Bacterial Diversity and Antimicrobial Resistance of Microorganisms Isolated from Teat Cup Liners in Dairy Farms in Shandong Province, China
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Bacterial Isolation and Identification
2.3. Antimicrobial Susceptibility Tests
2.4. Detection of Antimicrobial Resistance Genes
2.5. Statistical Analysis
3. Results
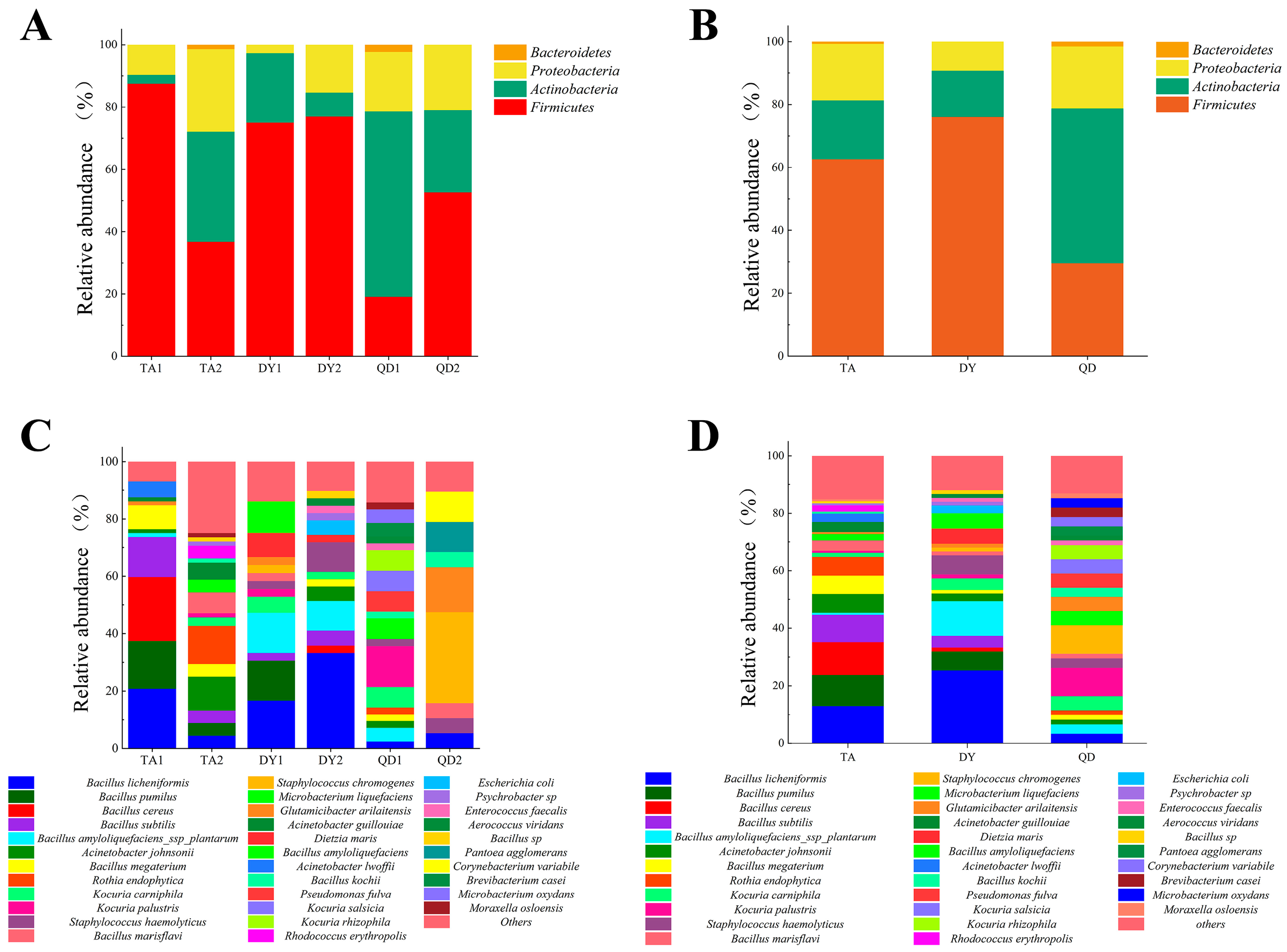
3.1. Bacterial Isolation
3.2. Antimicrobial Resistance and MDR Profile
3.3. Antimicrobial Resistance Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, N.W.; Fletcher, A.J.; Hill, J.P.; McNabb, W.C. Modeling the Contribution of Milk to Global Nutrition. Front. Nutr. 2021, 8, 716100. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Hu, Q.; Xu, F.; Ding, S.-Y.; Zhu, K. Characterization of Bacillus cereus in dairy products in China. Toxins 2020, 12, 454. [Google Scholar] [CrossRef]
- Antunes, P.; Novais, C.; Peixe, L. Food-to-Humans Bacterial Transmission. Microbiol. Spectr. 2020, 8. [Google Scholar] [CrossRef]
- Duse, A.; Persson-Waller, K.; Pedersen, K. Microbial aetiology, antibiotic susceptibility and pathogen-specific risk factors for udder pathogens from clinical mastitis in dairy cows. Animals 2021, 11, 2113. [Google Scholar] [CrossRef]
- Mola, I.; Onibokun, A.; Oranusi, S. Prevalence of multi-drug resistant bacteria associated with foods and drinks in Nigeria (2015–2020): A systematic review. Ital. J. Food Saf. 2021, 10, 9417. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U.; Maryam, L.; Zarrilli, R. Structure, Genetics and Worldwide Spread of New Delhi Metallo-beta-lactamase (NDM): A threat to public health. BMC Microbiol. 2017, 17, 101. [Google Scholar] [CrossRef]
- Yong, D.; Toleman, M.A.; Giske, C.G.; Cho, H.S.; Sundman, K.; Lee, K.; Walsh, T.R. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 2009, 53, 5046–5054. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Bell, B.G.; Schellevis, F.; Stobberingh, E.; Goossens, H.; Pringle, M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect Dis. 2014, 14, 13. [Google Scholar] [CrossRef]
- Junior, J.C.R.; Tamanini, R.; Soares, B.F.; de Oliveira, A.M.; de Godoi Silva, F.; da Silva, F.F.; Augusto, N.A.; Beloti, V. Efficiency of boiling and four other methods for genomic DNA extraction of deteriorating spore-forming bacteria from milk. Semin. Ciênc. Agrár. 2016, 37, 3069–3078. [Google Scholar] [CrossRef]
- Stenfors Arnesen, L.P.; Fagerlund, A.; Granum, P.E. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Rev. 2008, 32, 579–606. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Peng, C.; Zhang, G.; Shen, Y.; Zhang, Y.; Liu, C.; Liu, M.; Wang, F. Prevalence and characteristics of multidrug-resistant Proteus mirabilis from broiler farms in Shandong Province, China. Poult. Sci. 2022, 101, 101710. [Google Scholar] [CrossRef] [PubMed]
- Ehling-Schulz, M.; Lereclus, D.; Koehler, T.M. The Bacillus cereus Group: Bacillus Species with Pathogenic Potential. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Martlbauer, E.; Granum, P.E. Bacillus cereus Toxins. Toxins 2021, 13, 295. [Google Scholar] [CrossRef] [PubMed]
- Grutsch, A.A.; Nimmer, P.S.; Pittsley, R.H.; Kornilow, K.G.; McKillip, J.L. Molecular pathogenesis of Bacillus spp., with emphasis on the dairy industry. Fine Focus 2018, 4, 203–222. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018, 16, e05500. [Google Scholar] [CrossRef]
- Berthold-Pluta, A.; Pluta, A.; Garbowska, M.; Stefanska, I. Prevalence and toxicity characterization of Bacillus cereus in food products from Poland. Foods 2019, 8, 269. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Ding, Y.; Wu, Q.; Wang, J.; Zhang, J.; Yu, S.; Yu, P.; Liu, C.; Kong, L.; Feng, Z.; et al. Prevalence, Virulence Genes, Antimicrobial Susceptibility, and Genetic Diversity of Bacillus cereus Isolated From Pasteurized Milk in China. Front. Microbiol. 2018, 9, 533. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Cui, C.; Li, X.; Yan, J.; Sun, E.; Wang, C.; Guo, H.; Hao, Y. Prevalence, antimicrobial susceptibility, and antibiotic resistance gene transfer of Bacillus strains isolated from pasteurized milk. J. Dairy Sci. 2023, 106, 75–83. [Google Scholar] [CrossRef]
- Liu, B.G.; Xie, M.; Gong, Y.T.; Dong, Y.; Zheng, G.M.; Wu, H.; Hu, G.Z.; Bai, M.; Xu, E.P. Prevalence, resistance phenotypes, and fluoroquinolone resistance genes of Salmonella isolates from raw milk of healthy dairy cows in Henan province, China. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 6837–6844. [Google Scholar] [CrossRef]
- Wu, X.; Liu, J.; Feng, J.; Shabbir, M.A.B.; Feng, Y.; Guo, R.; Zhou, M.; Hou, S.; Wang, G.; Hao, H.; et al. Epidemiology, Environmental Risks, Virulence, and Resistance Determinants of Klebsiella pneumoniae from Dairy Cows in Hubei, China. Front. Microbiol. 2022, 13, 858799. [Google Scholar] [CrossRef] [PubMed]
- Abunna, F.; Ashenafi, D.; Beyene, T.; Ayana, D.; Mamo, B.; Duguma, R. Isolation, identification and antimicrobial susceptibility profiles of Salmonella isolates from dairy farms in and around Modjo town, Ethiopia. Ethiop. Vet. J. 2017, 21, 92–108. [Google Scholar] [CrossRef]
- Zhou, L.J.; Ying, G.G.; Liu, S.; Zhang, R.Q.; Lai, H.J.; Chen, Z.F.; Pan, C.G. Excretion masses and environmental occurrence of antibiotics in typical swine and dairy cattle farms in China. Sci. Total Environ. 2013, 444, 183–195. [Google Scholar] [CrossRef]
- Nielsen, T.K.; Browne, P.D.; Hansen, L.H. Antibiotic resistance genes are differentially mobilized according to resistance mechanism. Gigascience 2022, 11, giac072. [Google Scholar] [CrossRef]
- Achard, A.; Villers, C.; Pichereau, V.; Leclercq, R. New lnu(C) gene conferring resistance to lincomycin by nucleotidylation in Streptococcus agalactiae UCN36. Antimicrob. Agents Chemother. 2005, 49, 2716–2719. [Google Scholar] [CrossRef]
- Li, Y.; Qiu, Y.; She, J.; Wang, X.; Dai, X.; Zhang, L. Genomic Characterization of a Proteus sp. Strain of Animal Origin Co-Carrying bla(NDM-1) and lnu(G). Antibiotics 2021, 10, 1411. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.Y.; Liu, M.F.; Wang, M.S.; Zhao, X.X.; Jia, R.Y.; Chen, S.; Sun, K.F.; Yang, Q.; Wu, Y.; Chen, X.Y.; et al. A novel resistance gene, lnu(H), conferring resistance to lincosamides in Riemerella anatipestifer CH-2. Int. J. Antimicrob. Agents 2018, 51, 136–139. [Google Scholar] [CrossRef]
- Stepien-Pysniak, D.; Hauschild, T.; Dec, M.; Marek, A.; Brzeski, M.; Kosikowska, U. Antimicrobial resistance and genetic diversity of Enterococcus faecalis from yolk sac infections in broiler chicks. Poult. Sci. 2021, 100, 101491. [Google Scholar] [CrossRef]
- Poirel, L.; Walsh, T.R.; Cuvillier, V.; Nordmann, P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn. Microbiol. Infect. Dis. 2011, 70, 119–123. [Google Scholar] [CrossRef]
- Li, R.; Xie, M.; Zhang, J.; Yang, Z.; Liu, L.; Liu, X.; Zheng, Z.; Chan, E.W.; Chen, S. Genetic characterization of mcr-1-bearing plasmids to depict molecular mechanisms underlying dissemination of the colistin resistance determinant. J. Antimicrob. Chemother. 2017, 72, 393–401. [Google Scholar] [CrossRef]
- Doma, A.O.; Popescu, R.; Mituletu, M.; Muntean, D.; Degi, J.; Boldea, M.V.; Radulov, I.; Dumitrescu, E.; Muselin, F.; Puvaca, N.; et al. Comparative Evaluation of qnrA, qnrB, and qnrS Genes in Enterobacteriaceae Ciprofloxacin-Resistant Cases, in Swine Units and a Hospital from Western Romania. Antibiotics 2020, 9, 698. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, P.; Singh, A.; Chowdhary, P.; Pandey, A.; Gupta, P. Occurrence of emerging sulfonamide resistance (sul1 and sul2) associated with mobile integrons-integrase (intI1 and intI2) in riverine systems. Sci. Total Environ. 2021, 751, 142217. [Google Scholar] [CrossRef] [PubMed]
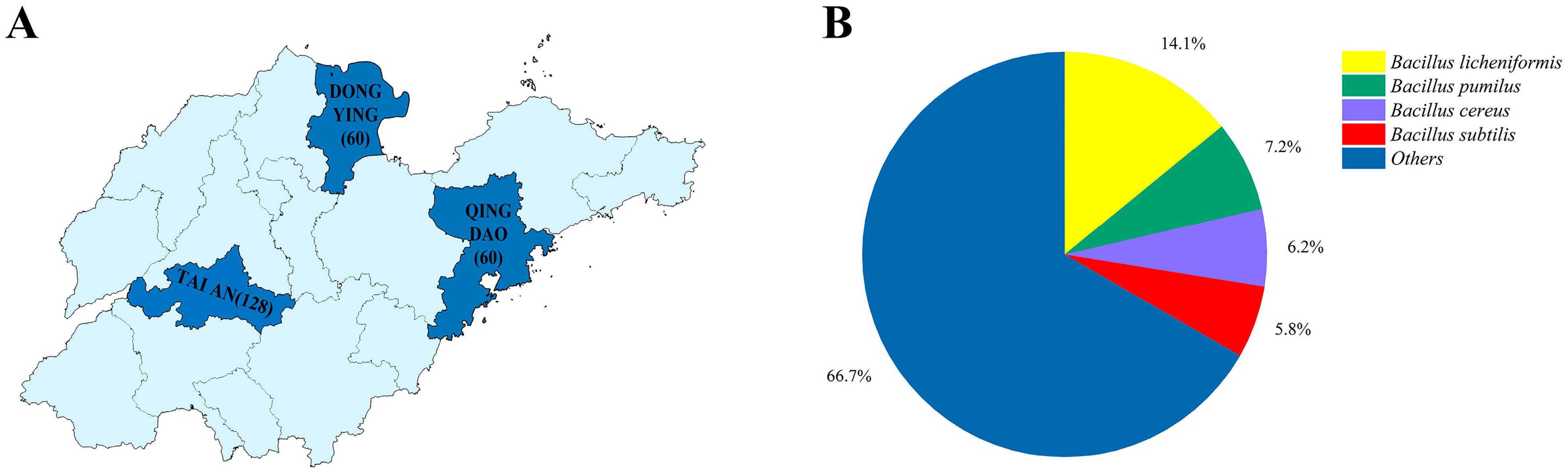



| Region | Farm 1 | Farm 2 | Total |
|---|---|---|---|
| Tai’an | 39.47% (30/76) | 63.46% (33/52) | 49.22% (63/128) |
| Dongying | 63.33% (19/30) | 73.33% (22/30) | 68.33% (41/60) |
| Qingdao | 100% (30/30) | 63.33% (19/30) | 81.67% (49/60) |
| Total | - | - | 61.69% (153/248) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, G.; Wang, S.; Cui, Y.; Xue, K.; Liu, Y.; Liu, J. Bacterial Diversity and Antimicrobial Resistance of Microorganisms Isolated from Teat Cup Liners in Dairy Farms in Shandong Province, China. Animals 2024, 14, 2167. https://doi.org/10.3390/ani14152167
Yan G, Wang S, Cui Y, Xue K, Liu Y, Liu J. Bacterial Diversity and Antimicrobial Resistance of Microorganisms Isolated from Teat Cup Liners in Dairy Farms in Shandong Province, China. Animals. 2024; 14(15):2167. https://doi.org/10.3390/ani14152167
Chicago/Turabian StyleYan, Guangwei, Shengnan Wang, Yuehui Cui, Kun Xue, Yongxia Liu, and Jianzhu Liu. 2024. "Bacterial Diversity and Antimicrobial Resistance of Microorganisms Isolated from Teat Cup Liners in Dairy Farms in Shandong Province, China" Animals 14, no. 15: 2167. https://doi.org/10.3390/ani14152167






