Integrating 16S rRNA Sequencing and LC-MS-Based Metabolomics to Evaluate the Effects of Dietary Crude Protein on Ruminal Morphology, Fermentation Parameter and Digestive Enzyme Activity in Tibetan Sheep
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal and Experimental Design
2.2. Sample Collection
2.3. Rumen Epithelial Morphology
2.4. Fermentation Parameters
2.5. Digestive Enzyme Activities
2.6. Microbiome Composition Analysis
2.7. Metabolomics Data Analysis
2.8. Statistical Analysis
3. Results
3.1. Histological Analysis of the Rumen
3.2. Rumen Fermentation Parameter
3.3. The Activity of Digestive Enzymes in the Rumen
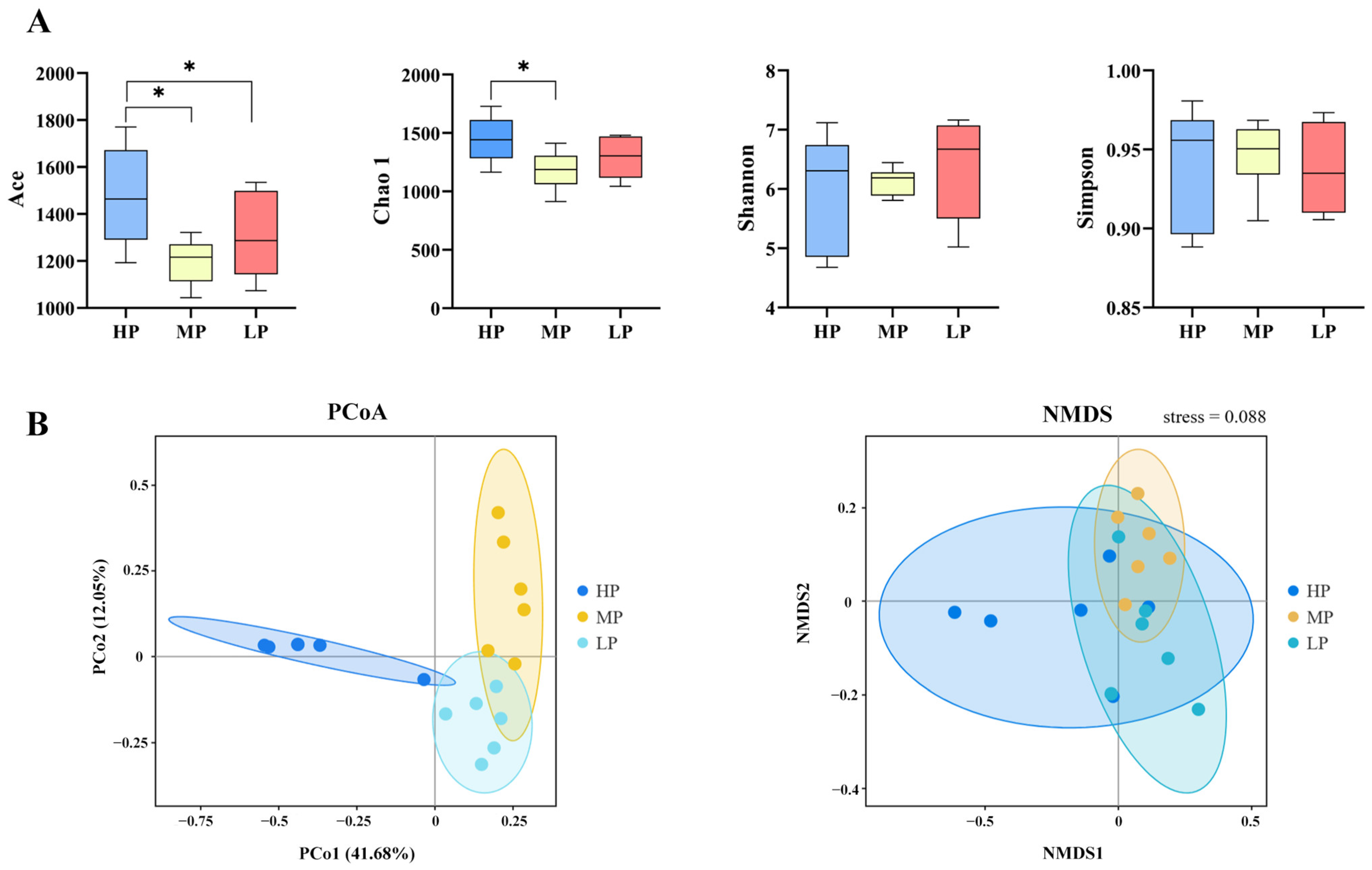
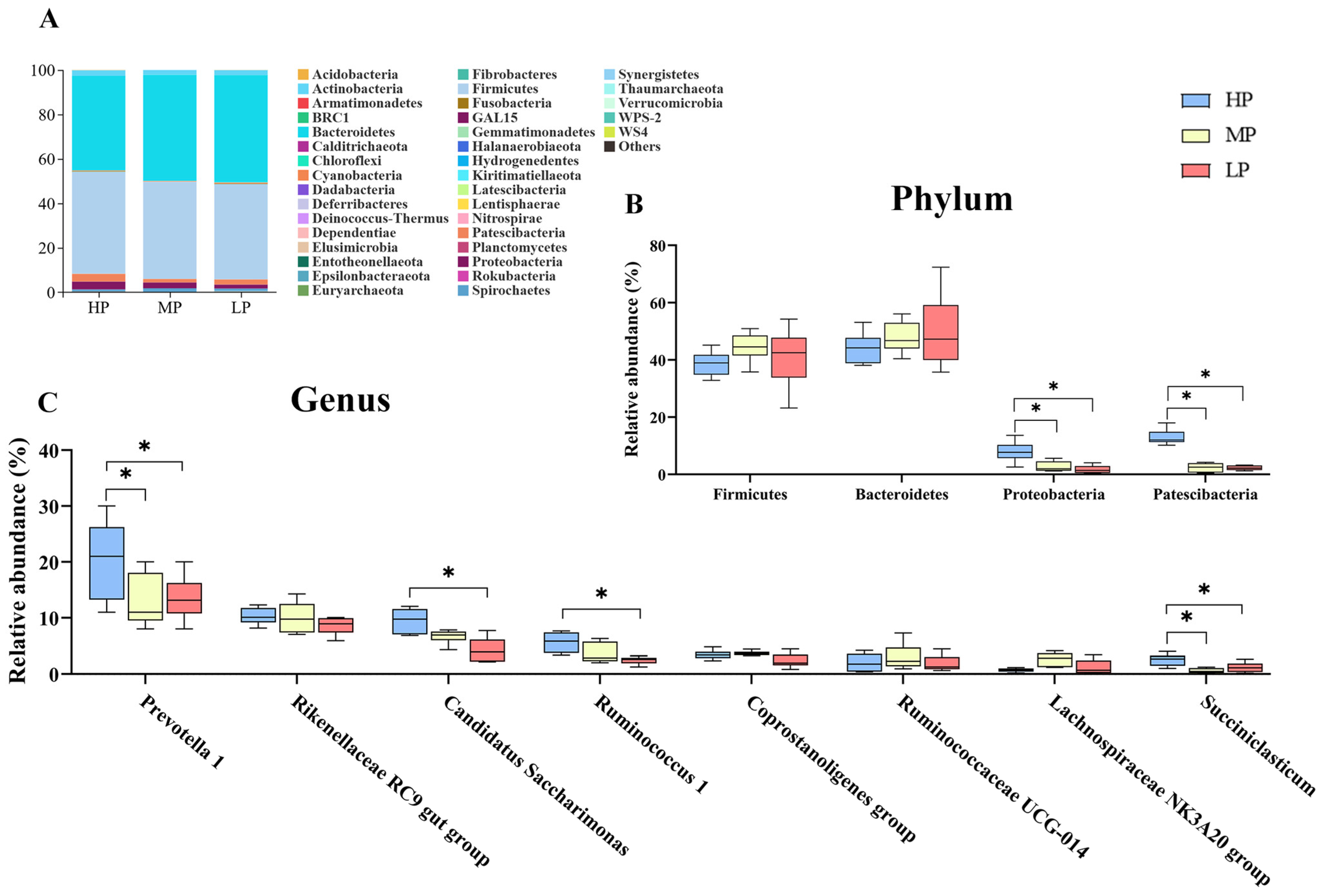
3.4. Bacterial Community
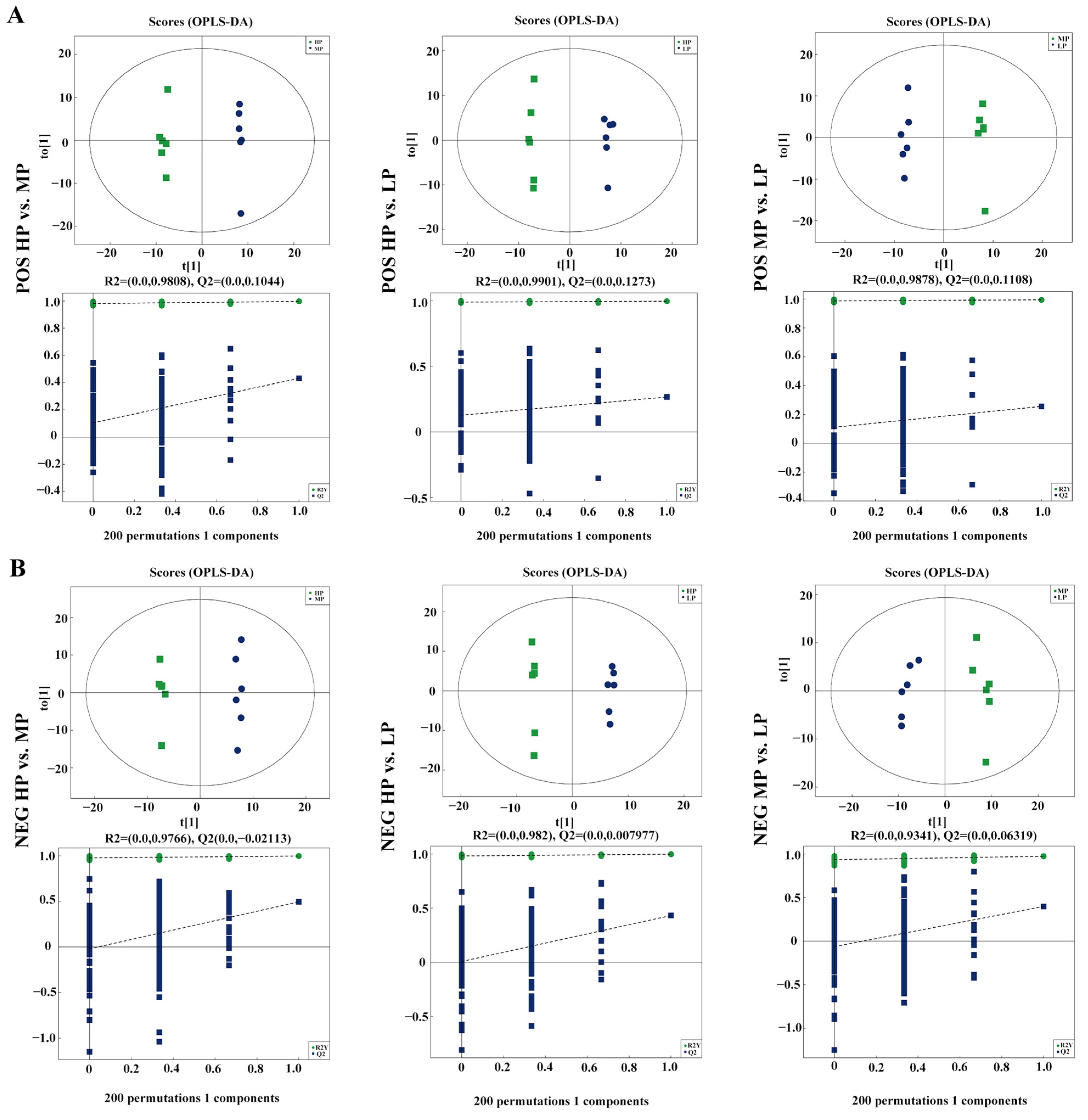
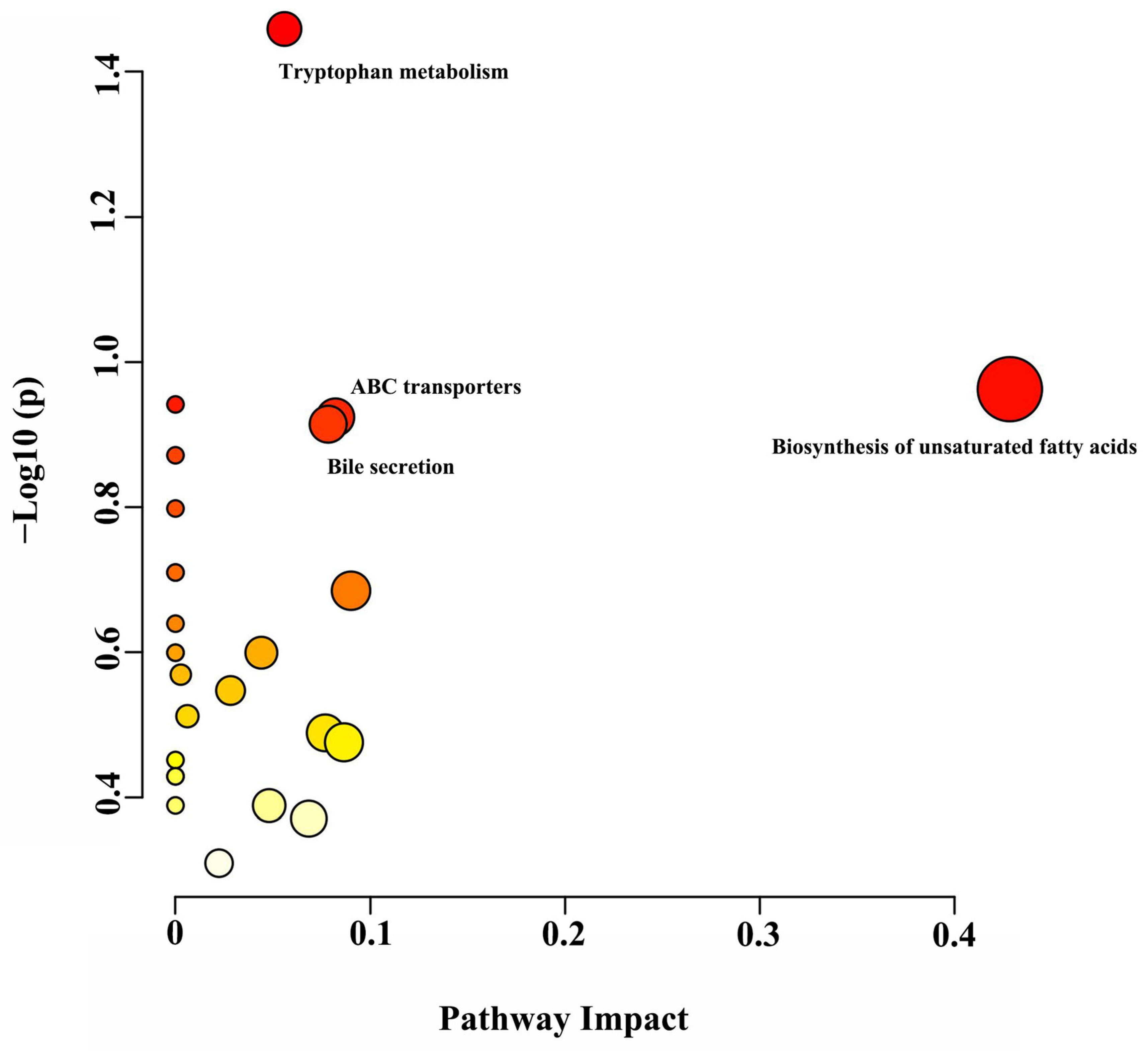
3.5. Metabolomics Profiling of Rumen
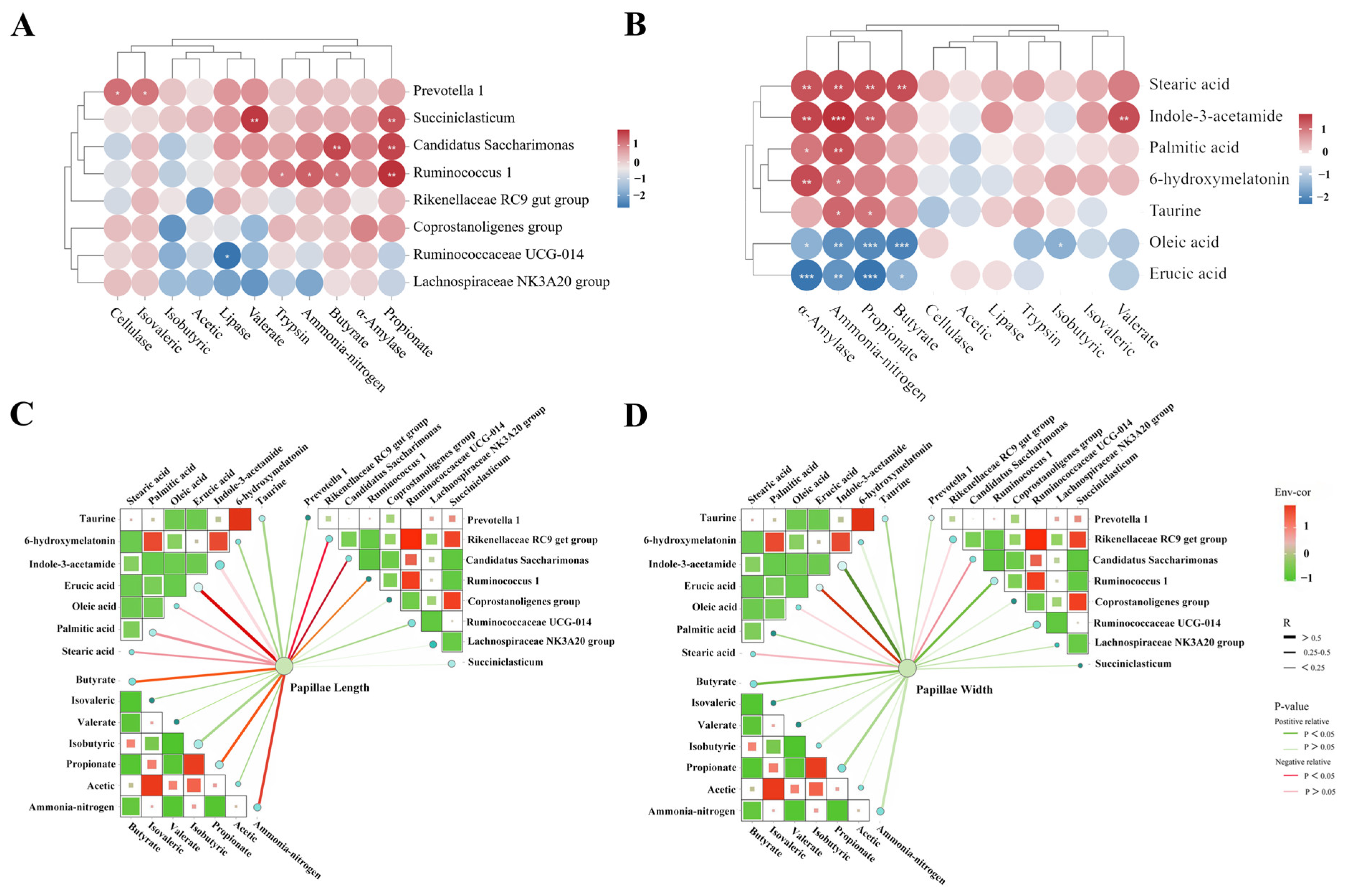
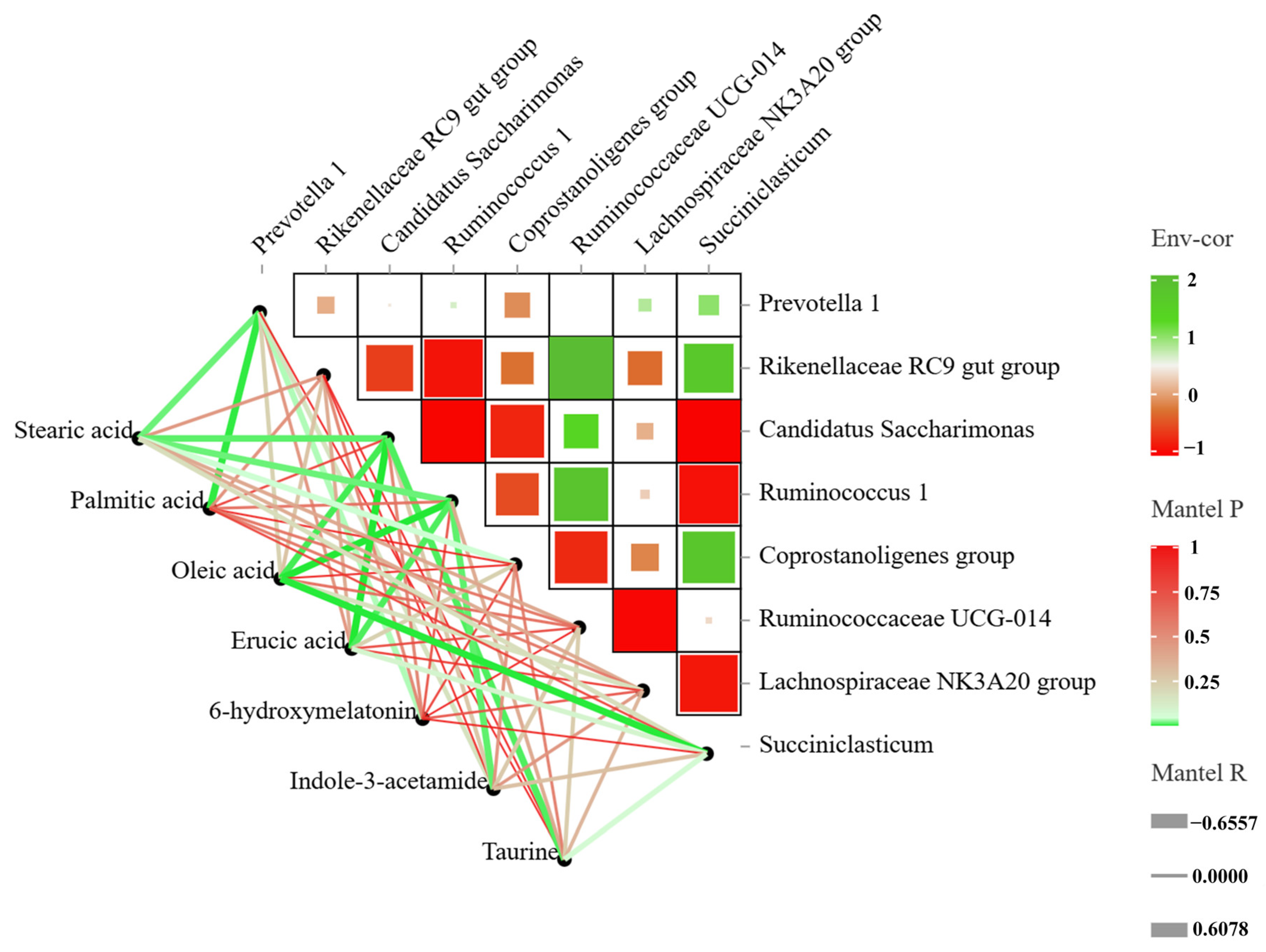
3.6. Correlation Analysis
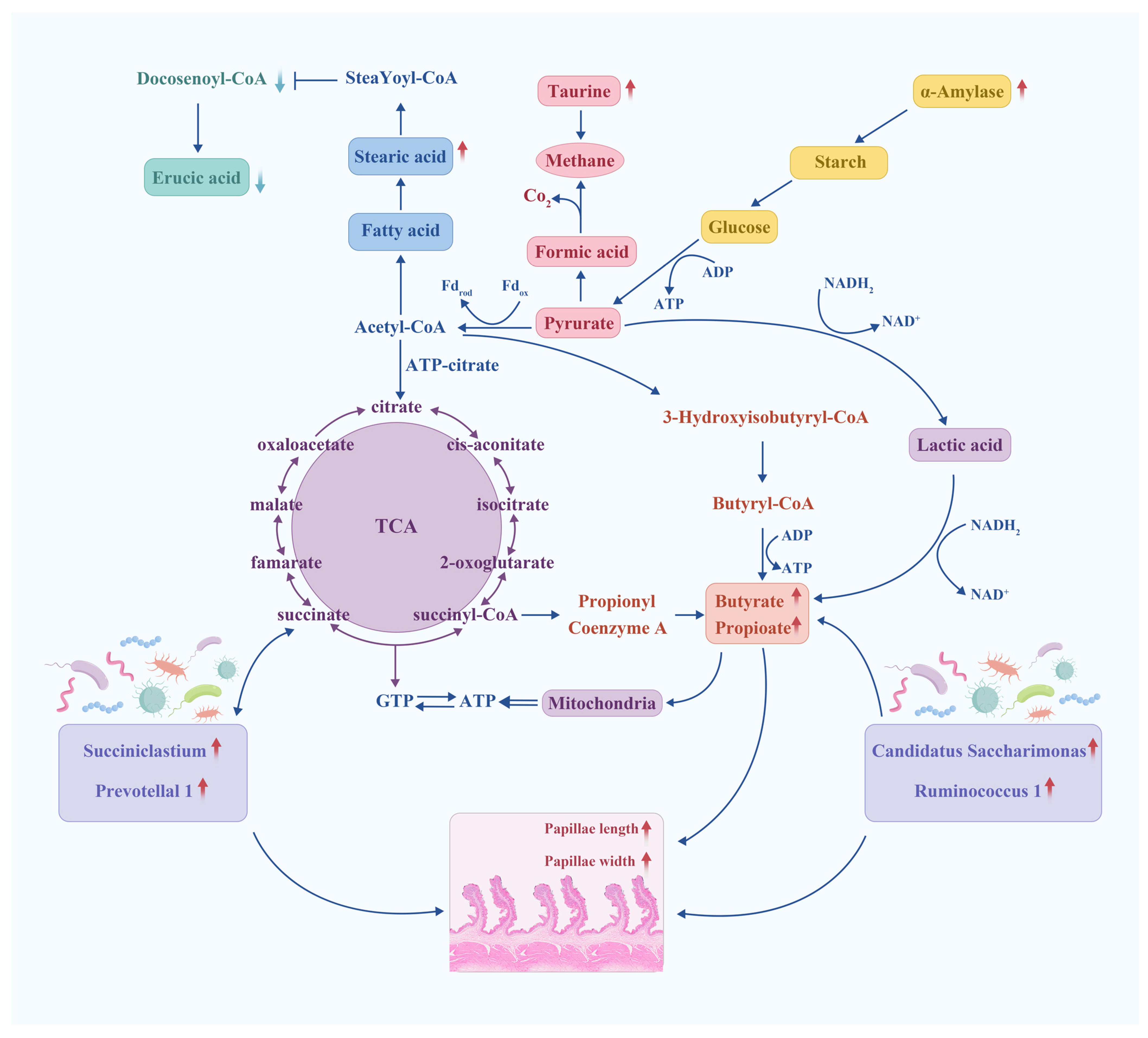
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, J.W.; Guo, X.S.; Degen, A.A.; Zhang, Y.; Liu, H.; Mi, J.D.; Ding, L.M.; Wang, H.C.; Qiu, Q.; Long, R.J. Urea kinetics and nitrogen balance and requirements for maintenance in Tibetan sheep when fed oat hay. Small Rumin. Res. 2015, 129, 60–68. [Google Scholar] [CrossRef]
- Sun, Y.; Angerer, J.; Hou, F.J. Effects of grazing systems on herbage mass and liveweight gain of Tibetan sheep in Eastern Qinghai-Tibetan Plateau, China. Rangel. J. 2015, 37, 181–190. [Google Scholar] [CrossRef]
- Dai, R.; Ma, X.; Dingkao, R.; Huang, C.; La, Y.; Li, X.; Ma, X.; Wu, X.; Chu, M.; Guo, X.; et al. Effects of dietary crude protein levels in the concentrate supplement after grazing on rumen microbiota and metabolites by using metagenomics and metabolomics in Jersey-yak. Front. Microbiol. 2023, 14, 1124917. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.Y.; Xie, Y.Y.; Zhong, Y.; Ma, X.J.; Liu, J.X. Integrated meta-omics reveals new ruminal microbial features associated with feed efficiency in dairy cattle. Microbiome 2022, 10, 32. [Google Scholar] [CrossRef]
- Pokhrel, B.; Jiang, H. Postnatal Growth and Development of the Rumen: Integrating Physiological and Molecular Insights. Biology 2024, 13, 269. [Google Scholar] [CrossRef] [PubMed]
- Saleem, F.; Bouatra, S.; Guo, A.C.; Psychogios, N.; Wishart, D.S. The Bovine Ruminal Fluid Metabolome. Metabolomics 2012, 9, 360–378. [Google Scholar] [CrossRef]
- Xu, C.; Liu, W.; Sun, B.; Zhang, S.; Zhang, S.; Yang, Y.; Lei, Y.; Chang, L.; Xie, P.; Suo, H. Multi-Omics Analysis Reveals a Dependent Relationship Between Rumen Bacteria and Diet of Grass- and Grain-Fed Yaks. Front. Microbiol. 2021, 12, 642959. [Google Scholar] [CrossRef]
- Huang, C.; Yao, X.; Ge, F.; Guo, X.; Liang, C.J. Microbiome and Metabolomics Reveal the Effects of Different Feeding Systems on the Growth and Ruminal Development of Yaks. Front. Microbiol. 2021, 12, 682989. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, P.; Xiao, Y.; Liu, J.; Chen, Y.; Yang, Y. Alterations in Rumen Bacterial Community and Metabolome Characteristics of Cashmere Goats in Response to Dietary Nutrient Density. Animals 2020, 10, 1193. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Broderick, G.A.; Kang, J.H. Automated Simultaneous Determination of Ammonia and Total Amino Acids in Ruminal Fluid and In Vitro Media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2. WIREs Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Zhang, X.; Han, L.; Hou, S.; Raza, S.H.A.; Wang, Z.; Yang, B.; Sun, S.; Ding, B.; Gui, L.; Simal-Gandara, J.; et al. Effects of different feeding regimes on muscle metabolism and its association with meat quality of Tibetan sheep. Food Chem. 2022, 374, 131611. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Xu, W.; Wei, C.; Zhang, Z.; Jiang, C.; Chen, X. Effects of Decreasing Dietary Crude Protein Level on Growth Performance, Nutrient Digestion, Serum Metabolites, and Nitrogen Utilization in Growing Goat Kids (Capra hircus). Animals 2020, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Wang, X.; Li, J.Z.; Huang, P.F.; Li, Y.L.; Ding, X.Q.; Huang, J.; Zhu, M.Z.; Yin, J.; Dai, C.P.; et al. The impact of lactating Hu sheep’s dietary protein levels on lactation performance, progeny growth and rumen development. Anim. Biotechnol. 2023, 34, 1919–1930. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.; Chai, S.; Yang, Y.; Wang, X.; Liu, S.; Wang, S. Dietary forage to concentrate ratios impact on yak ruminal microbiota and metabolites. Front. Microbiol. 2022, 13, 964564. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Liu, T.; Deng, J.; Wei, C.C.; Zhang, Z.J.; Wang, D.M.; Chen, X.Y. Microbiome-metabolomics analysis of the effects of decreasing dietary crude protein content on goat rumen mictobiota and metabolites. Anim. Biosci. 2022, 35, 1535–1544. [Google Scholar] [CrossRef]
- Ren, X.D.; Chen, X.S.; Tang, L.; Zeng, X.; Wang, L.; Mao, Z.G. Physiological mechanism of the overproduction of ε-poly-L-lysine by acidic pH shock in fed-batch fermentation. Bioprocess Biosyst. Eng. 2015, 38, 2085–2094. [Google Scholar] [CrossRef]
- Fei, Q.; Fu, R.; Shang, L.; Brigham, C.J.; Chang, H.N. Lipid production by microalgae Chlorella protothecoides with volatile fatty acids (VFAs) as carbon sources in heterotrophic cultivation and its economic assessment. Bioprocess Biosyst. Eng. 2015, 38, 691–700. [Google Scholar] [CrossRef]
- Fei, Q.; Chang, H.N.; Shang, L.; Choi, J.-D.; Kim, N.; Kang, J. The effect of volatile fatty acids as a sole carbon source on lipid accumulation by Cryptococcus albidus for biodiesel production. Bioresour. Technol. 2011, 102, 2695–2701. [Google Scholar] [CrossRef]
- Sha, Y.; He, Y.; Liu, X.; Zhao, S.; Hu, J.; Wang, J.; Li, S.; Li, W.; Shi, B.; Hao, Z. Rumen Epithelial Development- and Metabolism-Related Genes Regulate Their Micromorphology and VFAs Mediating Plateau Adaptability at Different Ages in Tibetan Sheep. Int. J. Mol. Sci. 2022, 23, 16078. [Google Scholar] [CrossRef]
- Zhou, J.W.; Jing, X.P.; Degen, A.A.; Liu, H.; Zhang, Y.; Yang, G.; Long, R. Effect of level of oat hay intake on apparent digestibility, rumen fermentation and urinary purine derivatives in Tibetan and fine-wool sheep. Anim. Feed. Sci. Technol. 2018, 241, 112–120. [Google Scholar] [CrossRef]
- Gui, H.; Shen, Z. Concentrate diet modulation of ruminal genes involved in cell proliferation and apoptosis is related to combined effects of short-chain fatty acid and pH in rumen of goats. J. Dairy Sci. 2016, 99, 6627–6638. [Google Scholar] [CrossRef]
- Greco, G.; Hagen, F.; Meißner, S.; Shen, Z.; Lu, Z.; Amasheh, S.; Aschenbach, J.R. Effect of individual SCFA on the epithelial barrier of sheep rumen under physiological and acidotic luminal pH conditions. J. Anim. Sci. 2018, 96, 126–142. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, X.; Chen, W.; Zhang, Y.; Jiang, Y.; Meng, Q.; Zhou, Z. Growth performance and development of internal organ, and gastrointestinal tract of calf supplementation with calcium propionate at various stages of growth period. PLoS ONE 2017, 12, e0179940. [Google Scholar] [CrossRef]
- Borthakur, A.; Saksena, S.; Gill, R.K.; Alrefai, W.A.; Ramaswamy, K.; Dudeja, P.K. Regulation of monocarboxylate transporter 1 (MCT1) promoter by butyrate in human intestinal epithelial cells: Involvement of NF-κB pathway. J. Cell. Biochem. 2008, 103, 1452–1463. [Google Scholar] [CrossRef]
- Trotta, R.J.; Keomanivong, F.E.; Peine, J.L.; Caton, J.S.; Swanson, K.C. Influence of maternal nutrient restriction and rumen-protected arginine supplementation on post-ruminal digestive enzyme activity of lamb offspring. Livest. Sci. 2020, 241, 104246. [Google Scholar] [CrossRef]
- Hao, Y.; Guo, C.; Gong, Y.; Sun, X.; Wang, W.; Wang, Y.; Yang, H.; Cao, Z.; Li, S. Rumen Fermentation, Digestive Enzyme Activity, and Bacteria Composition between Pre-Weaning and Post-Weaning Dairy Calves. Animals 2021, 11, 2527. [Google Scholar] [CrossRef]
- Yu, C.; Luo, Q.; Chen, Y.; Liu, S.; Zang, C. Impact of docusate and fauna-free on feed intake, ruminal flora and digestive enzyme activities of sheep. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1043–1051. [Google Scholar] [CrossRef]
- Moharrery, A.; Hymller, L.; Weisbjerg, M.R. The effect of rolled barley, sodium hydroxide-treated wheat or maize cob silage on digestive enzymes activity in the alimentary tract of dairy cows. J. Anim. Feed. Sci. 2017, 26, 303–310. [Google Scholar] [CrossRef]
- Gao, R.; Luo, Y.; Xu, S.; Wang, M.; Sun, Z.; Wang, L.; Yu, Z. Effects of Replacing Ensiled-Alfalfa with Fresh-Alfalfa on Dynamic Fermentation Characteristics, Chemical Compositions, and Protein Fractions in Fermented Total Mixed Ration with Different Additives. Animals 2021, 11, 572. [Google Scholar] [CrossRef]
- Gonzalez-Ronquillo, M.; Balcells, J.; Guada, J.A.; Vicente, F. Purine Derivative Excretion in Dairy Cows: Endogenous Excretion and the Effect of Exogenous Nucleic Acid Supply. J. Dairy Sci. 2003, 86, 1282–1291. [Google Scholar] [CrossRef]
- Zhang, R.; Wu, J.; Lei, Y.; Bai, Y.; Jia, L.; Li, Z.; Liu, T.; Xu, Y.; Sun, J.; Wang, Y.; et al. Oregano Essential Oils Promote Rumen Digestive Ability by Modulating Epithelial Development and Microbiota Composition in Beef Cattle. Front. Nutr. 2021, 8, 722557. [Google Scholar] [CrossRef]
- Hua, D.; Hendriks, W.H.; Xiong, B.; Pellikaan, W.F. Starch and Cellulose Degradation in the Rumen and Applications of Metagenomics on Ruminal Microorganisms. Animals 2022, 12, 3020. [Google Scholar] [CrossRef]
- Zhou, Z.; Fang, L.; Meng, Q.; Li, S.; Chai, S.; Liu, S.; Schonewille, J.T. Assessment of Ruminal Bacterial and Archaeal Community Structure in Yak (Bos grunniens). Front. Microbiol. 2017, 8, 179. [Google Scholar] [CrossRef]
- Bi, Y.; Zeng, S.; Zhang, R.; Diao, Q.; Tu, Y. Effects of dietary energy levels on rumen bacterial community composition in Holstein heifers under the same forage to concentrate ratio condition. BMC Microbiol. 2018, 18, 69. [Google Scholar] [CrossRef]
- Spirito, C.M.; Daly, S.E.; Werner, J.J.; Angenent, L.T. Redundancy in Anaerobic Digestion Microbiomes during Disturbances by the Antibiotic Monensin. Appl. Environ. Microbiol. 2018, 84, e02692-17. [Google Scholar] [CrossRef]
- Pang, K.; Wang, J.; Chai, S.; Yang, Y.; Wang, X.; Liu, S.; Ding, C.; Wang, S. Ruminal microbiota and muscle metabolome characteristics of Tibetan plateau yaks fed different dietary protein levels. Front. Microbiol. 2024, 15, 1275865. [Google Scholar] [CrossRef]
- Carberry, C.A.; Kenny, D.A.; Han, S.; McCabe, M.S.; Waters, S.M. Effect of Phenotypic Residual Feed Intake and Dietary Forage Content on the Rumen Microbial Community of Beef Cattle. Appl. Environ. Microbiol. 2012, 78, 4949–4958. [Google Scholar] [CrossRef]
- Aschalew, N.D.; Zhang, L.; Wang, Z.; Xia, Y.; Yin, G.; Dong, J.; Zhen, Y.; Zhang, X.; Wang, T.; Sun, Z.; et al. Effects of yeast culture and oxalic acid supplementation on in vitro nutrient disappearance, rumen fermentation, and bacterial community composition. Front. Veter. Sci. 2023, 10, 1330841. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, H.; Xu, J.; Zhai, Y.; Xia, H.; Xi, Y.; Han, Z. Characteristics of ruminal microbiota and metabolome in Holstein cows differing in milk protein concentrations. J. Anim. Sci. 2022, 100, skac253. [Google Scholar] [CrossRef]
- Guo, Y.Q.; Hu, Y.R.; Liu, S.R.; Wang, M.; Xian, Z.Y.; Liu, D.W.; Sun, B.L.; Li, Y.K.; Liu, G.B.; Deng, M.; et al. Effects of the Oat Hay Feeding Method and Compound Probiotic Supplementation on the Growth, Antioxidant Capacity, Immunity, and Rumen Bacteria Community of Dairy Calves. Antioxidants 2023, 12, 1851. [Google Scholar] [CrossRef]
- Pan, C.; Li, H.; Wang, F.; Qin, J.; Huang, Y.; Zhao, W. Dietary Supplementation with Bupleuri Radix Reduces Oxidative Stress Occurring during Growth by Regulating Rumen Microbes and Metabolites. Animals 2024, 14, 927. [Google Scholar] [CrossRef]
- Zhang, S.; Lv, H.; Cai, X.; Tang, S.; Zhong, R.; Chen, L.; Zhang, H. Effects of the compound extracts of Caprifoliaceae and Scutellaria baicalensis Georgi on the intestinal microbiota and antioxidant function. Front. Microbiol. 2023, 14, 1289490. [Google Scholar] [CrossRef]
- Ze, X.; Duncan, S.H.; Louis, P.; Flint, H.J. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J. 2012, 6, 1535–1543. [Google Scholar] [CrossRef]
- Flint, H.J.; Bayer, E.A.; Rincon, M.T.; Lamed, R.; White, B.A. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nat. Rev. Microbiol. 2008, 6, 121–131. [Google Scholar] [CrossRef]
- Liu, K.; Xu, Q.; Wang, L.; Wang, J.; Guo, W.; Zhou, M. The impact of diet on the composition and relative abundance of rumen microbes in goat. Asian-Australas. J. Anim. Sci. 2017, 30, 531–537. [Google Scholar] [CrossRef]
- Scano, P.; Murgia, A.; Pirisi, F.M.; Caboni, P. A gas chromatography-mass spectrometry-based metabolomic approach for the characterization of goat milk compared with cow milk. J. Dairy Sci. 2014, 97, 6057–6066. [Google Scholar] [CrossRef]
- Liu, C.; Wu, H.; Liu, S.; Chai, S.; Meng, Q.; Zhou, Z. Dynamic Alterations in Yak Rumen Bacteria Community and Metabolome Characteristics in Response to Feed Type. Front. Microbiol. 2019, 10, 1116. [Google Scholar] [CrossRef]
- Maia, M.R.; Chaudhary, L.C.; Bestwick, C.S.; Richardson, A.J.; McKain, N.; Larson, T.R.; Graham, I.A.; Wallace, R.J. Toxicity of unsaturated fatty acids to the biohydrogenating ruminal bacterium, Butyrivibrio fibrisolvens. BMC Microbiol. 2010, 10, 52. [Google Scholar] [CrossRef]
- Tian, K.E.; Aldian, D.; Luo, G.; Sossou, A.; Yayota, M. Condensed tannin-induced variations in the rumen metabolome and the correlation with fermentation characteristics in goats. Anim. Sci. J. 2024, 95, e13925. [Google Scholar] [CrossRef]
- Wu, D.; Xu, L.; Tang, S.; Guan, L.; He, Z.; Guan, Y.; Tan, Z.; Han, X.; Zhou, C.; Kang, J.; et al. Influence of Oleic Acid on Rumen Fermentation and Fatty Acid Formation In Vitro. PLoS ONE 2016, 11, e0156835. [Google Scholar] [CrossRef]
- Lopes, F.; Souza, S.M.D.; Filho, S.V.V.; Gama, M.; Agrarias, L.N.R.J.S.-C. Ruminal parameters and fatty acid composition of omasal digesta and milk in cows fed sugarcane-based diets supplemented with sunflower oil. Semin Cienc Agrar. 2020, 41, 2317–2334. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Feng, J.; Wan, B.; Tu, Q.; Cai, W.; Jin, F.; Tang, G.; Rodrigues, L.R.; Zhang, X.; et al. Modulation of chronic obstructive pulmonary disease progression by antioxidant metabolites from Pediococcus pentosaceus: Enhancing gut probiotics abundance and the tryptophan-melatonin pathway. Gut Microbes 2024, 16, 2320283. [Google Scholar] [CrossRef]
- Huang, S.; Zheng, G.; Men, H.; Wang, W.; Li, S. The Response of Fecal Microbiota and Host Metabolome in Dairy Cows Following Rumen Fluid Transplantation. Front. Microbiol. 2022, 13, 940158. [Google Scholar] [CrossRef]
- Yin, Q.; Yu, J.; Li, J.; Zhang, T.; Wang, T.; Zhu, Y.; Zhang, J.; Yao, J. Enhancing milk quality and modulating rectal microbiota of dairy goats in starch-rich diet: The role of bile acid supplementation. J. Anim. Sci. Biotechnol. 2024, 15, 7. [Google Scholar] [CrossRef]
- Bueno, I.C.; Brandi, R.A.; Franzolin, R.; Benetel, G.; Fagundes, G.M.; Abdalla, A.L.; Louvandini, H.; Muir, J.P. In vitro methane production and tolerance to condensed tannins in five ruminant species. Anim. Feed. Sci. Technol. 2015, 205, 1–9. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, J.; Li, M.M.; Zhao, G. Effects of taurine on rumen fermentation, nutrient digestion, rumen bacterial community and metabolomics and nitrogen metabolism in beef steers. J. Sci. Food Agric. 2023, 103, 3414–3426. [Google Scholar] [CrossRef]
- Malmuthuge, N.; Guan, L.L. Understanding host-microbial interactions in rumen: Searching the best opportunity for microbiota manipulation. J. Anim. Sci. Biotechnol. 2017, 8, 8. [Google Scholar] [CrossRef]
- Hua, C.; Tian, J.; Tian, P.; Cong, R.; Luo, Y.; Geng, Y.; Tao, S.; Ni, Y.; Zhao, R. Feeding a High Concentration Diet Induces Unhealthy Alterations in the Composition and Metabolism of Ruminal Microbiota and Host Response in a Goat Model. Front. Microbiol. 2017, 8, 138. [Google Scholar] [CrossRef]
- Yin, X.; Ji, S.; Duan, C.; Tian, P.; Ju, S.; Yan, H.; Zhang, Y.; Liu, Y. Age-Related Changes in the Ruminal Microbiota and Their Relationship With Rumen Fermentation in Lambs. Front. Microbiol. 2021, 12, 679135. [Google Scholar] [CrossRef]
- Kittelmann, S.; Seedorf, H.; Walters, W.A.; Clemente, J.C.; Knight, R.; Gordon, J.I.; Janssen, P.H. Simultaneous Amplicon Sequencing to Explore Co-Occurrence Patterns of Bacterial, Archaeal and Eukaryotic Microorganisms in Rumen Microbial Communities. PLoS ONE 2013, 8, e47879. [Google Scholar] [CrossRef]
- Wang, Z.; Elekwachi, C.; Jiao, J.; Wang, M.; Tang, S.; Zhou, C.; Tan, Z.; Forster, R.J. Changes in Metabolically Active Bacterial Community during Rumen Development, and Their Alteration by Rhubarb Root Powder Revealed by 16S rRNA Amplicon Sequencing. Front. Microbiol. 2017, 8, 159. [Google Scholar] [CrossRef]
- Haas, K.N.; Blanchard, J.L. Kineothrix alysoides, gen. nov., sp. nov., a saccharolytic butyrate-producer within the family Lachnospiraceae. Int. J. Syst. Evol. Microbiol. 2017, 67, 402–410. [Google Scholar] [CrossRef]
- Zened, A.; Combes, S.; Cauquil, L.; Mariette, J.; Klopp, C.; Bouchez, O.; Troegeler-Meynadier, A.; Enjalbert, F. Microbial ecology of the rumen evaluated by 454 GS FLX pyrosequencing is affected by starch and oil supplementation of diets. FEMS Microbiol. Ecol. 2013, 83, 504–514. [Google Scholar] [CrossRef]
- Lee, J.-H.; Wood, T.K.; Lee, J. Roles of Indole as an Interspecies and Interkingdom Signaling Molecule. Trends Microbiol. 2015, 23, 707–718. [Google Scholar] [CrossRef]
- Wyatt, M.; Greathouse, K.L. Targeting Dietary and Microbial Tryptophan-Indole Metabolism as Therapeutic Approaches to Colon Cancer. Nutrients 2021, 13, 1189. [Google Scholar] [CrossRef]
- Sun, D.; Bian, G.; Zhang, K.; Liu, N.; Yin, Y.; Hou, Y.; Xie, F.; Zhu, W.; Mao, S.; Liu, J. Early-life ruminal microbiome-derived indole-3-carboxaldehyde and prostaglandin D2 are effective promoters of rumen development. Genome Biol. 2024, 25, 64. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, S.C.; Oh, Y.G.; Kim, K.H.; Chung, I.B. Effects of Rumen Protected Oleic Acid in the Diet on Animal Performances, Carcass Quality and Fatty Acid Composition of Hanwoo Steers. Asian-Australasian J. Anim. Sci. 2003, 16, 1003–1010. [Google Scholar] [CrossRef]
- Polan, C.E.; McNeill, J.J.; Tove, S.B. Biohydrogenation of Unsaturated Fatty Acids by Rumen Bacteria. J. Bacteriol. 1964, 88, 1056–1064. [Google Scholar] [CrossRef]
- Hwang, I.H.; Kim, H.D.; Shim, S.S.; Lee, S.S.; Ha, J.K. Effect of Unsaturated Fatty Acids on Cellulose Degradation and Fermentation Characteristics by Mixed Ruminal Microbes. Asian-Australasian J. Anim. Sci. 2001, 14, 501–506. [Google Scholar] [CrossRef]
- Huws, S.A.; Kim, E.J.; Lee, M.R.; Scott, M.B.; Tweed, J.K.; Pinloche, E.; Wallace, R.J.; Scollan, N.D. As yet Uncultured Bacteria Phylogenetically Classified as Prevotella, Lachnospiraceae Incertae Sedis and Unclassified Bacteroidales, Clostridiales and Ruminococcaceae May Play a Predominant Role in Ruminal Biohydrogenation. Environ. Microbiol. 2011, 13, 1500–1512. [Google Scholar] [CrossRef]
- Fu, Y.; Yao, S.; Wang, T.; Lu, Y.; Han, H.; Liu, X.; Lv, D.; Ma, X.; Guan, S.; Yao, Y.; et al. Effects of melatonin on rumen microorganisms and methane production in dairy cow: Results from in vitro and in vivo studies. Microbiome 2023, 11, 196. [Google Scholar] [CrossRef]
- Wu, Q.; Liang, X.; Wang, K.; Lin, J.; Wang, X.; Wang, P.; Zhang, Y.; Nie, Q.; Liu, H.; Zhang, Z.; et al. Intestinal hypoxia-inducible factor 2α regulates lactate levels to shape the gut microbiome and alter thermogenesis. Cell Metab. 2021, 33, 1988–2003.e1987. [Google Scholar] [CrossRef]

| Items | Group | ||
|---|---|---|---|
| HP | MP | LP | |
| Ingredient | |||
| Oat hay | 15.00 | 15.00 | 15.00 |
| Oat silage | 15.00 | 15.00 | 15.00 |
| Corn | 32.41 | 32.20 | 37.10 |
| Wheat | 4.90 | 7.70 | 7.70 |
| Soybean meal | 5.60 | 1.40 | 0.70 |
| Rapeseed meal | 11.20 | 11.20 | 7.00 |
| Cottonseed meal | 1.40 | 0.70 | 0.70 |
| Maize germ meal | 1.40 | 0.70 | 0.70 |
| Palm meal | 8.40 | 11.20 | 11.20 |
| NaCl | 0.62 | 0.68 | 0.59 |
| Limestone | 0.70 | 0.70 | 0.70 |
| Baking soda | 0.07 | 0.07 | 0.07 |
| Premix 1 | 2.94 | 2.94 | 2.94 |
| Lysine | 0.29 | 0.39 | 0.48 |
| Methionine | 0.07 | 0.13 | 0.11 |
| Total | 100.00 | 100.00 | 100.00 |
| Nutrient levels 2 | |||
| Digestibility/MJ·kg−1 | 9.61 | 9.61 | 9.62 |
| Crude protein | 13.03 | 11.58 | 10.20 |
| Ether extract | 3.36 | 3.36 | 3.36 |
| Neutral detergent fiber | 32.95 | 33.03 | 32.82 |
| Acid detergent fiber | 15.25 | 15.43 | 15.04 |
| Calcium | 1.10 | 0.99 | 0.95 |
| Phosphorus | 0.60 | 0.57 | 0.55 |
| Item | HP | MP | LP | p-Value |
|---|---|---|---|---|
| pH | 6.24 ± 0.06 | 6.42 ± 0.13 | 6.67 ± 0.09 | 0.076 |
| Ammonia nitrogen (mg·L−1) | 329.53 ± 16.24 a | 235.83 ± 17.83 b | 225.25 ± 13.58 b | 0.001 |
| Total VFAs (mmol·mL−1) | 131.61 ± 7.23 a | 108.21 ± 4.80 b | 106.62 ± 1.38 b | 0.023 |
| VFA (mmol·mL−1) | ||||
| Acetate | 80.82 ± 2.87 | 78.79 ± 2.09 | 80.12 ± 3.41 | 0.806 |
| Propionate | 28.80 ± 0.87 a | 23.25 ± 1.25 ab | 17.00 ± 1.78 b | 0.001 |
| Butyrate | 12.77 ± 0.62 a | 7.30 ± 0.92 b | 9.11 ± 0.81 b | 0.001 |
| Isobutyrate | 1.95 ± 0.09 | 1.57 ± 0.11 | 1.99 ± 0.07 | 0.058 |
| Valerate | 2.32 ± 0.24 | 2.01 ± 0.12 | 1.69 ± 0.20 | 0.056 |
| Isovalerate | 4.17 ± 0.37 | 3.72 ± 0.62 | 3.95 ± 0.74 | 0.581 |
| Item | HP | MP | LP | p-Value |
|---|---|---|---|---|
| α-Amylase (μmol·L−1) | 194.52 ± 3.33 a | 183.45 ± 11.29 ab | 144.01 ± 4.38 b | 0.001 |
| Trypsin (ng·mL−1) | 490.10 ± 16.41 a | 442.26 ± 11.94 b | 446.67 ± 13.42 b | 0.039 |
| Cellulase (ng·L−1) | 153.61 ± 13.89 | 140.64 ± 9.66 | 138.98 ± 5.96 | 0.542 |
| Lipase (ng·L−1) | 471.09 ± 16.41 | 445.67 ± 21.94 | 446.48 ± 13.42 | 0.139 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Zhang, F.; Su, Q.; Ji, Q.; Zhu, K.; Zhang, Y.; Hou, S.; Gui, L. Integrating 16S rRNA Sequencing and LC-MS-Based Metabolomics to Evaluate the Effects of Dietary Crude Protein on Ruminal Morphology, Fermentation Parameter and Digestive Enzyme Activity in Tibetan Sheep. Animals 2024, 14, 2149. https://doi.org/10.3390/ani14152149
Wu Z, Zhang F, Su Q, Ji Q, Zhu K, Zhang Y, Hou S, Gui L. Integrating 16S rRNA Sequencing and LC-MS-Based Metabolomics to Evaluate the Effects of Dietary Crude Protein on Ruminal Morphology, Fermentation Parameter and Digestive Enzyme Activity in Tibetan Sheep. Animals. 2024; 14(15):2149. https://doi.org/10.3390/ani14152149
Chicago/Turabian StyleWu, Zhenling, Fengshuo Zhang, Quyangangmao Su, Qiurong Ji, Kaina Zhu, Yu Zhang, Shengzhen Hou, and Linsheng Gui. 2024. "Integrating 16S rRNA Sequencing and LC-MS-Based Metabolomics to Evaluate the Effects of Dietary Crude Protein on Ruminal Morphology, Fermentation Parameter and Digestive Enzyme Activity in Tibetan Sheep" Animals 14, no. 15: 2149. https://doi.org/10.3390/ani14152149
APA StyleWu, Z., Zhang, F., Su, Q., Ji, Q., Zhu, K., Zhang, Y., Hou, S., & Gui, L. (2024). Integrating 16S rRNA Sequencing and LC-MS-Based Metabolomics to Evaluate the Effects of Dietary Crude Protein on Ruminal Morphology, Fermentation Parameter and Digestive Enzyme Activity in Tibetan Sheep. Animals, 14(15), 2149. https://doi.org/10.3390/ani14152149






