostek-Mioduchowska, A.; Pinto-Bravo, P.; Galvao, A.; Skarzynski, D.J.; Ferreira-Dias, G. Impairment of the antifi-brotic prostaglandin E2 pathway may influence neutrophil extracellular trapsinduced fibrosis in the mare endometrium. Domest. Anim. Endocrinol. 2019, 67, 1–10. [Google Scholar] [CrossRef]
- Volk, S.W.; Bohling, M.W. Comparative wound healing--are the small animal veterinarian’s clinical patients an improved translational model for human wound healing research? Wound Repair Regen. 2013, 21, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Iacono, E.; Lanci, A.; Merlo, B.; Ricci, F.; Pirrone, A.; Antonelli, C.; Mariella, J.; Castagnetti, C. Effects of amniotic fluid mesenchymal stem cells in carboxymethyl cellulosegel on healing of spontaneous pressure sores: Clinical outcome in seven hospitalized neonatal foals. Turk. J. Biol. 2016, 40, 484–492. [Google Scholar] [CrossRef]
- Lanci, A.; Merlo, B.; Mariella, J.; Castagnetti, C.; Iacono, E. Heterologous Wharton’s jelly derived mesenchymal stem cells application on a large chronic skin wound in a 6-month-old filly. Front. Vet. Sci. 2019, 6, 9. [Google Scholar] [CrossRef]
- Enciso, N.; Avedillo, L.; Fermín, M.L.; Fragío, C.; Tejero, C. Cutaneous wound healing: Canine allogeneic ASC therapy. Stem Cell Res. Ther. 2020, 11, 261. [Google Scholar] [CrossRef]
- Cheng, Y.; Cao, X.; Qin, L. Mesenchymal Stem Cell-Derived Extracellular Vesicles: A Novel Cell-Free Therapy for Sepsis. Front. Immunol. 2020, 11, 647. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, T.; Saleem, A.; Farooq, N.; Dar, L.A.; Nazir, J.; Saleem, S.; Ismail, S.; Gugjoo, M.B.; Shiekh, P.A.; Ahmad, S.M. Extracellular vesicles derived from mesenchymal stem cells—A novel therapeutic tool in infectious diseases. Inflamm. Regen. 2023, 43, 17. [Google Scholar] [CrossRef]
- Palma, E.; Tilocca, B.; Roncada, P. Antimicrobial resistance in veterinary medicine: An overview. Int. J. Mol. Sci. 2020, 21, 1914. [Google Scholar] [CrossRef] [PubMed]
- Indira, V.; Corteling, R.; Stevanato, L.; Hicks, C.; Sinden, J. The development of stem cell-derived exosomes as a cell-free regenerative medicine. J. Circ. Biomark. 2014, 3, 2. [Google Scholar]
- Pollock, K.; Stroemer, P.; Patel, S.; Stevanato, L.; Hope, A.; Miljan, E.; Dong, Z.; Hodges, H.; Price, J.; Sinden, J.D. A conditionally immortal clonal stem cell line from human cortical neuroepithelium for the treatment of ischemic stroke. Exp. Neurol. 2006, 199, 143–155. [Google Scholar] [CrossRef]
- Yeo, R.W.; Lai, R.C.; Zhang, B.; Tan, S.S.; Yin, Y.; Teh, B.J.; Lim, S.K. Mesenchymal stem cell: An efficient mass producer of exosomes for drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Haraszti, R.A.; Miller, R.; Stoppato, M.; Sere, Y.Y.; Coles, A.; Didiot, M.C.; Wollacott, R.; Sapp, E.; Dubuke, M.L.; Li, X.; et al. Exosomes produced from 3D cultures of MSCs by tangential flow filtration show higher yield and improved activity. Mol. Ther. 2018, 26, 2838–2847. [Google Scholar] [CrossRef]
- Nordin, J.Z.; Lee, Y.; Vader, P.; Mager, I.; Johansson, H.J.; Heusermann, W.; Wiklander, O.P.B.; Hällbrink, M.; Seow, Y.; Bultema, J.J.; et al. Ultrafiltration with size-exclusion liquid chromatography for high yield isolation of extracellular vesicles preserving intact biophysical and functional properties. Nanomedicine 2015, 11, 879–883. [Google Scholar] [CrossRef]
- Benedikter, B.J.; Bouwman, F.G.; Vajen, T.; Heinzmann, A.C.A.; Grauls, G.; Mariman, E.C.; Wouters, E.F.M.; Savelkoul, P.H.; Lopez-Iglesias, C.; Koenen, R.R.; et al. Ultrafiltration combined with size exclusion chromatography efficiently isolates extracellular vesicles from cell culture media for compositional and functional studies. Sci. Rep. 2017, 7, 15297. [Google Scholar] [CrossRef]
- Mol, E.A.; Goumans, M.J.; Doevendans, P.A.; Sluijter, J.P.G.; Vader, P. Higher functionality of extracellular vesicles isolated using sizeexclusion chromatography compared to ultracentrifugation. Nanomedicine 2017, 13, 2061–2065. [Google Scholar] [CrossRef] [PubMed]
- Monguio-Tortajada, M.; Galvez-Monton, C.; Bayes-Genis, A.; Roura, S.; Borras, F.E. Extracellular vesicle isolation methods: Rising impact of size-exclusion chromatography. Cell Mol. Life Sci. 2019, 76, 2369–2382. [Google Scholar] [CrossRef] [PubMed]
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]
- Pachler, K.; Lener, T.; Streif, D.; Dunai, Z.A.; Desgeorges, A.; Feichtner, M.; Öller, M.; Schallmoser, K.; Rohde, E.; Gimona, M. A good manufacturing prac-tice-grade standard protocol for exclusively human mesenchymal stromal cell-derived extracellular vesicles. Cytotherapy 2017, 19, 458–472. [Google Scholar] [CrossRef]
- Burrello, J.; Monticone, S.; Gai, C.; Gomez, Y.; Kholia, S.; Camussi, G. Stem cell-derived extracellular vesicles and immune-modulation. Front. Cell Dev. Biol. 2016, 4, 83. [Google Scholar] [CrossRef]
| Animal | Source of EVs | District | Application | Mode of Administration | Reference |
|---|---|---|---|---|---|
| Pig | pBM-MSCs | Respiratory | Influenza virus | Intratracheal | [70] |
| pAT-MSCs | Cardiovascular | Myocardial infarction | Intraoperative insertion of EVs combined with biocompatible cardiac scaffolds | [81] | |
| pBM-MSCs | Cardiovascular | Myocardial infarction | Intraoperative insertion of EV collagen patch | [90] | |
| pAT-MSCs | Urinary | Model of metabolic syndrome and renal artery stenosis in cardiovascular complications | Intrarenal injection | [67,68,75,77,78,79,80,82,83] | |
| pAT-MSCs | Vertebral column | Inducted spinal cord injury | Intrathecal injection | [89] | |
| pAT-MSCs | Hepatic | Inducted liver injury (hemi-hepatectomy and hepatic ischemia-reperfusion injury) | Intravenous | [87,88] | |
| Sheep | hBM-MSCs | Neurological | Hypoxic ischemic encephalopathy (HIE) | Intravenous | [143] |
| hUC-MSCs | Musculoskeletal | Ligament injury | Application onto a type 1 collagen sponge | [144] | |
| hBM-MSCs | Systemic | Pneumonia/sepsis | Intravenous | [145] |
| Animal | Source of EVs | Application Culture | Effects | Reference |
|---|---|---|---|---|
| Horse | eAM-MSCs | Endometrial cells | reduced the apoptosis rate, increased cell proliferation values, downregulated pro-inflammatory gene expression, and decreased the secretion of pro-inflammatory cytokines | [134] |
| eAM-MSCs | Tenocytes | induced a down-regulation of MMP1, MMP9, MMP13 and TNFα expression | [130] | |
| eBM-MSCs eAT-MSCs ad Syn Fluid | Chondrocytes | reduced inflammation | [59] | |
| eBM-MSCs | Chondrocytes | increased the articular chondrocyte collagen protein amounts, mRNA levels of Prg4, and enhanced the proliferation and migratory capacities of chondrocytes | [99] | |
| eBM-MSCs (autologous) | Chondrocytes | anti-inflammatory effects on gene expression following chondrocyte exposure to tumor necrosis factor α and Interleukin 1β | [97] | |
| eBM-MSCs | Chondrocytes | induced a greater increase in equine articular chondrocyte-neosynthesized hyaline-like matrix by modulating collagen levels, increasing PCNA, and decreasing Htra1 synthesis | [104] | |
| eAM-MSCs | Alveolar macrophages | Modulatory-effect release of TGF-alfa and β and possibly IL-6 | [133] | |
| Bovine | bAM-MSCs | Blastocysts | addition of EVs during in vitro embryo production seemed to influence the developmental capacity and implantation potential of the embryos and regulate the expression of specific miRNAs that regulated blastocyst development | [146] |
| bAM-MSCs (CM) | Mammary epithelial cells | could attenuate bacterial growth, as evaluated by the number of CFUs. After 24 h of culture with S. aureus, 89.67% of mammary epithelial cells treated were still alive, whereas all cells cultured and not treated were dead | [147] | |
| Dog | cWJ-MSCs | Fibroblasts | suppressed the proliferation of cell T CD4+ using TGF-β and adenosin | [138] |
| cBM-MSCs | Murine microglia cells | decreased inflammation (decrease IL-1β) | [113] | |
| cAT-MSCs (Lyosecretoma) | Tenocytes, chondrocytes and AT-MSCs | induced proliferation of cells in dose-dependent manner and showed anti-elastase activity | [98] | |
| cAM-MSCs | Coculture with AM-MSCs and AT-MSCs | 15–20% increased expansion rate | [141] | |
| cAT-MSCs | Semen during cryopreservation | initiated damaged-sperm repair (higher motility, live sperm percentage, membrane and acrosome integrity; higher expression of genes related to the repair of plasma membrane and chromatin material) and decreased reactive oxygen species production | [106] | |
| Cat | fAT-MSCs and fibroblasts | Human THP-1 Macrophages | MSCs-EVs had lower levels of pro-inflammatory cytokines (IL-1β, TNF-α) and higher level of IL-10. MSCs-EVs played a crucial role in immune defense compared with EVs–fibroblasts | [118] |
| Animal | Source of EVs | District | Application | Mode of Administration | Reference |
|---|---|---|---|---|---|
| Horse | eAT-MSCs | Musculoskeletal | Ligament injury | Ultrasound-guided injection at the injury site | [96] |
| eAT-MSCs | Musculoskeletal | Osteoarthritis | Intra-articular injection | [101] | |
| eAM-MSCs | Reproductive | Chronic endometritis | Intrauterine | [148] | |
| eBM-MSCs | Reproductive | Chronic endometritis | Intrauterine | [103] | |
| eAM-MSCs (CM) | Reproductive | Persistent post-breeding-induced endometritis | Intrauterine | [149] | |
| Cattle | bAM-MSCs | Reproductive | Blastocyst development | In vitro during embryo production | [146] |
| bAM-MSCs (CM) | Mammary gland | Acute and chronic mastitis | Intramammarily | [147] | |
| Dog | cBM-MSCs | Skin | Inducted skin wound | Subcutaneous injection | [105] |
| cAT-MSCs (Lyosectetoma) | Musculoskeletal | Osteoarthritis | Intra-articular injection | [98] | |
| cAT-MSCs | Urinary | Renal ischemia-reperfusion injury | Renal cortex injection | [116] | |
| Cat | fAT-MSCs | Urinary | Post-renal acute kidney injury (PR-AKI) | Intravenous | [122] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanci, A.; Iacono, E.; Merlo, B. Therapeutic Application of Extracellular Vesicles Derived from Mesenchymal Stem Cells in Domestic Animals. Animals 2024, 14, 2147. https://doi.org/10.3390/ani14152147
Lanci A, Iacono E, Merlo B. Therapeutic Application of Extracellular Vesicles Derived from Mesenchymal Stem Cells in Domestic Animals. Animals. 2024; 14(15):2147. https://doi.org/10.3390/ani14152147
Chicago/Turabian StyleLanci, Aliai, Eleonora Iacono, and Barbara Merlo. 2024. "Therapeutic Application of Extracellular Vesicles Derived from Mesenchymal Stem Cells in Domestic Animals" Animals 14, no. 15: 2147. https://doi.org/10.3390/ani14152147
APA StyleLanci, A., Iacono, E., & Merlo, B. (2024). Therapeutic Application of Extracellular Vesicles Derived from Mesenchymal Stem Cells in Domestic Animals. Animals, 14(15), 2147. https://doi.org/10.3390/ani14152147






